Abstract
Background
Proximal gastrectomy (PG) is widely performed in Japan as a function-preserving surgical approach. Since esophagogastrostomy (EG) was associated with increased reflux symptoms and anastomotic strictures, we have chosen double-tract reconstruction (DTR) as the standard reconstruction method since March 2013. In this study, we described a novel method of laparoscopic DTR using detachable ENDO-PSD and compared its 1-year outcome with EG performed formerly in our institution.
Methods
Patients who underwent laparoscopic PG between May 2005 and July 2014 were retrospectively divided into two groups based on the type of reconstruction and were subsequently analyzed (19 patients in the DTR group and 22 in the EG group). All of them underwent a laparoscopic PG with regional lymph node dissection. In the DTR group, the lower left port site was extended to 4 cm, and an intracorporeal purse-string suture was performed using the detachable ENDO-PSD. The jejunogastrostomy was fashioned on the anterior side of the remnant stomach parallel to the transection line, 2 cm from the cut end. The EG group used the conventional purse-string suture instrument through the 6 cm upper midline mini-laparotomy incision. Patient characteristics, operative data, early operative complications and 1-year postoperative follow-up findings were compared between the two groups.
Results
The frequencies of reflux symptoms (10.5 vs. 54.5%, P = 0.003), usage of proton pump inhibitors (31.6 vs. 72.7%, P = 0.008), and anastomotic strictures (0 vs. 27%, P = 0.014) were significantly lower in the DTR group as compared to the EG group. There were no significant differences between the two groups with regard to operation time, blood loss, postoperative hospital stay, postoperative complications, average postoperative/preoperative weight loss ratio, and postoperative/preoperative ratio of biochemical markers (hemoglobin, total protein, albumin, cholesterol).
Conclusion
Our results indicate that DTR is a useful reconstruction method after PG, especially in terms of preventing reflux esophagitis and anastomotic strictures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Recently, the incidence of proximal gastric cancer has been increasing in both Western and Eastern countries [1,2,3]. Total or proximal gastrectomy is usually performed for gastric cancers affecting the upper third of the stomach. However, although many previous studies have compared total gastrectomy (TG) and proximal gastrectomy (PG), the ideal surgical approach for gastric cancer of the upper stomach is still a topic of debate. A recent multi-institutional study comparing long-term quality of life after PG and TG revealed that PG was better than TG in terms of preventing postoperative weight loss, the necessity for additional meals, diarrhea and dumping [4]. Several studies have shown that hemoglobin levels are significantly higher after PG when compared with TG in the long-term outcomes [5,6,7]. Although PG is a function-preserving surgery, with preservation of the distal stomach and pyloric ring, many surgeons choose TG for fear of reflux esophagitis and anastomotic stenosis postoperatively [8, 9]. Several types of reconstruction can be performed after PG, such as esophagogastrostomy (EG), double-tract reconstruction (DTR) (including a jejunogastrostomy and jejunojejunostomy), jejunal interposition (JI), and jejunal pouch interposition (JPI). In Japan, EG is the most commonly used method of reconstruction [10]. However, esophageal reflux and anastomotic stenosis are reported to be more frequent with EG than with DTR [11]. Recently, the laparoscopic approach has been adopted for both distal gastrectomy and PG with increasing frequency and with acceptable outcomes [11,12,13,14]. We have been performing laparoscopic PG (LPG) at our institution since the 1990s. We conducted this study to compare clinical outcomes between the two reconstruction methods, laparoscopic DTR and laparoscopic EG, especially in terms of the incidence of reflux esophagitis and anastomotic stenosis 1 year after operation.
Materials and methods
Between May 2005 and July 2014, we performed 51 LPGs for patients who had a confirmed pathologic diagnosis of primary gastric cancer. The indications for LPG were early gastric cancer of clinical stage IA and IB located in the upper third of the stomach, and also some advanced gastric cancers in high risk patients who had severe complications or were over 80 years of age, in whom reduction in the extent of gastrectomy and lymphadenectomy was considered preferable to total gastrectomy. Initially, we performed EG, but the rate of reflux symptoms was high, and we could not overcome the symptoms even though we performed anti-reflux procedures similar to Toupet fundoplication in the later five patients. Therefore, we started to perform DTR since March 2013. DTR was performed in 21 of the patients, 25 underwent EG using a circular stapler, and 5 underwent reconstruction with EG using a linear stapler. In this study, only patients who underwent reconstruction with a circular stapler, either DTR or EG, were included. We also excluded two patients in the DTR group and three patients in the EG group who were not followed up by endoscopy 1 year after operation. The resulting series of 41 cases included 8 female and 33 male patients, with a mean age of 70.2 years. There were 19 patients in the DTR group and 22 in the EG group.
The clinical characteristics of the patients (i.e., sex, age, BMI, tumor location [15], stage [16]), operation time, blood loss, postoperative hospital stay, and postoperative complications were analyzed. The primary outcome measure was the incidence of esophagitis and anastomotic strictures. Patients were routinely followed up at our outpatient clinic at 1, 3, 6 and 12 months postoperatively, and every 6 or 12 months thereafter. Endoscopy was routinely performed every 12 months after the surgery. Information on the presence of reflux symptoms, use of PPIs, and endoscopic findings at postoperative month 12 were obtained from our prospectively collected gastric cancer database and the patients’ medical records. PPIs were not used routinely and were only prescribed for patients who complained of reflux symptoms. Endoscopic findings of esophagitis were categorized by the Los Angeles classification [17], and the amount of food stagnation was classified using a previously reported method [18]. For patients who complained of dysphagia during postoperative follow-up, endoscopy was performed before the scheduled routine endoscopy to rule out anastomotic strictures.
In addition, postoperative digestive function was assessed based on weight loss at postoperative months 6 and 12, and by the postoperative/preoperative ratio of biochemical markers (hemoglobin, total protein, albumin and cholesterol) 1-year postoperatively.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Declaration of Helsinki 1964 and later versions. Informed consent or substitute for it was obtained from all patients for being included in the study.
This article does not contain any studies with animal subjects performed by any of the authors.
Surgical procedures
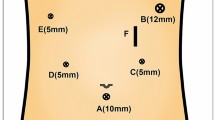
All the PG procedures were performed by the 5-port technique under general anesthesia with the patient in the lithotomy position. The surgeon stood on the patient’s right, the assistant stood on the patient’s left, and the camera operator sat between the patient’s legs. After 10–12 mmHg of pneumoperitoneum was established through 12-mm infra-umbilical camera port, additional four working ports were introduced into the right upper quadrant (5 mm), right middle quadrant (12 mm), left middle quadrant (12 mm), and left upper quadrant (7 mm) regions of the abdomen. The Nathanson liver retractor (Cook Surgical, Bloomington, IN, USA) was inserted in the midline of the upper region (Fig. 1). Vessels along the greater and lesser curvatures of the stomach, including the right gastroepiploic vessels and right gastric vessels, were preserved, and D1 + lymphadenectomy was performed [19]. The caudal side of the specimen was divided using a linear stapler.
Reconstruction with esophagogastrostomy
An approximately 6 cm upper midline mini-laparotomy incision was made, and a wound retraction system (WR; Alexis wound retractor, Applied Medical, CA, USA) was used. A purse-string suture instrument was inserted through a plastic glove, which was attached to the wound retraction system, re-establishing the pneumoperitoneum. The oral side of the specimen was cut after clamping the esophagus by the instrument, and followed by a purse-string suture intracorporeally. The anvil head of the 25-mm circular stapler (Proximate ILS CDH25; Ethicon Endo-Surgery, LLC., Cincinnati, OH, USA) was inserted into the esophageal stump and fixed in it. After taking out the specimen, an entry hole for the circular stapler was made on the anterior wall in the middle of the stomach. The circular stapler was inserted though the plastic glove, and the pneumoperitoneum was re-established. Esophagogastrostomy was performed intracorporeally. Subsequently, the incision site on the stomach was closed by hand sewing. Expecting an anti-reflux system, in some cases the remnant stomach was sutured to the crura of the diaphragm to form a pseudofornix, in some cases fundoplication by wrapping the stomach around the anastomosis was performed, and in others these procedures were combined.
Reconstruction by the double-tract method (Video)
After lymphadenectomy and resecting the proximal stomach with a linear stapler, the specimen was externalized from the lower left port site, which was extended to 4 cm. The wound retraction system (WR; Alexis wound retractor, Applied Medical, CA, USA) was used. The anvil head of a 25-mm circular stapler (Proximate ILS CDH25; Ethicon Endo-Surgery, LLC., Cincinnati, Ohio, USA) was inserted through the extended wound and was temporally placed inside the abdomen. An intracorporeal purse-string suture was performed using the detachable ENDO-PSD (TKZ-F3900, Takasago Medical Industry Co. Ltd, Japan). The detachable ENDO-PSD is a purse-string suture instrument which consists of two parts, the clamp and the rod. It enables the instrument to be inserted through a trocar by inserting the rod through the trocar before attaching the clamp to it. The assembled ENDO-PSD was brought inside the abdomen, and the pneumoperitoneum was re-established. The cut end of the esophagus was held by forceps to make sure that the clamp was parallel to the transection line. After clamping the esophageal stump, a double-armed straight needle suture was placed through the instrument. The staple line of the esophagus was cut off by laparosonic coagulating shears (LCS). The clamp can be detached intracorporeally, so that it is possible to advance to the next procedure without releasing the pneumoperitoneum. The anvil head of the circular stapler was fixed in the esophageal stump. The jejunum was divided by a linear stapler approximately 25 cm from the ligament of Treitz, in a length the esophagojejunostomy site would be tension-free. The distal jejunal stump was carried out of the peritoneal cavity, and the circular stapler was inserted through a plastic glove, into the distal jejunal stump and was fed distally to a point 5 cm from the stump. The jejunal stump was tied to the circular stapler to avoid slippage. The pneumoperitoneum was re-established again with the plastic glove, and the distal jejunum was brought up in an antecolic fashion. The center rod of the stapler was brought out to pierce the bowel wall intracorporeally and was connected to the corresponding anvil head fixed in the esophageal stump. Taking care not to twist the jejunum nor to bite the mesentery, an esophagojejunostomy was performed intracorporeally. The jejunal stump was closed by a linear stapler (Fig. 2). A silk thread of 15 cm length was used to measure 15 cm distal to the esophagojejunostomy, and crystal violet was used to leave a mark on the opposite side of the mesentery. An anastomosis was fashioned on the anterior side of the remnant stomach parallel to the transection line. To maintain blood flow between the staple lines, the anastomosis was created 2 cm from the cut end. The entry holes were opened by LCS, and a 60-mm linear stapler was inserted through the lower left port site. The bigger side of the jaw was inserted into the entry hole of the jejunum, and then the smaller side of the jaw was carried into the hole of the remnant stomach. The operator controls the stomach, and the assistant controls the jejunum so that the stapler runs on the opposite side of the mesentery. The antimesenteric border of the jejunum and the anterior wall of the remnant stomach were approximated. The common entry hole was closed with an absorbable running suture (Fig. 3). Whenever possible, the entry hole was closed extracorporeally. A side-to-side jejunojejunostomy was created 20 cm distal to the jejunogastrostomy. The antimesenteric borders of the proximal and distal jejunum were approximated by a linear stapler, and the common entry hole was closed by another linear stapler. The mesenteric gap and the Petersen’s defect were manually closed with non-absorbable running sutures to prevent internal hernia.
Esophagojejunostomy was performed using detachable ENDO-PSD. A Double-armed straight needle suture was placed through the instrument. B The anvil head of the circular stapler was inserted into the esophageal stump. C The anvil head was fixed in it. D The center rod of the circular stapler pierced the bowel wall at a point 5 cm from the stump and was connected to the anvil head intracorporeally. E The jejunal stump was closed using a linear stapler. F Esophagojejunostumy completed
Jejunogastrostomy side-to-side was performed 15 cm distal to esophagojejunostomy. A The antimesenteric border of the jejunum and B the anterior side of the remnant stomach, parallel to the transection line of the stomach, 2 cm away from the cut end were C approximated by a 6 cm linear stapler. D The entry hole was closed with an absorbable running suture (D)
Statistical analysis
The statistical analyses were performed with SPSS statistical software version 20 for Windows (SPSS, Chicago, IL, USA). Categorical variables were compared using the Pearson χ 2 test, and continuous variables were compared using Student’s t test. All the values are expressed as mean ± standard deviation (SD) of the mean. Statistical significance was set at P < 0.05.
Results
Patient characteristics and clinical findings are shown in Table 1. There were no significant differences between the two groups with respect to age (71 ± 9.9 vs. 69 ± 10.2 years, mean ± SD, P = 0.627), sex (16:3 vs. 17:5, male/female, P = 0.576) and BMI (21.4 ± 2.2 vs. 22.0 ± 1.9 kg/m2, mean ± SD, P = 0.627). The DTR group tended to have a higher proportion of patients with advanced gastric cancer than the EG group (P = 0.045). 47.4% of the patients in the DTR group were diagnosed as Stage IIA or higher, while only 4.5% (one patient) was in the EG group. Six patients in the DTR group underwent adjuvant chemotherapy and none in the EG group.
The frequencies of reflux symptoms (10.5 vs. 54.5%, P = 0.003), usage of proton pump inhibitors (31.6 vs. 72.7%, P = 0.008), anastomotic strictures (0 vs. 27%, P = 0.014), and food stagnation in the remnant stomach (5.3 vs. 59.1%, P < 0.001) were significantly lower in the DTR group as compared to the EG group (Table 2).
There were no significant differences between the two groups with regard to operation time (325 ± 66.9 vs. 290.3 ± 55.1 min, mean ± SD, P = 0.074), blood loss (131.4 ± 118.7 vs. 132.0 ± 129.7 ml, mean ± SD, P = 0.988), hospital stay after operation (10.2 ± 4.6 vs. 9.8 ± 2.7 days, mean ± SD, P = 0.705), postoperative complications and average weight loss ratio at both 6 months (13.2 ± 6.1 vs. 12.6 ± 6.3%, mean ± SD, P = 0.775) and 12 months (12.4 ± 6.6 vs. 12.2 ± 6.4%, mean ± SD, P = 0.930) after the surgery, and in the postoperative/preoperative ratio of biochemical markers (hemoglobin, total protein, albumin and cholesterol), even though six patients in the DTR group received adjuvant chemotherapy (Tables 3, 4).
Fluoroscopy using diatrizoate meglumine and diatrizoate sodium as the contrast medium showed half of the medium passing through the remnant stomach and the rest directly to the jejunum (Fig. 4).
Discussion
The results of this study proved our hypothesis that DTR following proximal gastrectomy more effectively prevents reflux esophagitis and anastomotic strictures as compared to EG. In our study, the incidence of both reflux esophagitis and anastomotic stenosis was lower in the DTR group than in the EG group. There was no significant difference in the incidence of endoscopic reflux esophagitis, although the usage of PPIs may have influenced these results.
In the present study, the incidence of reflux symptoms 1 year after operation in the DTR group was 10.5% (two patients), which was significantly lower than that in the EG group (54.5%). It is also lower than the incidence in the EG group (32.0%) in the study by Ahn et al. [20]. However, the incidence seems to be a little higher than those in the DTR group (4.65%) in the study by Ahn et al. [11], the JI group (5.5%) of the study by Katai et al. [21], and the JI group (6.5%) of Zhao et al.’s study [5]. Nomura et al. evaluated the presence of esophagitis using endoscopic findings, and found that esophagitis was observed in 10% of patients in both the DTR and JI groups [22], which is similar to the results in our DTR group, in which 10.5% of the patients were diagnosed with reflux esophagitis by endoscopy. According to the previous studies and our present study, DTR and JI seem to be associated with a lower incidence of reflux symptoms compared to EG. With both the DTR and JI methods, the jejunal interposition substitutes for the cardiac sphincter and reduces gastroesophageal reflux. In our study, the location of the cancer in the two patients who suffered from reflux symptoms despite the use of PPI drugs was the gastroesophageal junction (Siewert type II), and the oral end of the resected specimen was 2–3 cm more proximal to that in patients whose cancer did not involve the gastroesophageal junction. In these patients, the lower esophageal sphincter is resected, and the anastomosis site is located in the lower mediastinum, where negative intrathoracic pressure may increase gastroesophageal reflux [23]. Hence, the type of reconstruction operation must be selected after careful consideration in patients with gastroesophageal junction cancers.
Previously, the length of the interposed jejunum in the DTR method used to be longer than 30 cm, since formerly PG was only done for cases in which the stomach remnant was less than one-third the size of the original stomach [10]. This long jejunal interposition segment made it difficult for the endoscope to reach the remnant stomach during postoperative follow-up evaluations. However, now PG is mostly performed when the stomach remnant is likely to be more than half the original size of the stomach, allowing for a shorter jejunal interposition segment. The length of the interposed jejunum was 15 cm in the present study, and the endoscope reached the duodenum via the remnant stomach postoperatively in all cases. The interposed jejunum was 10 cm in the DTR group in the study by Ahn et al. [11], and 15 cm in Nomura et al.’s study [22]. Theoretically, a longer interposed jejunum can reduce the incidence of reflux esophagitis, although a jejunal loop of 20 cm did not result in a lower incidence of reflux esophagitis in the previous studies that assessed the jejunal interposition technique [5, 6, 21, 24]. Therefore, a 10–15 cm long interposed jejunal segment may be the appropriate length to prevent reflux esophagitis, while not being a concern in terms of endoscopic observation of the remnant stomach during follow-up evaluations.
In our study, not all patients with reflux symptoms had food stagnation in the remnant stomach, although food stagnation cannot be ignored because it interferes with thorough endoscopic observation of the remnant stomach postoperatively, leading to a delay in detecting second primary gastric cancers in the remnant stomach, the incidence of which seems to be higher than that in patients after distal gastrectomy [21].
Although DTR requires three anastomoses and the procedures seem to be more complicated, there was no significant difference in the operation time or blood loss between the two types of reconstruction procedures. However, the results of our study are limited by its retrospective nature and small sample size.
The frequency of anastomotic stenosis was higher in the EG group in this study, similar to that reported by Johansson et al., who reported that esophagogastric anastomotic sites following esophagectomy were narrower and developed more benign strictures than esophagojejunal anastomoses following total gastrectomy. They opined that this could be the result of different vascularization and the erosive effect of the refluxed duodenal and gastric contents [25]. According to previous literature, the main risk factors associated with benign anastomotic strictures seem to be anastomotic technique, limited circular stapler diameter, poor vascular supply, and anastomotic leak [26]. In our study, leakage was not observed in any of the patients analyzed, and the diameter of the circular stapler used (25 mm) was the same in all patients. All the six patients who suffered anastomotic stenosis could eat 50% of the served meal before they were discharged from the hospital. (We teach patients not to eat more than 50% of the meal offered.) However, after they were discharged, their loss of appetite gradually occurred, and 21–50 days after operation, they were diagnosed as anastomotic stenosis by endoscopy. Their stomach was full with food stagnation, which may have caused tension on the esophagogastric anastomosis, leading to poorer vascular supply with esophagogastric anastomosis and stenosis in the EG group. All the six patients with stenosis were successfully treated with endoscopic balloon dilatations.
Difficulty in maintaining body weight is a defining characteristic of the post-gastrectomy syndrome. In the present study, the mean weight loss 12 months after PG with DTR was 12.4%, whereas an average weight loss of 13.8–15.8% has been reported after total gastrectomy [4, 7, 21]. Various mechanisms have been postulated for this, but reduced food intake due to early satiety is the most conceivable explanation for weight loss after total gastrectomy [11, 27, 28]. In the previous studies, PG was mostly reconstructed by EG, although DTR was also associated with a tendency toward less weight loss and rapid recovery of total protein and albumin levels [20]. Low rate of food stagnation in the DTR group in our study might mean little inflow to the remnant stomach, and actually our postoperative fluoroscopy showed half of the medium passing directly through the jejunum. However, in our study, even though the DTR procedure has an esophagojejunostomy and on imaging, half of the contrast remains in the small bowel, none of the 19 patients in the DTR group suffered from dumping syndrome or diarrhea. Patients in the DTR and EG groups did not have significantly different postoperative weight loss and nutritional indicators. PG is probably associated with lower postoperative weight loss by maintaining gastric reserve [29], which suggests that preservation of the stomach rather than the reconstruction method affects the production of gastric acid, pepsin and intrinsic factor, and hence, the postoperative nutritional status and weight loss.
In conclusion, our results indicate that DTR is a useful reconstruction method after PG. Although this study is limited by its retrospective nature and short follow-up period, it suggests the value of DTR, use of which should be encouraged. Randomized studies to determine the most desirable reconstruction method following PG are required to confirm this conclusion.
References
Ahn HS, Lee HJ, Yoo MW, Jeong SH, Park DJ, Kim HH et al (2011) Changes in clinicopathological features and survival after gastrectomy for gastric cancer over a 20-year period. Br J Surg 98:255–260
Harrison LE, Karpeh MS, Brennan MF (1998) Total gastrectomy is not necessary for proximal gastric cancer. Surgery 123:127–130
Kim JH, Park SS, Kim J, Boo YJ, Kim SJ, Mok YJ, Kim CS (2006) Surgical outcomes for gastric cancer in the upper third of the stomach. World J Surg 30:1870–1876 (discussion 1877–1878)
Takiguchi N, Takahashi M, Ikeda M, Inagawa S, Ueda S, Nobuoka T et al (2015) Long-term quality-of-life comparison of total gastrectomy and proximal gastrectomy by Postgastrectomy Syndrome Assessment Scale (PGSAS-45): a nationwide multi-institutional study. Gastric Cancer 18:407–416
Zhao P, Xiao SM, Tang LC, Ding Z, Zhou X, Chen XD (2014) Proximal gastrectomy with jejunal interposition and TGRY anastomosis for proximal gastric cancer. World J Gastroenterol 20:8268–8273
Nozaki I, Hato S, Kobatake T, Ohta K, Kubo Y, Kurita A (2013) Long-term outcome after proximal gastrectomy with jejunal interposition for gastric cancer compared with total gastrectomy. World J Surg 37:558–564
Masuzawa T, Takiguchi S, Hirao M, Imamura H, Kimura Y, Fujita J et al (2014) Comparison of perioperative and long-term outcomes of total and proximal gastrectomy for early gastric cancer: a multi-institutional retrospective study. World J Surg 38:1100–1106
Wen L, Chen XZ, Wu B, Chen XL, Wang L, Yang K et al (2012) Total vs. proximal gastrectomy for proximal gastric cancer: a systematic review and meta-analysis. Hepatogastroenterology 59:633–640
Pu YW, Gong W, Wu YY, Chen Q, He TF, Xing CG (2013) Proximal gastrectomy versus total gastrectomy for proximal gastric carcinoma. A meta-analysis on postoperative complications, 5-year survival, and recurrence rate. Saudi Med J 34:1223–1228
Kumagai K, Shimizu K, Yokoyama N, Aida S, Arima S, Aikou T (2012) The Japanese Society for the Study of Postoperative Morbidity after Gastrectomy. Questionnaire survey regarding the current status and controversial issues concerning reconstruction after gastrectomy in Japan. Surg Today 42:411–418
Ahn SH, do Jung H, Son SY, Lee CM, do Park J, Kim HH (2014) Laparoscopic double-tract proximal gastrectomy for proximal early gastric cancer. Gastric Cancer 17:562–570
Pavlidis TE, Pavlidis ET, Sakantamis AK (2012) The role of laparoscopic surgery in gastric cancer. J Minim Access Surg 8:35–38
Tanimura S, Higashino M, Fukunaga Y, Kishida S, Ogata A, Fujiwara Y, Osugi H (2007) Laparoscopic gastrectomy with regional lymph node dissection for upper gastric cancer. Br J Surg 94:204–207
Uyama I, Sugioka A, Fujita J, Komori Y, Matsui H, Hasumi A (2000) Completely laparoscopic proximal gastrectomy with jejunal interposition and lymphadenectomy. J Am Coll Surg 191:114–119
Siewert JR, Feith M, Stein HJ (2005) Biologic and clinical variations of adenocarcinoma at the esophago-gastric junction: relevance of a topographic-anatomic subclassification. J Surg Oncol 90:139–146
Sobin LH, Gospodarowicz MK, Wittekind Ch (eds) (2009) International Union Against Cancer (UICC). TNM classification of malignant tumors, 7th edn. Wiley-Blackwell, Oxford
Dent J, Brun J, Fendrick AM, Fennerty MB, Janssens J, Kahrilas PJ et al (1999) An evidence-based appraisal of reflux disease management—the Genval Workshop Report. Gut 44:1–16
Kubo M, Sasako M, Gotoda T, Ono H, Fujishiro M, Saito D et al (2002) Endoscopic evaluation of the remnant stomach after gastrectomy: proposal for a new classification. Gastric Cancer 5:83–89
Association Japanese Gastric Cancer (2011) Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 14:113–123
Ahn SH, Lee JH, Park DJ, Kim HH (2013) Comparative study of clinical outcomes between laparoscopy-assisted proximal gastrectomy (LAPG) and laparoscopy-assisted total gastrectomy (LATG) for proximal gastric cancer. Gas Cancer 16:282–289
Katai H, Morita S, Saka M, Taniguchi H, Fukagawa T (2010) Longterm outcome after proximal gastrectomy with jejunal interposition for suspected early cancer in the upper third of the stomach. Br J Surg 97:558–562. doi:10.1002/bjs.6944
Nomura E, Lee SW, Kawai M, Yamazaki M, Nabeshima K, Nakamura K, Uchiyama K (2014) Functional outcomes by reconstruction technique following laparoscopic proximal gastrectomy for gastric cancer: double tract versus jejunal interposition. World J Surg Oncol 12:20
Aly A, Jamieson GG (2004) Reflux after oesophagectomy. Br J Surg 91:137–141. doi:10.1002/bjs.4508
Tokunaga M, Ohyama S, Hiki N, Hoshino E, Nunobe S, Fukunaga T et al (2008) Endoscopic evaluation of reflux esophagitis after proximal gastrectomy: comparison between esophagogastric anastomosis and jejunal interposition. World J Surg 32:1473–1477
Johansson J, Zilling T, von Holstein CS, Johnsson F, Oberg S, Walther B (2000) Anastomotic diameters and strictures following esophagectomy and total gastrectomy in 256 patients. World J Surg 24:78–84 (discussion 84–85)
Wang WP, Gao Q, Wang KN, Shi H, Chen LQ (2013) A prospective randomized controlled trial of semi-mechanical versus hand-sewn or circular stapled esophagogastrostomy for prevention of anastomotic stricture. World J Surg 37:1043–1050
Braga M, Zuliani W, Foppa L, Di Carlo V, Cristallo M (1988) Food intake and nutritional status after total gastrectomy: results of a nutritional follow-up. Br J Surg 75:477–480
Bergh C, Sjostedt S, Hellers G, Zandian M, Sodersten P (2003) Meal size, satiety and cholecystokinin in gastrectomized humans. Physiol Behav 78:143–147
Katai H, Sano T, Fukagawa T, Shinohara H, Sasako M (2003) Prospective study of proximal gastrectomy for early gastric cancer in the upper third of the stomach. Br J Surg 90:850–853
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosures
Tomoki Aburatani, Kazuyuki Kojima, Sho Otsuki, Hideaki Murase, Keisuke Okuno, Kentaro Gokita, Chiharu Tomii, Toshiro Tanioka and Mikito Inokuchi have no conflicts of interest or financial ties to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (WMV 353094 kb)
Rights and permissions
About this article
Cite this article
Aburatani, T., Kojima, K., Otsuki, S. et al. Double-tract reconstruction after laparoscopic proximal gastrectomy using detachable ENDO-PSD. Surg Endosc 31, 4848–4856 (2017). https://doi.org/10.1007/s00464-017-5539-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-017-5539-4