Abstract
Purpose
Low-intensity venous blood flow restriction (vBFR) resistance training has been shown to promote increases in muscle strength and size. Eccentric-only muscle actions are typically a more potent stimulus to increase muscle strength and size than concentric-only muscle actions performed at the same relative intensities. Therefore, the purpose of this investigation was to examine the time-course of changes in muscle strength, hypertrophy, and neuromuscular adaptations following 4 weeks of unilateral forearm flexion low-intensity eccentric vBFR (Ecc-vBFR) vs. low-intensity concentric vBFR (Con-vBFR) resistance training performed at the same relative intensity.
Methods
Thirty-six women were randomly assigned to either Ecc-vBFR (n = 12), Con-vBFR (n = 12) or control (no intervention, n = 12) group. Ecc-vBFR trained at 30% of eccentric peak torque and Con-vBFR trained at 30% of concentric peak torque. All training and testing procedures were performed at an isokinetic velocity of 120° s−¹.
Results
Muscle strength increased similarly from 0 to 2 and 4 weeks of training as a result of Ecc-vBFR (13.9 and 35.0%) and Con-vBFR (13.4 and 31.2%), but there were no changes in muscle strength for the control group. Muscle thickness increased similarly from 0 to 2 and 4 weeks of training as a result of Ecc-vBFR (11.4 and 12.8%) and Con-vBFR (9.1 and 9.9%), but there were no changes for the control group. In addition, there were no changes in any of the neuromuscular responses.
Conclusions
The Ecc-vBFR and Con-vBFR low-intensity training induced comparable increases in muscle strength and size. The increases in muscle strength, however, were not associated with neuromuscular adaptations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent studies (Abe et al. 2006; Fujita et al. 2008; Laurentino et al. 2012) have examined the effects of venous blood flow restriction (vBFR) vs. non-vBFR resistance training on muscle strength and hypertrophy. For example, 1 week of low-intensity [20% of one-repetition maximum (1RM)] vBFR leg extension resistance training increased 1RM and muscle cross-sectional area by 6.7 and 3.5%, respectively (Fujita et al. 2008). Low-intensity non-vBFR resistance training at the same intensity, however, had no effects on 1RM or muscle cross-sectional area (Fujita et al. 2008). In addition, 8 weeks of low-intensity (20% of 1RM) vBFR leg extension resistance training increased 1RM and muscle cross-sectional area by 40.1 and 6.3%, respectively, while low-intensity non-vBFR resistance training at the same intensity resulted in smaller increases of 20.7% for 1RM and no significant changes in muscle cross-sectional area (Laurentino et al. 2012).
Previous investigations (Karabulut et al. 2010; Takarada et al. 2000; Ellefsen et al. 2015) have also demonstrated that low-intensity (≤ 50% of 1RM) vBFR resistance training elicited comparable increases in muscle strength and hypertrophy as high-intensity (≥ 50% of 1RM) non-vBFR resistance training. For example, Takarada et al. (2000) reported no differences for training-induced increases in muscle strength (18.4–22.6%) and muscle cross-sectional area (18.4–20.3%) following 16 weeks of low-intensity (30–50% of 1RM) vBFR vs. high-intensity (50–80% of 1RM) non-vBFR forearm flexion resistance training. In addition, Ellefsen et al. (2015) found no differences between 12 weeks of low-intensity (30% of 1RM) vBFR leg extension resistance training and high-intensity (60–80% of 1RM) non-vBFR resistance training for increases in 1RM (10–12%) or muscle cross-sectional area (6–7%).
It has been hypothesized that the increases in muscle strength and hypertrophy associated with low-intensity vBFR resistance training are related to cell swelling (Loenneke et al. 2012a) and/or metabolite accumulation (Loenneke et al. 2011) that stimulate the mTOR pathway via an intrinsic volume sensor (Haussinger 1996). Hoffmann et al. (2009) suggested that the changes in intracellular pH associated with cell hydration and/or swelling likely affect the anabolic responses by enhancing the activity of ion exchange pumps. In addition, unlike high-intensity non-vBFR resistance training (Phillips 2000; Moritani and deVries 1979; Staron et al. 1994), the early phase increases in muscle strength as a result of low-intensity vBFR resistance training appear to be driven primarily by hypertrophy and to a lesser extent, neuromuscular adaptations (Loenneke et al. 2012b).
Yasuda et al. (2013) reported that 6 weeks of low-intensity (30% of 1RM) concentric-only vBFR (Con-vBFR) resistance training resulted in greater increases in muscle strength (8.6 vs. 3.8%) and hypertrophy (11.7 vs. 3.9%) than eccentric-only vBFR (Ecc-vBFR) resistance training. These findings (Yasuda et al. 2013) were contrary to high-intensity non-vBFR resistance training where the increases in muscle strength and hypertrophy are typically greater during eccentric-only than concentric-only resistance training performed at a similar relative intensity (i.e., maximal eccentric vs. maximal concentric training) (Roig et al. 2009). The differences in training adaptions reported by Yasuda et al. (2013), however, may have been due to the relative training intensity that was lower during Ecc-vBFR (approximately 10% of eccentric 1RM) than Con-vBFR (30% of concentric 1RM). In addition, Yasuda et al. (2013) examined low-intensity Ecc-vBFR vs. Con-vBFR isotonic resistance training. During isotonic resistance training, the time under tension that the external load is resisted changes due to acceleration and deceleration phases and based on the trajectory of the external load relative to gravitational pull. These changes in the time duration that the external load is resisted may affect training-induced adaptations as a result of Ecc-vBFR vs. Con-vBFR. For example, Burd et al. (2012) demonstrated that muscle protein synthesis (a precursor to hypertrophy and strength adaptations) was enhanced when time under tension was increased (slow vs. fast repetitions) during work-matched leg extension muscle actions performed at 30% of 1RM. In addition, Popov et al. (2006) reported that increasing the time that the external load was maintained throughout a range of motion during leg press resistance training elicited greater increases in blood lactate, growth hormone, insulin-like growth factor, and cortisol. Together, these findings (Burd et al. 2012; Popov et al. 2006) indicated that time under tension affects the training response. During isotonic resistance training, however, it is difficult to maintain time under tension or consistent force against the external load throughout a range of motion. Thus, the present study examined the effects of low-intensity Ecc-vBFR vs. Con-vBFR isokinetic resistance training where the resistance of the external load was consistent throughout the range of motion.
Therefore, the purpose of this investigation was to examine the time-course of changes in muscle strength, hypertrophy, and neuromuscular adaptations following 4 weeks of unilateral forearm flexion low-intensity Ecc-vBFR vs. low-intensity Con-vBFR resistance training performed at the same relative intensity. Based on previous investigations (Roig et al. 2009; Loenneke et al. 2012b; Yasuda et al. 2013), we hypothesized that Ecc-vBFR would result in greater increases in muscle strength and hypertrophy than Con-vBFR, but there would be no changes in the neuromuscular responses for either mode of training.
Methods
Subjects
Thirty-six women volunteered to participate in this investigation and were randomly assigned to one of three groups: Ecc-vBFR (n = 12; mean age ± SD = 21.7 ± 1.0 years; body mass = 56.0 ± 6.6 kg; height = 166.4 ± 6.7 cm), Con-vBFR (n = 12; mean age ± SD = 22.1 ± 1.7 years; body mass = 55.4 ± 5.0 kg; height = 165.9 ± 5.2 cm), or control (n = 12; mean age ± SD = 23.3 ± 2.0 years; body mass = 55.7 ± 5.1 kg; height = 165.7 ± 5.5 cm). The subjects had no known cardiovascular, pulmonary, metabolic, muscular, and/or coronary heart disease, or regularly used prescription medication. All subjects were recreationally active at the time of testing, but no subjects had been actively participating in resistance training for at least the past six months. The subjects visited the laboratory on 15 occasions (familiarization, baseline, 13 testing/training visits) within a 5-week period and performed the testing procedures at the same time of day. The study was approved by the University Institutional Review Board for Human Subjects and all subjects completed a health history questionnaire and signed a written informed consent prior to testing.
Experimental design
A randomized, repeated measures, between-group, parallel design was used for this study. Thirty-six women were randomly assigned to one of three groups: (1) low-intensity Ecc-vBFR; (2) low-intensity Con-vBFR; or (3) a control group that received no intervention. Currently, women are an understudied population in the resistance training literature and less is known regarding the effects of vBFR on muscle strength and muscle hypertrophy (Counts et al. 2016b). Venous BFR was applied using a KAATSU resistance band and vBFR was determined for each subject as 40% of the lowest amount of pressure needed to completely occlude the brachial artery as indicated by ultrasound. Subjects assigned to Ecc-vBFR trained at 30% of eccentric peak torque and Con-vBFR trained at 30% of concentric peak torque and training was performed three times per week for 4 weeks. Training consisted of 75 eccentric (Ecc-vBFR) or concentric (Con-vBFR) isokinetic muscle actions of the forearm flexors performed over four sets (1 × 30, 3 × 15) and each set was separated by 30 s of rest. The subjects in the control group did not perform resistance training or receive vBFR. All subjects performed testing procedures that were completed at the baseline, 0, 2, and 4 weeks testing visits and all testing and training procedures were performed using an isokinetic dynamometer performed at a velocity of 120° s−1 and were performed at the same time of day (± 2 h). During each testing session, ultrasound, muscle strength, and electromyography (EMG) were measured.
Procedures
Familiarization
The first laboratory visit consisted of an orientation session to familiarize the subjects with the testing protocols. During the orientation, subjects performed submaximal and maximal isometric muscle actions as well as submaximal and maximal concentric and eccentric isokinetic muscle actions of the forearm flexors at 120° s−1 on a Cybex 6000 isokinetic dynamometer. To familiarize the subjects with the training protocols, the subjects also practiced performing concentric or eccentric isokinetic muscle actions at 30% of their concentric or eccentric peak torque, respectively. Torque was visually tracked using real-time torque displayed on a computer monitor.
Determination of eccentric peak torque, concentric peak torque, and maximal voluntary isometric contraction
During the baseline, 0, 2, and 4 weeks testing visits, the subjects performed a warm-up consisting of 10 submaximal (approximately 50% effort), concentric and eccentric muscle actions of the forearm flexors performed at 120° s−1. Following the warmup, the subjects rested for five minutes and then performed two randomly ordered maximal eccentric, concentric, and isometric muscle actions of the forearm flexors at 120° s−1 to determine the pretest eccentric peak torque, concentric peak torque, and maximal voluntary isometric contraction (MVIC) values, respectively. The highest peak torque and MVIC force produced during each of the two attempts was used for further analyses. The eccentric and concentric muscle actions were performed through a 120° range of motion (0°–120° of elbow flexion, where 0° corresponds to full extension at the elbow) and the MVIC muscle actions were performed at 45° sustained for a period of 3-s.
Eccentric and concentric training interventions
The subjects in the Ecc-vBFR and Con-vBFR groups completed 4 weeks of training at a frequency of three training sessions per week (separated by 48-h) for a total of 12 training sessions. Each training session consisted of 75 eccentric or concentric muscle actions of the forearm flexors performed over four sets (1 × 30, 3 × 15) and each set was separated by 30 s of rest (Thiebaud et al. 2013; Loenneke et al. 2016; Counts et al. 2016a; Yasuda et al. 2013). The training intervention was randomly assigned to either the dominant or non-dominant arm. All muscle actions were performed at a velocity of 120° s−1 and all eccentric or concentric muscle actions were followed by a passive concentric or eccentric muscle action, respectively, that was assisted by the investigator (E.C.H). The Ecc-vBFR and Con-vBFR training interventions were performed at the same relative intensity. Specifically, the Ecc-vBFR training group performed 75 eccentric muscle actions of the forearm flexors at 30% of eccentric peak torque and the Con-vBFR training group performed 75 concentric forearm flexion muscle actions at 30% of concentric peak torque. Thus, the relative training intensity, velocity and tempo, number of repetitions performed, and rest between sets were identical between the Ecc-vBFR and Con-vBFR interventions. The relative training intensity, repetitions, rest between sets, and frequency of training were consistent with previous investigations (Thiebaud et al. 2013; Loenneke et al. 2016; Counts et al. 2016a; Yasuda et al. 2013) that have examined low-intensity vBFR and were selected to optimize the training-induced adaptations on muscle strength and hypertrophy. Furthermore, a recent meta-analysis (Loenneke et al. 2012b) reported that effects sizes for increasing muscle strength and hypertrophy as a result of vBFR resistance training were greatest using training loads of 15–30% of 1RM, performing 60–70 repetitions with 30 s between sets, performed 2–3 days per week. In addition, there were no differences in muscle thickness, whole body lactate, or muscle activation using vBFR arterial occlusion pressures of 40–90% when combined with a training load of 30% of 1RM (Loenneke et al. 2016; Counts et al. 2016a).
Venous blood flow restriction
Venous blood flow restriction was applied using a 30 mm wide cuff (KAATSU Master, Sato Sports Plaza, Tokyo, Japan) placed on the most proximal portion of the upper arm (Fig. 1). The cuff pressure was initially applied at 30 mmHg and progressively inflated and deflated over a 60-s period until the target pressure was reached. Target pressure was calculated during the baseline, 0, and 2 weeks testing visits as 40% of the lowest amount of pressure needed to completely occlude the brachial artery as indicated by ultrasound (Counts et al. 2016a; Loenneke et al. 2016, 2013). Previous investigations (Counts et al. 2016a; Loenneke et al. 2016, 2013) have indicated that 40% of vBFR induces similar training-induced responses as 90% of vBFR when combined with low-intensity training (30% 1RM). The cuff remained inflated during the duration of the training bout and was deflated immediately after completing the 75 repetitions. The total duration of vBFR was approximately five minutes.
Venous blood flow restriction (vBFR) was applied using a 30-mm wide cuff (KAATSU Master, Sato Sports Plaza, Tokyo, Japan) placed on the most proximal portion of the upper arm. The cuff pressure was initially applied at 30 mmHg and progressively inflated and deflated over a 60-s period until the target pressure was reached. Target pressure was calculated during the baseline, 0, and 2 weeks testing visits as 40% of the lowest amount of pressure needed to completely occlude the brachial artery as indicated by ultrasound (Counts et al. 2016a; Loenneke et al. 2016, 2013). The cuff remained inflated during the duration of the training bout and was deflated immediately after completing the 75 repetitions. The total duration of vBFR was approximately five minutes
Neuromuscular
During baseline, 0, 2, and 4 weeks testing visits, pre-gelled surface electrodes (Ag/AgCl, AccuSensor, Lynn Medical, Wixom, MI, USA) were placed in a bipolar arrangement (30 mm center-to-center) on the biceps brachii muscle of the trained arm according to the recommendations of Barbero et al. (2012). The reference electrode was placed over the acromion process and prior to each electrode placement, the skin was shaved, carefully abraded, and cleaned with alcohol. The raw EMG signals were digitized at 2000 Hz with a 32-bit analog-to-digital converter (Model MP150, Biopac Systems, Inc.) and stored in a personal computer (ATIV Book 9 Intel Core i7 Samsung Inc., Dallas, TX, USA) for subsequent analyses. The EMG signals were amplified (gain: × 1000) using differential amplifiers (EMG 100, Biopac Systems, Inc., Santa Barbara, CA, USA) with a common mode rejection ratio of 110 dB min and an impedance of 2M Ω. The signals were digitally bandpass filtered (fourth-order Butterworth, zero-phase shift) at 10–500 Hz and all signal processing was performed in LabVIEW (National Instruments, Austin, TX, USA) using custom written programs. The amplitude of the EMG (µV root-mean-square, µVrms) signals were calculated from 40° to 80° of flexion at the elbow (0° corresponds to full extension of the elbow). Thus, signal epochs of 0.33 s (667 data points) were used to calculate the EMG values associated with the eccentric and concentric muscle actions. Similarly, the EMG values during the MVIC muscle actions were calculated for a time period that corresponds to 0.33 s (667 data points) over the middle one-ninth of the muscle action.
To examine potential trained-induced neural adaptations, electrical efficiency was determined during the baseline, 0, 2, and 4 weeks testing visits. Electrical efficiency was calculated as the ratio of EMG amplitude to torque production (i.e., μVRMS per Nm), whereby a decrease in electrical efficiency reflected improved efficiency (Pasquet et al. 2000; Lenman 1959; deVries 1968).
Ultrasound measurements
Muscle thickness and echo intensity were assessed via ultrasound prior to each testing and training visit. Ultrasound images of the trained arm (biceps brachii) were obtained using a portable brightness mode (B-mode) ultrasound-imaging device (GE Logiqe, USA) and a multi-frequency linear-array probe (12L-Rs; 5–13 MHz; 38.4 mm field-of-view). All ultrasound measurements were performed at a sampling rate of 10 MHz and at a gain of 58 dB. Ultrasound images were analyzed using ImageJ software (Version 1.47v., National Institutes of Health, Bethesda, MD, USA) and prior to all analyses, images were scaled from pixels to centimeters using the straight line function in ImageJ. Muscle thickness and echo intensity were assessed at 66% of the distance from the medial acromion of the scapula to the fossa cubit. Muscle thickness was determined as the distance from the adipose tissue–muscle interface to the muscle–bone interface. Echo intensity, as assessed by gray-scale analysis (0 arbitrary units (AU) corresponds to black image, 255 AU corresponds to white image) was performed using the histogram function and was determined from the same region of interest as muscle thickness. Great care was taken to ensure that consistent, minimal pressure was applied with the probe to limit compression of the tissue. To enhance acoustic coupling and reduce near field artifacts, a generous amount of water-soluble transmission gel was applied to the skin prior to each measurement.
Blood flow measurements were assessed at an insonation angle of 60° to the brachial artery. All measurements were taken while the subjects were lying in the supine position on the isokinetic dynamometer with both arms and legs supported. Blood flow was assessed from the brachial artery proximal to the antecubital fossa using Pulsed Wave Doppler. Blood flow was used to determine the vBFR pressure needed to completely occlude the brachial artery (assessed at baseline, 0, and 2 weeks).
Data analysis
Reliability
Test–retest reliability for eccentric peak torque, concentric peak torque, MVIC, muscle thickness, echo intensity, and neuromuscular responses (EMG amplitude and electrical efficiency assessed during the eccentric peak, concentric peak torque, and MVIC muscle actions) were assessed from the baseline and 0-week testing visits. Repeated measures ANOVAs were used to assess systematic error, and model 2,k (26) was used to calculate intraclass correlation coefficients (ICCs), standard errors of measurement (SEM), and minimal difference (MD) needed to consider a change as real (29). The 95% confidence intervals for the means of the dependent variables were calculated with the Student’s t distribution.
Normalization
The absolute EMG amplitude values at the baseline, 0, 2, and 4 weeks testing visits during the eccentric peak torque, concentric peak torque, and MVIC muscle actions were normalized to the EMG amplitude values obtained during the baseline MVIC muscle actions. Thus, all EMG amplitude values for each group (Ecc-vBFR, Con-vBFR, and control) and for each mode (eccentric peak torque, concentric peak, and MVIC) were expressed as percent changes from the EMG amplitude values obtained during the baseline MVIC muscle actions (Fig. 6).
Statistical analyses
Torque, EMG amplitude, and electrical efficiency were examined using separate 3 [Group (Ecc-vBFR, Con-vBFR, control)] × 4 [Time (baseline, 0 week, 2 weeks, 4 weeks)] × 3 [Mode (eccentric peak torque, concentric peak torque, and MVIC)] mixed factorial ANOVAs. Muscle thickness and echo intensity were examined using separate 3 [Group (Ecc-vBFR, Con-vBFR, control)] × 4 [Time (baseline, 0 week, 2 weeks, 4 weeks)] mixed factorial ANOVAs. In addition, a 2 [Group (Ecc-vBFR, Con-vBFR)] × 2 [Time (2 weeks, 4 weeks)] mixed factorial ANOVA was used to compare exercise volume performed during the first 2 weeks (2-week) and the second 2 weeks (4-week) of the training interventions. Significant interactions were decomposed with follow-up mixed factorial or repeated measures ANOVAs and Bonferonni-corrected independent or dependent samples t tests. Greenhouse–Geisser corrections were applied when sphericity was not met according to Mauchly’s Test of Sphericity and partial eta squared effect sizes (\(\eta _{p}^{2}\)) were calculated for each ANOVA. All statistical analyses were performed using IBM SPSS v. 25 (Armonk, NY, USA) and an alpha of p ≤ 0.05 considered statistically significant for all comparisons.
Results
Reliability
Table 1 includes the test–retest reliability and MD values from the baseline and 0-week measurements of muscle thickness, echo intensity, eccentric peak torque, concentric peak torque, MVIC, EMG amplitude, and electrical efficiency determined during each of the eccentric peak torque, concentric peak torque, and MVIC muscle actions. There were no mean differences for baseline vs. 0-week testing visits (p > 0.05) for any of the variables. The ICC values for all measured variables ranged from 0.719 to 0.971 and the SEM values ranged from 2.2 to 22.2% of the grand mean. For each measurement, the ICC and SEM are provided in Table 1.
Torque responses
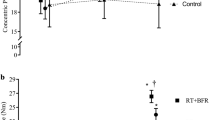
There was no significant three-way interaction (Group × Time × Mode), but there was a significant two-way interaction (Group × Time) and a significant main effect for Mode (Table 2). As a result of Ecc-vBFR, torque increased from baseline and 0 week to 2 weeks (9.1 and 13.9%) and 4 weeks (29.4 and 35.0%), respectively, and increased 18.6% from 2 to 4 weeks (collapsed across Mode) (Figs. 2, 3). For Con-vBFR, torque increased from baseline and 0 week to 2 weeks (14.9 and 13.4%) and 4 weeks (32.9 and 31.2%) and increased 15.7% from 2 to 4 weeks (collapsed across Mode). There were no changes in torque across Time for the control group.
Absolute (Nm) mean (± SE) changes (∆ = change) in eccentric peak torque, concentric peak torque, and maximal voluntary isometric contraction (MVIC) from baseline, 0, 2, and 4 weeks of training for the eccentric venous blood flow restriction (Ecc-vBFR = solid circles) training group, concentric vBFR (Con-vBFR = solid squares) training group, and control (solid triangles) group. For each Mode of torque measurement (eccentric peak torque, concentric peak torque, and MVIC) the minimal difference (MD = empty diamonds) needed for a change to be considered “real” is plotted and derived using standard error of measurement (SEM) values from the reliability data in Table 1 and using the equation, MD = SEM × 21/2 × df (Weir 2005)
Absolute (Nm) individual changes in strength [collapsed across Mode (eccentric peak torque, concentric peak torque, and maximal voluntary isometric contraction)] from baseline, 0, 2, and 4 weeks of training for the eccentric venous blood flow restriction (Ecc-vBFR = solid circles) training group, concentric vBFR (Con-vBFR = solid squares) training group, and control (solid triangles) group
There were no Group differences in torque at baseline or at 0 week. At 2 weeks, torque was greater as a result of Ecc-vBFR (27.4 Nm) compared to the control groups (25.7 Nm), and at 4 weeks torque was greater as a result of Ecc-vBFR (32.5 Nm) and Con-vBFR (30.9 Nm) compared to the control group (25.5 Nm) (collapsed across Mode).
The main effect for Mode indicated that MVIC torque (23.5 Nm) was greater than concentric peak torque (20.1 Nm), and eccentric peak torque (36.1 Nm) was greater than both MVIC and concentric peak torque (collapsed across Group and Time).
Muscle thickness
There was a significant two-way interaction (Group × Time) for muscle thickness (Table 2). Follow-up analyses indicated that muscle thickness increased from baseline and 0 week to 2 weeks (13.3 and 11.4%) and 4 weeks (14.6 and 12.8%) as a result of Ecc-vBFR, but there were no differences between 2 and 4 weeks (Fig. 4a). Similarly, muscle thickness increased from baseline and 0 week to 2 weeks (9.9 and 9.1%) and 4 weeks (10.7 and 9.9%), but there were no differences between 2 and 4 weeks (Figs. 4a, 5). In addition, at 2 weeks of training muscle thickness was greater as a result of Ecc-vBFR (2.41 cm) compared to control (2.15 cm) and at 4 weeks of training muscle thickness was greater as a result of Ecc-vBFR (2.44 cm) and Con-vBFR (2.35 cm) compared to control (2.15 cm).
Absolute (cm) mean (± SE) values for muscle thickness and echo intensity across 4 weeks of eccentric venous blood flow restriction (Ecc-vBFR = solid circles) training group, concentric vBFR (Con-vBFR = solid squares) training group, and control group (solid triangles). For each Group (Ecc-vBFR, Con-vBFR, and control), the minimal difference (MD = empty diamonds) needed for a change to be considered “real” is plotted and derived using standard error of measurement (SEM) values from the reliability data in Table 1 and using the equation, MD = SEM × 21/2 × df (Weir 2005)
Echo intensity
There was no significant two-way interaction (Group × Time) or significant main effects for Group or Time (Table 2). In addition, there were no changes in echo intensity at any of the time points (Fig. 4b). Thus, echo intensity was not affected by either Ecc-vBFR or Con-vBFR training. These findings suggested that neither Ecc-vBFR or Con-vBFR resulted in exercise-induced edema.
EMG amplitude
There was no significant three-way interaction (Group × Time × Mode), but there was a significant two-way interaction (Group × Mode) and no significant main effect for Time (Table 2). There were not, however, any significant follow-up analyses. Thus, there were no changes in EMG amplitude (muscle activation) as a result of either Ecc-vBFR or Con-vBFR (Fig. 6).
Normalized (to baseline maximal voluntary isometric contraction [MVIC]) mean (± SE) changes (∆ = change) in electromyographic (EMG) amplitude during the eccentric peak torque, concentric peak torque, and MVIC muscle actions from baseline, 0, 2, and 4 weeks of training for the eccentric venous blood flow restriction (Ecc-vBFR = solid circles) training group, concentric vBFR (Con-vBFR = solid squares) training group, and control (solid triangles) group. For each Mode of EMG amplitude measurement (eccentric peak torque, concentric peak torque, and MVIC) the minimal difference (MD = empty diamonds) needed for a change to be considered “real” is plotted and derived using standard error of measurement (SEM) values from the reliability data in Table 1 and using the equation, MD = SEM × 21/2 × df and normalized to baseline MVIC (MD/baseline MVIC EMG amplitude) (Weir 2005)
Electrical efficiency
There was no significant three-way interaction (Group × Time × Mode) or significant two-way interactions (Group × Time, Group × Mode, Time × Mode), but there were significant main effects for Time and Mode (Table 2). Specifically, electrical efficiency improved from baseline and 0 week to 2 weeks (20.6 and 15.0%) and improved from baseline and 0 week to 4 weeks (22.1 and 16.6%) (collapsed across Group and Mode). In addition, electrical efficiency was lower (more efficient) during the MVIC muscle actions (33.3 ± 10.4 μVRMS per Nm) than during the concentric peak torque muscle actions (37.4 ± 12.9 μVRMS per Nm), and electrical efficiency was lower (more efficient) during the eccentric peak torque muscle actions (19.7 ± 5.8 μVRMS per Nm) than both MVIC and concentric peak torque muscle actions (collapsed across Group and Time) (Fig. 7).
Absolute (μVRMS/Nm) mean (± SE) changes (∆ = change) in electrical efficiency during the eccentric peak torque, concentric peak torque, and maximal voluntary isometric contraction (MVIC) muscle actions from baseline, 0, 2, and 4 weeks of training for the eccentric venous blood flow restriction (Ecc-vBFR = solid circles) training group, concentric vBFR (Con-vBFR = solid squares) training group, and control (solid triangle) group. For each Mode of EMG amplitude measurement (eccentric peak torque, concentric peak torque, and MVIC), the minimal difference (MD = empty diamond) needed for a change to be considered “real” is plotted and derived using standard error of measurement (SEM) values from the reliability data in Table 1 and using the equation, MD = SEM × 21/2 × df (Weir 2005)
Exercise volume
There was no significant two-way interaction (Group × Time), but there were significant main effects for Group and Time (Table 2). Specifically, exercise volume per session was greater during Ecc-vBFR (777.2 ± 138.2 lbs) compared Con-vBFR (449.1 ± 83.3 lbs) (collapsed across Time) and exercise volume per session increased from 2 weeks (571.9 ± 115.1 lbs) to 4 weeks (731.3 ± 144.7 lbs) (collapsed across Group). Thus, the Ecc-vBFR group performed a greater volume of exercise compared to the Con-vBFR group, but there were increases in exercise volume from 2 to 4 weeks of training for Ecc-vBFR and Con-vBFR.
Discussion
Muscle strength and size
In the present study, there were no mode-specific (eccentric peak torque vs. concentric peak torque vs. MVIC) increases in strength as a result of the Ecc-vBFR or Con-vBFR training. There were increases in eccentric peak torque of 34.6 and 28.2%, concentric peak torque of 26.0 and 30.2%, and MVIC of 35.0 and 37.6% as a result of the Ecc-vBFR and Con-vBFR training, respectively (Figs. 2, 3). For both modes of training, the increases in eccentric peak torque, concentric peak torque, and MVIC exceeded the MD necessary to be considered “real” (Weir 2005) after 4 weeks of training. Thus, contrary to our hypothesis, the increases in muscle strength were similar as a result of the Ecc-vBFR and Con-vBFR training interventions. No previous investigations have compared the strength increases from isokinetic Ecc-vBFR vs. Con-vBFR training, but Yasuda et al. (2013) examined the effects of isotonic Ecc-vBFR vs. Con-vBFR training. Yasuda et al. (2013) reported a smaller increase in MVIC strength as a result of Ecc-vBFR (3.8%) than Con-vBFR (8.6%) following 6 weeks of training. In the study by Yasuda et al. (2013), the Ecc-vBFR training group trained at an intensity of 30% of 1RM which corresponded to approximately 10% of eccentric peak torque, compared to the 30% of eccentric peak that was used in the present study. These findings indicated that Ecc-vBFR training at 30% of 1RM (Yasuda et al. 2013) did not elicit increases in muscle strength that were comparable to isokinetic Ecc-vBFR training at 30% of eccentric peak torque. In addition, vBFR resistance training at 30% of isokinetic peak torque elicited comparable increases in muscle strength, regardless of training modality (Ecc-vBFR or Con-vBFR) when the relative training intensity, velocity and tempo, number of repetitions performed, and rest between sets were identical.
The increase in MVIC as a result of Con-vBFR training in the present study was also greater (37.6 vs. 8.6%) than that reported by Yasuda et al. (2013). The differences in training adaptations associated with Con-vBFR in the present study vs. those of Yasuda et al. (2013) may have been due to the type of training (isokinetic vs. isotonic) (Guilhem et al. 2010; Pipes and Wilmore 1975) and/or the joint angle at which MVIC was assessed (120° vs. 90°, where 180° corresponds to full extension at the elbow) (Kang et al. 2013; Yang et al. 2014). Thus, the present findings indicated that 4 weeks of low-intensity isokinetic Ecc-vBFR and Con-vBFR resulted in similar increases in strength across all modes of assessment (eccentric peak torque, concentric peak torque, and MVIC).
As a result of both the Ecc-vBFR and Con-vBFR training in the present study, there were increases in muscle thickness that exceeded the MD at 2 weeks and continued to increase to 4 weeks of training (Fig. 4a). The increases in muscle thickness, however, were not accompanied by changes in echo intensity which is thought to be related to edema (DeFreitas et al. 2011; Damas et al. 2016) (Fig. 4b). Therefore, the increases in muscle thickness were likely the result of muscle hypertrophy and not attributable to exercise-induced edema (DeFreitas et al. 2011; Damas et al. 2016). The present findings indicated that low-intensity vBFR training resulted in early phase increases in muscle hypertrophy within 2 weeks of training that was earlier than the 3.9–11.7% increases in muscle thickness reported by Yasuda et al. (2013) following 6 weeks of isotonic, forearm flexion Ecc-vBFR and Con-vBFR training. Like the effects of Ecc-vBFR training on MVIC strength, the smaller increases in muscle thickness after 6 weeks of training reported by Yasuda et al. (2013) compared to those of the present study at 2 and 4 weeks were likely due to the lower intensity of Ecc-vBFR training (approximately 10% of eccentric peak torque) compared to 30% of eccentric peak torque. Thus, the present findings indicated that both low-intensity Ecc-vBFR and Con-vBFR stimulated muscle hypertrophy during the early phases of training as evidenced by the increases in muscle thickness and lack of changes in exercise-induced edema.
Neuromuscular adaptations
In the present study, EMG amplitude remained unchanged (collapsed across modes of testing) from baseline to 0, 2, and 4 weeks as a result of low-intensity Ecc-vBFR and Con-vBFR training, and EMG amplitude was not different between the Ecc-vBFR group, Con-vBFR group, or control group (collapsed across Time). Furthermore, there were no “real” changes in electrical efficiency which has been used to track training-induced improvements in force production per unit of muscle activation (Nm/μVRMS) (Lenman 1959; deVries 1968). Together, these findings indicated that the training-induced increases in muscle strength in the present study were not associated with neural changes as assessed by EMG amplitude and electrical efficiency.
The present findings were consistent with previous investigations (Takarada et al. 2002, 2000; Fujita et al. 2008; Yasuda et al. 2011) that have also reported increases in muscle strength as a result of low-intensity vBFR training that were associated with increases in muscle size, but were not associated with neural changes. For example, Yasuda et al. (2011) reported increases in muscle strength as a result of 30% of 1RM vBFR bench press resistance training that were not associated with neural adaptations, but were likely the result of muscle hypertrophy. It is possible that low-intensity vBFR resistance training is not sufficient to elicit comparable neural adaptions as high-intensity non-BFR resistance training (Loenneke et al. 2012b). In support of this, previous investigations (Jenkins et al. 2016, 2017) have reported a dissociation in the training-induced increases in muscle strength and neural adaptations that were greater as a result of high-intensity non-vBFR vs. low-intensity non-vBFR resistance training. In addition, there were no significant increases in muscle strength per unit of muscle cross-sectional area during the early phases (< 8 weeks) of low-intensity vBFR resistance training studies (Loenneke et al. 2012b). Thus, the early phase increases in muscle strength as a result of low-intensity vBFR resistance training were coupled with or could be explained by muscle hypertrophy. The training-induced increases in muscle strength and size, but lack of neural changes may be a unique characteristic of low-intensity vBFR resistance training. Loenneke et al. (2012b) postulated that “…the traditional training adaption paradigm is reversed with low-intensity BFR exercise” (page 1856) as indicated by early phase increases in muscle strength and muscle hypertrophy, without neural changes. It is plausible, however, that neural adaptations may facilitate increases in muscle strength during longer duration low-intensity vBFR resistance training studies or that low-intensity vBFR and low-intensity non-vBFR resistance training is not of sufficient intensity to induce neural adaptations (Loenneke et al. 2012b). Collectively, the present findings indicated that there were no differences between low-intensity Ecc-vBFR and Con-vBFR training for the early phase changes in muscle strength, size, and muscle activation. Therefore, in conjunction with previous investigations (Takarada et al. 2002, 2000; Fujita et al. 2008; Yasuda et al. 2011), the present findings indicated the low-intensity Ecc-vBFR and Con-vBFR training resulted in early phase increases in muscle strength and size, without changes in muscle activation or efficiency.
Summary
The present findings indicated that 4 weeks of low-intensity isokinetic Ecc-vBFR and Con-vBFR resulted in similar increases in strength across all modes of assessment (eccentric peak torque, concentric peak torque, and MVIC). In addition, as a result of both the Ecc-vBFR and Con-vBFR training in the present study, there were increases in muscle thickness that exceeded the MD at 2 weeks and continued to increase to 4 weeks of training. There were, however, no changes in muscle activation (EMG amplitude) or electrical efficiency at any of the time points. Together, these findings indicated that the training-induced increases in muscle strength in the present study were not associated with neural changes as assessed by EMG amplitude and electrical efficiency, but were likely due to muscle hypertrophy (as indicated by the increases in muscle thickness without changes in echo intensity). Therefore, unlike non-vBFR resistance training, the present findings indicated that early phase increases in muscle strength as a result of low-intensity Ecc-vBFR and Con-vBFR training were due to increases in muscle size and not neural adaptations.
Limitations
In the present study, the women were not asked to provide information regarding their phase within the ovarian cycle or if they were using a contraceptive. It has been suggested (Wikstrom-Frisen et al. 2017; Sung et al. 2014; Sakamaki et al. 2012; Gil et al. 2017) that different phases of the ovarian cycle may enhance the effects of resistance training. For example, previous investigations (Wikstrom-Frisen et al. 2017; Sung et al. 2014) have reported that training-induced increases in muscle strength and size were greatest during the follicular phase, while other investigations (Sakamaki et al. 2012; Gil et al. 2017) have reported that training-induced increases in muscle strength and size were greatest during luteal phase. It has been suggested (Wikstrom-Frisen et al. 2017; Sung et al. 2014) that estrogen, which is highest during the follicular phase promotes anabolic signaling pathways, while progesterone, which is highest during the luteal phase supports catabolic activities. There were, however, no correlations for estrogen or progesterone and training-induced increases in muscle strength or size (Sakamaki et al. 2012). For women taking contraceptives, however, there appears to be no effect on the training induces increases in muscle strength or size (Nichols et al. 2008; Sarwar et al. 1996).
In the present study, all testings were performed within 30 ± 2 days that were consistent with the typical ovarian cycle of 27–31 days (Sung et al. 2014). Therefore, it is likely that all women completed the initial and final testing procedures at similar phases within their ovarian cycle across the 4 weeks of testing and training. Furthermore, the women were randomly assigned to each condition (Ecc-vBFR, Con-vBFR, and control group) to counterbalance the potential effects of the phase of ovarian cycle on the training-induced adaptations to muscle strength and size.
Abbreviations
- Ecc-vBFR:
-
Eccentric venous blood flow restriction
- Con-vBFR:
-
Concentric venous blood flow restriction
- vBFR:
-
Venous blood flow restriction
- EMG:
-
Electromyography
- 1RM:
-
One-repetition maximum
- MVIC:
-
Maximal voluntary isometric contraction
- SE:
-
Standard error
- ICC:
-
Intraclass correlation coefficient
- MD:
-
Minimal difference
- SEM:
-
Standard error of measurement
References
Abe T, Kearns CF, Sato Y (2006) Muscle size and strength are increased following walk training with restricted venous blood flow from the leg muscle, Kaatsu-walk training. J Appl Physiol 100(5):1460–1466
Barbero M, Merletti R, Rainoldi A (2012) Atlas of muscle innervation zones understanding surface electromyography and its applications. Springer, New York
Counts BR, Dankel SJ, Barnett BE, Kim D, Mouser JG, Allen KM, Thiebaud RS, Abe T, Bemben MG, Loenneke JP (2016a) Influence of relative blood flow restriction pressure on muscle activation and muscle adaptation. Muscle Nerve 53(3):438–445
Counts BR, Rossow LM, Mattocks KT, Mouser JG, Jessee MB, Buckner SL, Dankel SJ, Loenneke JP (2016b) Let’s talk about sex: where are the young females in blood flow restriction research? Clin Physiol Funct Imaging 38(1):1–3
Damas F, Phillips SM, Lixandrao ME, Vechin FC, Libardi CA, Roschel H, Tricoli V, Ugrinowitsch C (2016) Early resistance training-induced increases in muscle cross-sectional area are concomitant with edema-induced muscle swelling. Eur J Appl Physiol 116(1):49–56
DeFreitas JM, Beck TW, Stock MS, Dillon MA, Kasishke PR II (2011) An examination of the time course of training-induced skeletal muscle hypertrophy. Eur J Appl Physiol 111(11):2785–2790
deVries HA (1968) “Efficiency of electrical activity” as a physiological measure of the functional state of muscle tissue. Am J Phys Med 47(1):10–22
Ellefsen S, Hammarström D, Strand TA, Zacharoff E, Whist JE, Rauk I, Nygaard H, Vegge G, Hanestadhaugen M, Wernbom M, Cumming KT, Rønning R, Raastad T, Rønnestad BR (2015) Blood flow-restricted strength training displays high functional and biological efficacy in women: a within-subject comparison with high-load strength training. Am J Physiol Regul Integr Comp Physiol 309(7):R767–R779
Fujita T, Brechue WF, Kurita K, Sato Y, Abe T (2008) Increased muscle volume and strength following six days of low-intensity resistance training with restricted muscle blood flow. Int J KAATSU Train Res 4(1):1–8
Gil AL, Neto GR, Sousa MS, Dias I, Vianna J, Nunes RA, Novaes JS (2017) Effect of strength training with blood flow restriction on muscle power and submaximal strength in eumenorrheic women. Clin Physiol Funct Imaging 37(2):221–228
Guilhem G, Cornu C, Guevel A (2010) Neuromuscular and muscle-tendon system adaptations to isotonic and isokinetic eccentric exercise. Ann Phys Rehabil Med 53(5):319–341
Haussinger D (1996) The role of cellular hydration in the regulation of cell function. Biochem J 313(Pt 3):697–710
Hoffmann EK, Lambert IH, Pedersen SF (2009) Physiology of cell volume regulation in vertebrates. Physiol Rev 89(1):193–277
Jenkins ND, Housh TJ, Buckner SL, Bergstrom HC, Cochrane KC, Hill EC, Smith CM, Schmidt RJ, Johnson GO, Cramer JT (2016) Neuromuscular adaptations after 2 and 4 weeks of 80% versus 30% 1 repetition maximum resistance training to failure. J Strength Cond Res 30(8):2174–2185
Jenkins NDM, Miramonti AA, Hill EC, Smith CM, Cochrane-Snyman KC, Housh TJ, Cramer JT (2017) Greater neural adaptations following high- vs. low-load resistance training. Front Physiol 8:331
Kang T, Seo Y, Park J, Dong E, Seo B, Han D (2013) The effects of elbow joint angle change on the elbow flexor muscle activation in pulley with weight exercise. J Phys Ther Sci 25(9):1133–1136
Karabulut M, Abe T, Sato Y, Bemben MG (2010) The effects of low-intensity resistance training with vascular restriction on leg muscle strength in older men. Eur J Appl Physiol 108(1):147–155
Laurentino GC, Ugrinowitsch C, Roschel H, Aoki MS, Soares AG, Neves M Jr, Aihara AY, Fernandes Ada R, Tricoli V (2012) Strength training with blood flow restriction diminishes myostatin gene expression. Med Sci Sports Exerc 44(3):406–412
Lenman JAR (1959) Quantitative electromyographic changes associated with muscular weakness. J Neurol Neurosurg Psychiatry 22(4):306–310
Loenneke JP, Fahs CA, Wilson JM, Bemben MG (2011) Blood flow restriction: the metabolite/volume threshold theory. Med Hypotheses 77(5):748–752
Loenneke JP, Fahs CA, Rossow LM, Abe T, Bemben MG (2012a) The anabolic benefits of venous blood flow restriction training may be induced by muscle cell swelling. Med Hypotheses 78(1):151–154
Loenneke JP, Wilson JM, Marin PJ, Zourdos MC, Bemben MG (2012b) Low intensity blood flow restriction training: a meta-analysis. Eur J Appl Physiol 112(5):1849–1859
Loenneke JP, Thiebaud RS, Fahs CA, Rossow LM, Abe T, Bemben MG (2013) Blood flow restriction does not result in prolonged decrements in torque. Eur J Appl Physiol 113(4):923–931
Loenneke JP, Kim D, Fahs CA, Thiebaud RS, Abe T, Larson RD, Bemben DA, Bemben MG (2016) The influence of exercise load with and without different levels of blood flow restriction on acute changes in muscle thickness and lactate. Clin Physiol Funct Imaging 37:734–740
Moritani T, deVries HA (1979) Neural factors versus hypertrophy in the time course of muscle strength gain. Am J Phys Med 58(3):115–130
Nichols AW, Hetzler RK, Villanueva RJ, Stickley CD, Kimura IF (2008) Effects of combination oral contraceptives on strength development in women athletes. J Strength Cond Res 22(5):1625–1632
Pasquet B, Carpentier A, Duchateau J, Hainaut K (2000) Muscle fatigue during concentric and eccentric contractions. Muscle Nerve 23(11):1727–1735
Phillips SM (2000) Short-term training: when do repeated bouts of resistance exercise become training? Can J Appl Physiol 25(3):185–193
Pipes TV, Wilmore JH (1975) Isokinetic vs isotonic strength training in adult men. Med Sci Sports 7(4):262–274
Popov DV, Swirkun DV, Netreba AI, Tarasova OS, Prostova AB, Larina IM, Borovik AS, Vinogradova OL (2006) Hormonal adaptation determines the increase in muscle mass and strength during low-intensity strength training without relaxation. Human Physiol 32(5):609–614
Roig M, O’Brien K, Kirk G, Murray R, McKinnon P, Shadgan B, Reid WD (2009) The effects of eccentric versus concentric resistance training on muscle strength and mass in healthy adults: a systematic review with meta-analysis. Br J Sports Med 43(8):556–568
Sakamaki M, Yasuda T, Abe T (2012) Comparison of low-intensity blood flow-restricted training-induced muscular hypertrophy in eumenorrheic women in the follicular phase and luteal phase and age-matched men. Clin Physiol Funct Imaging 32(3):185–191
Sarwar R, Niclos BB, Rutherford OM (1996) Changes in muscle strength, relaxation rate and fatiguability during the human menstrual cycle. J Physiol 493(Pt 1):267–272
Staron RS, Karapondo DL, Kraemer WJ, Fry AC, Gordon SE, Falkel JE, Hagerman FC, Hikida RS (1994) Skeletal muscle adaptations during early phase of heavy-resistance training in men and women. J Appl Physiol 76(3):1247–1255
Sung E, Han A, Hinrichs T, Vorgerd M, Manchado C, Platen P (2014) Effects of follicular versus luteal phase-based strength training in young women. SpringerPlus 3(1):668
Takarada Y, Takazawa H, Sato Y, Takebayashi S, Tanaka Y, Ishii N (2000) Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol 88(6):2097–2106
Takarada Y, Sato Y, Ishii N (2002) Effects of resistance exercise combined with vascular occlusion on muscle function in athletes. Eur J Appl Physiol 86(4):308–314
Thiebaud RS, Yasuda T, Loenneke JP, Abe T (2013) Effects of low-intensity concentric and eccentric exercise combined with blood flow restriction on indices of exercise-induced muscle damage. Interv Med Appl Sci 5(2):53–59
Weir JP (2005) Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J Strength Cond Res 19(1):231–240
Wikstrom-Frisen L, Boraxbekk CJ, Henriksson-Larsen K (2017) Effects on power, strength and lean body mass of menstrual/oral contraceptive cycle based resistance training. J Sports Med Phys Fit 57(1–2):43–52
Yang J, Lee J, Lee B, Kim S, Shin D, Lee Y, Lee J, Han D, Choi S (2014) The effects of elbow joint angle changes on elbow flexor and extensor muscle strength and activation. J Phys Ther Sci 26(7):1079–1082
Yasuda T, Ogasawara R, Sakamaki M, Ozaki H, Sato Y, Abe T (2011) Combined effects of low-intensity blood flow restriction training and high-intensity resistance training on muscle strength and size. Eur J Appl Physiol 111(10):2525–2533
Yasuda T, Loenneke JP, Thiebaud RS, Abe T (2013) Effects of blood flow restricted low-intensity concentric or eccentric training on muscle size and strength. PLoS One 7(12):e52843
Acknowledgements
This research was supported by the National Strength and Conditioning Association Doctoral Research Grant and NASA Nebraska Space Grant. All data are presented honestly, without fabrication, falsification, or data manipulation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by William J. Kraemer.
Rights and permissions
About this article
Cite this article
Hill, E.C., Housh, T.J., Keller, J.L. et al. Early phase adaptations in muscle strength and hypertrophy as a result of low-intensity blood flow restriction resistance training. Eur J Appl Physiol 118, 1831–1843 (2018). https://doi.org/10.1007/s00421-018-3918-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-018-3918-8