Abstract
Skeletal muscle hypertrophy is typically considered to be a slow process. However, this is partly because the time course for hypertrophy has not been thoroughly examined. The purpose of this study was to use weekly testing to determine a precise time course of skeletal muscle hypertrophy during a resistance training program. Twenty-five healthy, sedentary men performed 8 weeks of high-intensity resistance training. Whole muscle cross-sectional area (CSA) of the dominant thigh was assessed using a peripheral quantitative computed tomography scanner during each week of training (W1–W8). Isometric maximum voluntary contractions (MVC) were also measured each week. After only two training sessions (W1), the mean thigh muscle CSA increased by 5.0 cm2 (3.46%; p < 0.05) from the pre-testing (P1) and continued to increase with each testing session. It is possible that muscular edema may have influenced the early CSA results. To adjust for this possibility, with edema assumedly at its highest at W1, the next significant increase from W1 was at W3. W4 was the first significant increase of MVC over P1. Therefore, significant skeletal muscle hypertrophy likely occurred around weeks 3–4. Overall, from the pre-testing to W8, there was an increase of 13.9 cm2 (9.60%). These findings suggested that training-induced skeletal muscle hypertrophy may occur early in a training program.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Skeletal muscle hypertrophy can be defined as an increase in skeletal muscle mass due to more contractile protein content and connective tissue. Skeletal muscle hypertrophy can be induced by any of a number of stimuli, such as resistance training, chronic stretching, synergist ablation, and chronic electrical stimulation (MacIntosh et al. 2006; Miyazaki and Esser 2009). However, of these stimuli, resistance training is the only one that can be appropriately applied to human models in a non-clinical, practical setting.
Typically, training-induced hypertrophy is considered to be a slow process, often taking 6–7 weeks before significant hypertrophy at the whole muscle level can be demonstrated (Phillips 2000). However, recent studies have found hypertrophy earlier in their training programs. Seynnes et al. (2007) found a significant increase in quadriceps femoris cross-sectional area (CSA) after only 20 days of training. Abe et al. (2005) reported increases in mid-thigh muscle-bone CSA and muscle volume after 2 weeks of low-intensity training when combined with vascular occlusion. Despite these findings, whole muscle hypertrophy is still considered to be a slow process. This could be due in part to the large amount of conflicting findings that fail to show training-induced changes in muscle size. For example, Blazevich et al. (2007) were unable to detect any muscle size or architectural changes in the quadriceps femoris muscles after 5 weeks of training. It should be noted, however, that many of the studies that were unable to show hypertrophy, such as Blazevich et al. (2007), may not have used a sufficient stimulus to induce changes (e.g., low-intensity, unilateral training). Previous studies have shown that the total amount of muscle mass recruited and/or the total work performed affects the systemic anabolic hormone responses that are necessary for muscle growth (Hansen et al. 2001; Mulligan et al. 1996). Another reason the time course for hypertrophy is poorly documented is because the few studies that have implemented a high-intensity resistance training program either do not frequently test during the study (e.g., pre and post testing only) or used measurement techniques that are less sensitive to change than current technology (e.g., anthropometry).
The purpose of this study was to determine the time course of skeletal muscle hypertrophy during a resistance training program. There were three requirements that we felt were particularly important in this investigation: (a) a sufficient stimulus (i.e., high-intensity training) must be used to maximize the chances for hypertrophy, (b) frequent testing (i.e., weekly) must be utilized to identify the precise time course for hypertrophy, and (c) a sensitive measurement technique must be used to detect small changes in whole muscle CSA (i.e., medical imaging technology rather than anthropometry). We hypothesized that if those three important testing requirements are met, it may be possible to identify hypertrophy early in a training program.
Methods
Subjects
Twenty-five healthy men (mean ± SD age = 21.5 ± 3.6 years; stature = 1.81 ± 0.01 m; mass = 76.5 ± 13.2 kg) volunteered to participate in this investigation. Each participant completed an informed consent, and a pre-exercise health and exercise status questionnaire. The questionnaire had to indicate no current or recent (within the past 6 months) neuromuscular or musculoskeletal problems to the knees, hips, or lower back for the subject to be considered eligible for the study. In addition, each subject had to be untrained in resistance exercise (i.e., no participation in an organized weight training program for at least the last 6 months prior to the study). Two of the subjects had performed aerobic exercise on a regular basis prior to enrolling in the study. However, both of them ceased running during the 8 weeks of training so as not to hinder hypertrophy. The study was approved by the University Institutional Review Board for Human Subjects prior to testing.
Research design
Each subject came to the laboratory for a total of 26 visits over 9 weeks. During these 26 visits, the subjects completed 10 testing sessions and 24 training sessions (over 8 weeks). A schematic of the schedule for the study can be seen in Fig. 1. There was always 48 h of rest between each visit. The subjects came into the laboratory at the same time of day on each visit (±1 h).
Resistance training and testing
The training program consisted of the bilateral incline leg press, leg extension, and bench press exercises. For each exercise, 3 sets to failure were performed, with approximately 2-min rest between each set. The training load was adjusted on a set-by-set basis to assure that the subject was failing between 8 and 12 repetitions (i.e., if the subject performed 16 repetitions, the weight was increased accordingly before the next set). During the first pre-testing session (P1), the subjects were familiarized with the leg press and bench press exercises. This allowed the inexperienced participants to become accustomed to proper lifting technique and also allowed the investigator to find an approximate estimate of the one-repetition maximums (1-RMs) for each participant. During the second pre-testing session (P2), the subjects performed the 1-RM test. Approximately 80% of each subject’s 1-RM (to the nearest 5 lb increment) was used as their starting weight for training session #1.
Muscle CSA
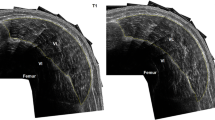
The CSA of the right thigh muscles was measured using a peripheral quantitative computed tomography (pQCT; XCT 3000, Stratec Medizintechnik GmbH, Pforzheim, Germany) scanner. Cramer et al. (2007) showed that when compared with the magnetic resonance imaging (MRI) scanner (the current gold-standard), the pQCT provided a reliable and valid measurement of muscle CSA with a correlation between measurements of r = 0.991. The subject sat upright with their leg fully extended (180°), and the scan was taken at the midpoint of the thigh (i.e., at 50% of the distance between the greater trochanter and lateral epicondyle of the femur). All of the scans were performed by the same investigator. Muscle CSA was calculated using the software provided by the manufacturer. This procedure has been described in more detail in a previous publication (DeFreitas et al. 2010).
Isometric strength assessment
The subjects were tested for unilateral isometric strength of the dominant leg extensors during each testing session (P1–2 and W1–8). The strength measurements were performed with the subjects seated in a customized chair with the leg attached to a load cell (LC101 Series, Omega Engineering, Inc., Stamford, CT) to measure isometric leg extension force (lb). All isometric muscle actions were performed at a joint angle of 120° between the thigh and the leg (180° = full extension). Following a warm-up of four, 6-s submaximal isometric muscle actions at approximately 50% of the subject’s maximum, they performed two maximum voluntary contractions (MVC). Each MVC was separated by 2 min of rest, and the highest force value was designated as their MVC for that testing session.
Statistical analyses
Two separate one-way repeated measures analyses of variance were used to examine the muscle CSA measurements and isometric MVC across the training program. Follow-up analysis included Bonferroni post-hoc comparisons. An alpha level of 0.05 was used for all comparisons. Test–retest reliability between the two pre-testing sessions was calculated using Pearson correlations, a paired samples t-test, 2-way fixed-effect intra-class correlation coefficients (ICCs; model 3,1), as well as the standard error of measurement (SEM), and minimal difference needed for a change to be considered real (MD) (Weir 2005).
Results
The mean ± SEM (standard error of the mean) thigh muscle CSA and isometric MVC across the 8 weeks of training can be seen in Fig. 2. Separate one-way repeated measures ANOVAs revealed a significant increase in both muscle CSA and MVC across time. Follow-up Bonferroni pairwise comparisons and percent changes are provided in Table 1. All of the test–retest reliability statistics are also provided in Table 1. Measures of muscle quality, defined as force per unit CSA, can be seen in Table 2.
Discussion
The results from the present study demonstrated a significant increase in muscle CSA after just two training sessions. Prior to this investigation, the earliest statistically significant training-induced increase in muscle size was reported by Seynnes et al. (2007), who found a significant increase in quadriceps femoris CSA after 20 days of training, as well as an increase in fascicle length after 10 days. It should be noted, however, that their subjects only performed one exercise (leg extensions), so the training stimulus was not as great as that in the present study. Other investigations have shown increases in protein synthesis rate much earlier into a resistance training program (MacDougall et al. 1995; Yarasheski et al. 1999). However, an increased protein synthesis rate only suggests that hypertrophy could occur soon and does not guarantee any actual change to skeletal muscle size.
It should be noted that the imaging technique used in the present study is not the gold-standard for measuring muscle CSA. Despite not being considered a gold-standard, the pQCT is both valid and highly reliable (Cramer et al. 2007). One of the limitations of a pQCT scanner, however, is that it is unable to distinguish between muscle tissue and intramuscular fluid. As a consequence, it is possible that the increases in CSA not only reflected increases in contractile protein, but training-induced inflammatory responses as well. Although we did not measure it in our study, many of the subjects did complain of muscle soreness during the first week. These complaints drastically decreased during week 2 and had ceased prior to week 3. Since there is a strong link between training-induced muscle soreness and swelling (Bobbert et al. 1986), it is reasonable to speculate that the periods of reported soreness were also accompanied by some degree of muscular edema. However, we do not believe that edema and/or inflammation alone could have accounted for these initial increases in CSA. First, as stated above, the perceptions of soreness reported by the subjects decreased throughout the study. Second, CSA showed an increase with each subsequent week. It is reasonable to speculate that muscular edema would be at its highest during the week-1 testing session. Since the edema response would likely have either remained constant or decreased from week to week, this continual increase in CSA over time suggests that there were also increases in contractile protein. The Bonferroni pairwise comparisons show that the first testing session in which CSA was significantly higher than week 1, which would likely have the highest degree of edema, was week 3. Additionally, it is to be expected that significant hypertrophy should also be accompanied by significant increases in strength. Week 4 was the first testing session in which the subjects were significantly stronger than their pre-testing values. This finding, in conjunction with the CSA data (including the adjustment for possible edema) suggests that significant skeletal muscle hypertrophy occurred around weeks 3–4. This timeline is further supported by the measures of muscle quality shown in Table 2. Week 3 was the first week in which the muscle quality was higher than pre-testing values.
Some of the first literature to report training-induced human skeletal muscle hypertrophy suggested that hypertrophy was a slow process that often took weeks or even months to occur. For example, the National Strength and Conditioning Association (NSCA) teaches its coaches and practitioners that it takes 6–8 weeks of training before a sedentary individual will demonstrate hypertrophy (Harris and Dudley 2000). In a highly cited study, Moritani and deVries (1979) reported that it took 4–6 weeks of training before hypertrophy became the primary contributor to strength gains. Neural factors were believed to be responsible for most of the early strength gains. Generally speaking, neural factors (e.g., agonist muscle activation, neuron excitability, motor unit synchronization, antagonist muscle coactivation, etc.…) have been shown to be the primary contributor to the strength gains that occur during the first few weeks of a resistance training program in previously untrained individuals (Del Balso and Cafarelli 2007; Hakkinen et al. 1998; Ishida et al. 1990; Moritani and deVries 1979). Collectively, these findings of neural adaptations may have led to the belief that muscle hypertrophy was not simultaneously occurring. There is little reason to believe that hypertrophy would wait for the neural adaptations to occur, as these two adaptations are not mutually exclusive. Perhaps some of the early findings that were suggestive of hypertrophy being a slow process may have been hindered by methodological limitations. These limitations include the use of anthropometric measurement techniques that are less sensitive to change (Moritani and deVries 1979) than present imaging technology, insufficient training stimulus to induce early hypertrophy (Blazevich et al. 2007), and long layovers between testing sessions [i.e., pre + post testing only; (Higbie et al. 1996; Housh et al. 1992; Narici et al. 1989, 1996)].
Furthermore, this study includes the MD, which is an underutilized statistic. MD is the minimal difference required for a change to be considered real (Weir 2005). The MD for thigh muscle CSA was 3.37% in the present study, which means that if an individual has a pre- to post-training increase in CSA that is less than 3.37%, then the change was not real. The change in that scenario could be attributed to the measurement error of the instrument. However, an increase in CSA greater than 3.37% (in total change) should be attributed to the intervention, which is typically resistance training. It should be noted that MD typically should only be inferred to measurements taken using the same methodology. This is because the calculation is based on the measurement error of that specific device (in this case, a pQCT). However, given the particularly high test–retest reliability of the pQCT (ICC3,1 = 0.995, SEM = 1.21%), it is doubtful that an MRI or CT measurement would provide a much different MD.
Despite the large amount of research concerning skeletal muscle hypertrophy, the mechanisms that are responsible are still largely unknown. For example, there is still a dispute on whether satellite cells (muscle precursor cells) are necessary for hypertrophy to occur (McCarthy and Esser 2007; O’Connor and Pavlath 2007). One of the most important issues in the satellite cell debate is the legitimacy of the myonuclear domain theory, which states: “the volume of cytoplasm managed by a nucleus within a myofiber is finite” (Hawke 2005). As a consequence, in order to increase CSA, the muscle would also have to increase the number of myonuclei per fiber (the proposed role of satellite cells). Despite the need for more research on skeletal muscle hypertrophy at the cellular level, our findings suggest that whole-muscle hypertrophy may occur earlier than originally suggested. The largest limitations to detecting earlier signs of whole-muscle hypertrophy may be the sensitivity of the measurement techniques and the efficacy of the stimuli. As mentioned earlier, muscle protein synthesis rates have been shown to increase as early as 4 h post-exercise (MacDougall et al. 1995). Additional research is needed to determine how quickly human skeletal muscle can add contractile and/or connective tissue protein: a research question that current imaging techniques for measuring muscle CSA are not sensitive enough to determine.
In conclusion, the findings in the present study showed significant increases in whole muscle CSA after just two training sessions. Furthermore, muscle CSA continued to increase from week to week throughout the study. The isometric MVC results in conjunction with the adjusting of the CSA comparisons to account for the possibility of edema led us to the conclusion that significant skeletal muscle hypertrophy occurred around weeks 3–4. It is possible that human skeletal muscle hypertrophy may occur even earlier than that shown in this study. However, further examination of the precise time course of hypertrophy may require advancements in imaging technology.
References
Abe T, Yasuda T, Midorikawa T, Sato Y, Kearns CF, Inoue K, Koizumi K, Ishii N (2005) Skeletal muscle size and circulating IGF-1 are increased after two weeks of twice daily Kaatsu resistance training. Int J Kaatsu Train Res 1:6–12
Blazevich AJ, Gill ND, Deans N, Zhou S (2007) Lack of human muscle architectural adaptation after short-term strength training. Muscle Nerve 35:78–86
Bobbert MF, Hollander AP, Huijing PA (1986) Factors in delayed onset muscular soreness of man. Med Sci Sports Exerc 18:75–81
Cramer JT, Palmer IJ, Ryan ED, Herda TJ, Bemben DA, Bemben MG, Stratemeier PH (2007) Validity and reliability of a peripheral quantitative computed tomography scanner for measuring muscle cross-sectional area. American College of Sports Medicine, Annual Meeting, New Orleans
DeFreitas J, Beck TW, Stock MS, Dillon MA, Sherk VD, Stout JR, Cramer JT (2010) A comparison of techniques for estimating training-induced changes in muscle cross-sectional area. J Strength Cond Res 24:2383–2389
Del Balso C, Cafarelli E (2007) Adaptations in the activation of human skeletal muscle induced by short-term isometric resistance training. J Appl Physiol 103:402–411
Hakkinen K, Kallinen M, Izquierdo M, Jokelainen K, Lassila H, Malkia E, Kraemer WJ, Newton RU, Alen M (1998) Changes in agonist-antagonist EMG, muscle CSA, and force during strength training in middle-aged and older people. J Appl Physiol 84:1341–1349
Hansen S, Kvorning T, Kjaer M, Sjogaard G (2001) The effect of short-term strength training on human skeletal muscle: the importance of physiologically elevated hormone levels. Scand J Med Sci Sports 11:347–354
Harris RT, Dudley G (2000) Neuromuscular anatomy and adaptations to conditioning. In: Baechle TR, Earle RW (eds) Essentials of strength training and conditioning. Human Kinetics, Champaign, pp 15–24
Hawke TJ (2005) Muscle stem cells and exercise training. Exerc Sport Sci Rev 33:63–68
Higbie EJ, Cureton KJ, Warren GL 3rd, Prior BM (1996) Effects of concentric and eccentric training on muscle strength, cross-sectional area, and neural activation. J Appl Physiol 81:2173–2181
Housh DJ, Housh TJ, Johnson GO, Chu WK (1992) Hypertrophic response to unilateral concentric isokinetic resistance training. J Appl Physiol 73:65–70
Ishida K, Moritani T, Itoh K (1990) Changes in voluntary and electrically induced contractions during strength training and detraining. Eur J Appl Physiol Occup Physiol 60:244–248
MacDougall JD, Gibala MJ, Tarnopolsky MA, MacDonald JR, Interisano SA, Yarasheski KE (1995) The time course for elevated muscle protein synthesis following heavy resistance exercise. Can J Appl Physiol 20:480–486
MacIntosh BR, Gardiner PF, McComas AJ (2006) Muscle training. Skeletal muscle: form and function. Human Kinetics, Champaign, pp 298–312
McCarthy JJ, Esser KA (2007) Counterpoint: satellite cell addition is not obligatory for skeletal muscle hypertrophy. J Appl Physiol 103:1100–1102; discussion 1102–1103
Miyazaki M, Esser KA (2009) Cellular mechanisms regulating protein synthesis and skeletal muscle hypertrophy in animals. J Appl Physiol 106:1367–1373
Moritani T, deVries HA (1979) Neural factors versus hypertrophy in the time course of muscle strength gain. Am J Phys Med 58:115–130
Mulligan SE, Fleck SJ, Gordon SE, Koziris P, Triplett-McBride T, Kraemer WJ (1996) Influence of resistance exercise volume on serum growth hormone and cortisol concentrations in women. J Strength Cond Res 10:256–262
Narici MV, Roi GS, Landoni L, Minetti AE, Cerretelli P (1989) Changes in force, cross-sectional area and neural activation during strength training and detraining of the human quadriceps. Eur J Appl Physiol Occup Physiol 59:310–319
Narici MV, Hoppeler H, Kayser B, Landoni L, Claassen H, Gavardi C, Conti M, Cerretelli P (1996) Human quadriceps cross-sectional area, torque and neural activation during 6 months strength training. Acta Physiol Scand 157:175–186
O’Connor RS, Pavlath GK (2007) Point: satellite cell addition is obligatory for skeletal muscle hypertrophy. J Appl Physiol 103:1099–1100
Phillips SM (2000) Short-term training: when do repeated bouts of resistance exercise become training? Can J Appl Physiol 25:185–193
Seynnes OR, de Boer M, Narici MV (2007) Early skeletal muscle hypertrophy and architectural changes in response to high-intensity resistance training. J Appl Physiol 102:368–373
Weir JP (2005) Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J Strength Cond Res 19:231–240
Yarasheski KE, Pak-Loduca J, Hasten DL, Obert KA, Brown MB, Sinacore DR (1999) Resistance exercise training increases mixed muscle protein synthesis rate in frail women and men >/=76 yr old. Am J Physiol 277:E118–E125
Acknowledgments
The funding for this study was received by the NSCA Foundation through their Master’s Student Research Grant.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by William J. Kraemer.
Rights and permissions
About this article
Cite this article
DeFreitas, J.M., Beck, T.W., Stock, M.S. et al. An examination of the time course of training-induced skeletal muscle hypertrophy. Eur J Appl Physiol 111, 2785–2790 (2011). https://doi.org/10.1007/s00421-011-1905-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-011-1905-4