Abstract
Purpose
The purpose of this investigation was to examine the individual and composite patterns of responses and time-course of changes in muscle size, strength, and edema throughout a 4 week low-load blood flow restriction (LLBFR) resistance training intervention.
Methods
Twenty recreationally active women (mean ± SD; 23 ± 3 years) participated in this investigation and were randomly assigned to 4 weeks (3/week) of LLBFR (n = 10) or control (n = 10) group. Resistance training consisted of 75 reciprocal isokinetic forearm flexion–extension muscle actions performed at 30% of peak torque. Strength and ultrasound-based assessments were determined at each training session.
Results
There were quadratic increases for composite muscle thickness (R2 = 0.998), concentric peak torque (R2 = 0.962), and maximal voluntary isometric contraction (MVIC) torque (R2 = 0.980) data for the LLBFR group. For muscle thickness, seven of ten subjects exceeded the minimal difference (MD) of 0.16 cm during the very early phase (laboratory visits 1–7) of the intervention compared to three of ten subjects that exceeded MD for either concentric peak torque (3.7 Nm) or MVIC (2.2 Nm) during this same time period. There was a linear increase for composite echo intensity (r2 = 0.563) as a result of LLBFR resistance training, but eight of ten subjects never exceeded the MD of 14.2 Au.
Conclusions
These findings suggested that the increases in muscle thickness for the LLBFR group were not associated with edema and changes in echo intensity should be examined on a subject-by-subject basis. Furthermore, LLBFR forearm flexion–extension resistance training elicited real increases in muscle size during the very early phase of training that occurred prior to real increases in muscle strength.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increases in muscle strength as a result of resistance training are attributed to muscle hypertrophy and neural adaptations (Moritani and deVries 1979; Sale 1988; DeFreitas et al. 2011; Jenkins et al. 2016, 2017; Colomer-Poveda et al. 2017). It is generally believed that during resistance training the early phase (< 4 weeks) increases in muscle strength are driven primarily by neural adaptations and muscle hypertrophy becomes the primary contributor to further increases in muscle strength after approximately 4 weeks of resistance training. This initial understanding of resistance training on muscle adaptation was based on the work of Moritani and deVries (1979) who proposed an electromyographic (EMG) technique called efficiency of electrical activity for estimating the relative contributions of neural and hypertrophic adaptations to resistance training-induced increases in muscle strength. Using this technique, Moritani and deVries (1979) reported that the relative contributions of muscle hypertrophy and neural adaptations to the increases in muscle strength were approximately 15% versus 85% at 2 weeks, 52% versus 48% at 4 weeks, 80% versus 20% at 6 weeks, and 85% versus 15% at 8 weeks, respectively. More recently, the time-course of training-induced adaptations in skeletal muscle and neuromuscular adaptations have commonly been investigated using computed tomography, ultrasound, mechanomyography, and interpolated twitch technique (DeFreitas et al. 2011; Jenkins et al. 2016, 2017; Colomer-Poveda et al. 2017).
The early phase increases in muscle size as a result of resistance training may reflect exercise-induced edema as a result of muscle damage, particularly in previously untrained individuals (Damas et al. 2016; Buckner et al. 2017). For example, a single bout of resistance training increased muscle thickness that was attributed to exercise-induced muscle edema possibly the result of muscle damage (Buckner et al. 2017). Interestingly, however, this response was finite and was not further increased by additional training sessions performed over an 8-day period (Buckner et al. 2017). Similarly, muscle cross-sectional area increased within 1 week of resistance training that was attributed to muscle edema, but the subsequent increases in muscle size beyond 1 week reflected muscle hypertrophy apart from muscle edema (DeFreitas et al. 2011). Therefore, to differentiate early phase increases in muscle size as it relates to muscle hypertrophy, it was suggested to simultaneously assess muscle size and exercise-induced edema via echo intensity from ultrasound (Damas et al. 2016). In addition, muscle size should be assessed prior to exercise under resting conditions as acute changes in muscle blood flow may result in an overestimation of exercise-induced edema (Hill et al. 2018).
Little is known regarding the patterns of responses and time-course of changes in muscle hypertrophy associated with low-load blood flow restriction (LLBFR) resistance training. This is of particular interest as LLBFR resistance training increases muscle strength and elicits muscle hypertrophy without the use of heavy training loads or exercising to volitional exhaustion. For example, we previously demonstrated that LLBFR resistance training performed at 30% of peak torque increased muscle strength and size of the forearm flexors within 2 weeks, but was not associated with delayed onset muscle soreness or high perceived exertion (Hill et al. 2018, 2019c). Furthermore, unlike high-load non-blood flow restriction (BFR) resistance training, the increases in muscle strength as a result of LLBFR were not associated with neural adaptations (Loenneke et al. 2012). For example, there were no changes in muscle activation following 4–6 weeks of LLBFR forearm flexion or bench press resistance training performed at 20–30% of maximum strength (Yasuda et al. 2011; Hill et al. 2018). Similarly, there were no changes in afferent excitability or cortical activation following 4 weeks of LLBFR plantarflexion resistance training performed at 20% of maximum strength (Colomer-Poveda et al. 2017). Therefore, the early phase increases in muscle strength as a result of LLBFR appear to occur independent of neural adaptations, but may be associated with increased muscle size. No previous investigations, however, have examined the patterns of responses and the time-course of changes in muscle size, strength, and edema as a result of LLBFR resistance training on a visit-by-visit and subject-by-subject basis. Together, these findings may provide further insight to the utility or limitations of LLBFR resistance training. Therefore, the purpose of this investigation was to examine the individual and composite patterns of responses and the time-course of changes in muscle size, strength, and edema throughout a 4-week LLBFR resistance training intervention. Based on previous investigations (Hill et al. 2018, 2019a), it was hypothesized that LLBFR resistance training will increase muscle size and strength for the individual and group responses within 2 weeks of training without changes in muscle edema.
Methods
Subjects
Twenty women volunteered to participate in this investigation and were randomly assigned to either LLBFR (n = 10; mean age ± SD = 22 ± 2 years; body mass = 60.1 ± 5.3 kg; height = 165.1 ± 7.4 cm) or control (n = 10; mean age ± SD = 23 ± 3 years; body mass = 62.6 ± 8.0 kg; height = 164.8 ± 9.2 cm). The data from these subjects have been examined in a previous investigation for purposes unrelated to the present study (Hill et al. 2019a). The participants had no known cardiovascular, pulmonary, metabolic, muscular, and/or coronary heart disease, or regularly used prescription medication. All participants were recreationally active at the time of testing (Progression Models in Resistance Training for Healthy Adults 2009), but no participants had been actively participating in resistance training for at least the past 6 months. To minimize the influence that extrinsic variables may exhibit on individual responses to the interventions, the subjects were asked to maintain current eating, drinking, sleeping, and exercise habits throughout the duration of the study and to avoid starting dieting, new exercise habits, and major lifestyle changes. Additionally, subjects were excluded if they were currently taking any nutritional supplements. All eligible participants were currently taking an oral contraceptive and started the testing and training procedures during the menses phase of their ovulatory cycle and all testing and training procedures were performed at the same time of day (± 2 h). The study was approved by the University Institutional Review Board for Human Participants (IRB #20180918559EP) and all participants completed a health history questionnaire and signed a written informed consent prior to testing.
Experimental design
A randomized, repeated measures, between-group, parallel design was used for this study. Twenty women were randomly assigned to either LLBFR or control. A KAATSU resistance band was used to achieve BFR and the BFR pressure was determined individually for each subject as 40% of the pressure needed to completely occlude the brachial artery as indicated by ultrasound. The subjects assigned to LLBFR performed reciprocal concentric forearm flexion–extension resistance training (dominant arm) at 30% of forearm flexion peak torque and forearm extension peak torque, respectively. Training was performed 3 times per week for 4 weeks which consisted of 75 reciprocal forearm flexion–extension isokinetic muscle actions of the forearm flexors and extensors performed over 4 sets (1 × 30, 3 × 15) and each set was separated by 30 s of rest. The relative training intensity, repetitions, rest between sets, and frequency of training were consistent with previous investigations (Thiebaud et al. 2013; Loenneke et al. 2017; Counts et al. 2016; Yasuda et al. 2013) that have examined low-load blood flow restriction and were selected to optimize the training-induced adaptations on muscle strength and hypertrophy. Furthermore, meta-analyses (Loenneke et al. 2012; Patterson et al. 2019) reported that effects sizes for increasing muscle strength and hypertrophy as a result of blood flow restriction resistance training were greatest using training loads of 20–40% of maximum strength, performing 75 repetitions (1 × 30, 3 × 15) with 30 s between sets, and performed 2–3 days per week. The control group also visited the laboratory three times per week for 4 weeks, but did not perform any training. During each laboratory visit, ultrasound and strength assessments were measured from the dominant arm for both the LLBFR and control groups. All procedures were performed using a calibrated isokinetic dynamometer performed at a velocity of 120° s−1.
Procedures
Prior to the baseline and subsequent laboratory visits, the participants completed an orientation session to familiarize the participants with the testing procedures. During the orientation, participants performed submaximal and maximal isometric and concentric isokinetic muscle actions of the forearm extensors at 120° s−1 on an isokinetic dynamometer (Biodex System 3; Biodex Medical Systems, Inc. Shirley, NY, USA). To familiarize the participants with the training protocols, the participants also practiced performing reciprocal concentric-only forearm flexion–extension muscle actions at 30% of their forearm flexion concentric peak torque and forearm extension concentric peak torque, respectively. Torque was visually tracked using real-time torque displayed on a computer monitor.
The participants in the LLBFR group completed 4 weeks of training at a frequency of 3 training sessions per week (separated by 48-h) for a total of 12 training sessions. Each training session consisted of 75 reciprocal concentric forearm flexion–extension muscle actions performed over 4 sets (1 × 30, 3 × 15) and each set was separated by 30 s of rest. All muscle actions were performed at a velocity of 120° s−1 on a Biodex System 3 Pro isokinetic dynamometer. The reciprocal concentric forearm flexion–extension muscle actions were performed at 30% of forearm flexion concentric peak torque and forearm extension concentric peak torque, respectively. The concentric muscle actions were performed through a 120° range of motion (0°–120° of elbow flexion, where 0° corresponds to full extension at the elbow).
BFR was applied using a 30-mm wide cuff (KAATSU Master, Sato Sports Plaza, Tokyo, Japan) placed on the proximal portion of the arm. The cuff pressure was initially applied at 30 mmHg and progressively inflated and deflated over a 60 s period until the target pressure was reached. Target pressure was calculated as 40% of the lowest pressure needed to completely occlude the brachial artery as indicated by ultrasound (Counts et al. 2016; Loenneke et al. 2017; Loenneke et al. 2013). Previous investigations (Counts et al. 2016; Loenneke et al. 2016, 2013) have indicated that 40% of arterial occlusion results in the same training-induced responses as 50–90% of arterial occlusion when combined with low-load training (30% one-repetition maximum). The cuff remained inflated during the duration of the training bout and was deflated immediately after completing the 75 repetitions. The total duration of BFR was approximately 5 min.
Measurements
Muscle strength
During each laboratory visit, the participants (LLBFR and control) performed a warm-up consisting of 10 submaximal (approximately 50% effort), reciprocal concentric forearm flexion–extension muscle actions performed at 120° s−1. Following the warmup, the participants rested for 5 min and then performed two randomly ordered maximal concentric (120° s−1) and isometric muscle actions of the forearm flexors and extensors to determine the concentric peak torque and maximal voluntary isometric contraction (MVIC) values, respectively. The highest forearm extension concentric peak torque and MVIC torque produced during each of the two attempts were used for further analyses. The forearm extension MVIC muscle actions were performed at 45° of flexion at the elbow (where 0° corresponds to full extension at the elbow) sustained for a period of 3 s.
Ultrasound-based assessments of muscle size
To examine the time-course of muscle hypertrophy, ultrasound assessments of muscle thickness and echo intensity were performed at the beginning of each laboratory visit prior to the warmup for both the LLBFR and control groups. Ultrasound images of the trained arm (triceps brachii) were obtained using a portable brightness mode (B-mode) ultrasound-imaging device (GE Logiq, USA) and a multi-frequency linear-array probe (12 L-Rs; 5–13 MHz; 38.4 mm field-of-view). All ultrasound measurements were performed at a sampling rate of 10 MHz and at a gain of 58 dB. Ultrasound images were analyzed using ImageJ software (Version 1.47v., National Institutes of Health, Bethesda, MD, USA) and prior to all analyses, images were scaled from pixels to centimeters using the straight-line function in ImageJ. Muscle thickness and echo intensity were assessed at 20% of the distance from the infraglenoid tubercle to the olecranon. Muscle thickness was determined as the distance from the adipose-muscle interface to the muscle-bone interface. Echo intensity, as assessed by gray-scale analysis (0 arbitrary units [Au]) corresponds to black image, 255 Au corresponds to white image) was determined from the same region of interest as muscle thickness. Great care was taken to ensure that consistent, minimal pressure was applied with the probe to limit compression of the artery. To enhance acoustic coupling and reduce near field artifacts, a generous amount of water-soluble transmission gel was applied to the skin prior to each measurement.
Data analysis
Reliability
Test–retest reliability for muscle thickness, concentric peak torque, MVIC, and echo intensity were assessed from the baseline visit and first laboratory visit. Repeated measures ANOVAs were used to assess systematic error, and model 2, 1 was used to calculate intraclass correlation coefficients (ICCs), standard errors of measurement (SEM), and minimal difference (MD = SEM × 21/2 × df) needed to consider a change as real (Weir 2005). The MD was used to determine at which visits real changes in each of the dependent variables (muscle thickness, MVIC, and echo intensity) were observed. The visit-by-visit changes for each dependent variable were compared relative to the average of the baseline visit and first visit values. The 95% confidence intervals for the means of the dependent variables were calculated with the Studentized t distribution.
Statistical analyses
Polynomial regression analyses (first, second, and third order) were used to examine the individual and composite patterns of responses for muscle thickness, concentric peak torque, MVIC, and echo intensity across the 13 laboratory visits (visit 1 proceeds the orientation and baseline visits). The F test was used to determine if the increment in proportion of variance accounted for by a higher-order polynomial was significant at p ≤ 0.05. All statistical analyses were performed using IBM SPSS v. 27 (Armonk, NY) and an alpha of p ≤ 0.05 considered statistically significant for all comparisons.
Results
Reliability
Table 1 includes the test–retest reliability and MD values from the baseline and first laboratory visits for muscle thickness, concentric peak torque, MVIC, and echo intensity. There were no mean differences for baseline versus first laboratory visits (p > 0.05) for any of the variables. The ICC values for muscle thickness, concentric peak torque, MVIC, and echo intensity ranged from 0.836 to 0.981 and the SEM values were 2.9, 6.0, 3.3, and 5.8% of the grand mean, respectively. Furthermore, the MD for a change to be considered real was 0.16 cm for muscle thickness, 3.7 Nm for concentric peak torque, 2.2 Nm for MVIC, and 14.2 Au for echo intensity.
Muscle thickness
LLBFR group
For the individual muscle thickness versus laboratory visits, there was a significant linear increase for one of the 10 subjects, significant quadratic increases for five (one quadratic decrease) of the 10 subjects, and significant cubic increases for three of the 10 subjects (Table 2). For the composite muscle thickness versus laboratory visits, there was a significant quadratic increase (Fig. 1a). Additionally, two subjects exceeded the MD of 0.16 cm on the fourth laboratory visit, three subjects on the fifth laboratory visit, two subjects on the sixth laboratory visit, one subject on the 10th visit, one subject on the 11th visit, and one of the 10 subjects never exceeded the MD of 0.16 cm across the 13 laboratory visits (Table 3, Fig. 2a).
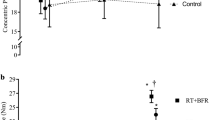
The patterns of responses (± 95% confidence intervals) for muscle thickness (a), concentric peak torque (b), maximal voluntary isometric contraction (MVIC) torque (c), and echo intensity (d) as a result of low-load blood flow restriction (LLBFR, filled squares) and control (filled triangles). Laboratory visit 1 represents the first day of intervention following the orientation and baseline visits. For echo intensity (c), the 95% confidence intervals are shaded in light gray (LLBFR) and dark gray (control) to delineate the two data sets from each other
The individual absolute changes in muscle thickness (cm) as a result of low-load blood flow restriction (LLBFR, a) and control (b). Laboratory visit 1 represents the first day of intervention following the orientation and baseline visits. All subsequent visits represent absolute changes relative to laboratory visit 1. The minimal difference (MD, open circles) needed for a change to be considered real is plotted and derived using standard error of measurement (SEM) values from the reliability data and using the equation, MD = SEM × 21/2 × df (Weir 2005)
Control group
For the individual muscle thickness versus laboratory visits, there were significant linear increases for two subjects and no significant relationships for eight of the 10 subjects (Table 2). For the composite muscle thickness versus laboratory visits, there was no significant relationship (Fig. 1a). Additionally, none of the 10 subjects ever exceeded the MD of 0.16 cm across the 13 laboratory visits (Table 3, Fig. 2b).
Concentric peak torque
LLBFR group
For the individual forearm extension concentric peak torque versus laboratory visits, there were significant linear increases for five subjects, significant quadratic increases for four subjects, and no significant relationship for one of the 10 subjects (Table 2). For the composite forearm extension concentric peak torque versus laboratory visits, there was a significant quadratic increase (Fig. 1b). Additionally, one subject exceeded the MD of 3.7 Nm on the fifth laboratory visit, one subject on the sixth laboratory visit, one subject on the seventh laboratory visit, one subject on the eighth laboratory visit, one subject on the ninth laboratory visit, two subjects on the 10th laboratory visit, two subjects on the 13th visit, and one of 10 subjects never exceeded the MD of 3.7 Nm across the 13 laboratory visits (Table 3, Fig. 3a).
The individual absolute changes in forearm extension concentric peak torque (Nm) as a result of low-load blood flow restriction (LLBFR, a) and control (b). Laboratory visit 1 represents the first day of intervention following the orientation and baseline visits. All subsequent visits represent absolute changes relative to laboratory visit 1. The minimal difference (MD, open circles) needed for a change to be considered real is plotted and derived using standard error of measurement (SEM) values from the reliability data and using the equation, MD = SEM × 21/2 × df (Weir 2005)
Control group
For the individual forearm extension concentric peak torque versus laboratory visits, there were significant linear increases for two subjects and no significant relationships for eight of the 10 subjects (Table 2). For the composite forearm extension concentric peak torque versus laboratory visits, there was no significant relationship (Fig. 1b). Additionally, two subjects exceeded the MD of 3.7 Nm on the 13th visit, while eight of the 10 subjects never exceeded the MD of 3.7 Nm across the 13 laboratory visits (Table 3, Fig. 3b).
MVIC
LLBFR group
For the individual forearm extension MVIC versus laboratory visits, there were significant linear increases for seven subjects, significant quadratic increases for two subjects, and a significant cubic increase for one of the 10 subjects (Table 2). For the composite forearm extension MVIC versus laboratory visits, there was a significant quadratic increase (Fig. 1c). Additionally, one subject exceeded the MD of 2.2 Nm on the third laboratory visit, one subject on the fourth laboratory visit, one subject on the fifth laboratory visit, one subject on the seventh laboratory visit, one subject on the eighth laboratory visit, four subjects on the ninth laboratory visit, and one of the 10 subjects on the 10th laboratory visit (Table 3, Fig. 4a).
The individual absolute changes in forearm extension maximal voluntary isometric contraction (MVIC) torque (Nm) as a result of low-load blood flow restriction (LLBFR, a) and control (b). Laboratory visit 1 represents the first day of intervention following the orientation and baseline visits. All subsequent visits represent absolute changes relative to laboratory visit 1. The minimal difference (MD, open circles) needed for a change to be considered real is plotted and derived using standard error of measurement (SEM) values from the reliability data and using the equation, MD = SEM × 21/2 × df (Weir 2005)
Control group
For the individual forearm extension MVIC versus laboratory visits, there were significant linear increases for two subjects and no significant relationships for eight of the 10 subjects (Table 2). For the composite forearm extension MVIC versus laboratory visits, there was a significant linear increase (Fig. 1c). Additionally, one of the 10 subjects exceeded the MD of 2.2 Nm, while nine of the 10 subjects never exceeded the MD of 2.2. Nm across the 13 laboratory visits (Table 3, Fig. 4b).
Echo intensity
LLBFR group
For the individual echo intensity versus laboratory visits, there were significant linear increases for three subjects and no significant relationships for seven of the 10 subjects (Table 2). For the composite echo intensity versus laboratory visits, there was a significant linear increase (Fig. 1d). Additionally, one subject exceeded the MD of 14.2 Au on the third laboratory visit but no longer exceeded the MD on the sixth laboratory visit. Furthermore, one subject exceeded the MD of 14.2 Au on the ninth laboratory visit and eight of the 10 subjects never exceeded the MD of 14.2 Au across the 13 laboratory visits (Table 3).
Control group
For the individual echo intensity versus laboratory visits, there was a significant linear increase for one subject, a significant quadratic increase for one subject, and no significant relationships for eight of the 10 subjects (Table 2). For the composite echo intensity versus laboratory visits, there was a significant linear increase (Fig. 1d). Additionally, one subject exceeded the MD of 14.2 Au on the fourth laboratory visit and nine of the 10 subjects never exceeded the MD of 14.2 Au across the 13 laboratory visits (Table 3).
Discussion
The results of the present study indicated that 4 weeks of LLBFR forearm flexion–extension resistance training resulted in a 17.4% increase in muscle thickness, a 32.3% increase in forearm extension concentric peak torque, a 29.5% increase in forearm extension MVIC torque, and no change in echo intensity. These findings were consistent with previous investigations (Laurentino et al. 2012; Cook et al. 2017; Hill et al. 2018, 2019b) that have examined the early phase changes in muscle size, strength, and echo intensity as a result of LLBFR resistance training. For example, in previously untrained women, 4 weeks of LLBFR forearm flexion resistance performed at 30% of peak torque increased muscle thickness by 9.9–12.8% and muscle cross-sectional area by 26.6%, increased peak torque by 31.4–35.0%, but had no effects on echo intensity (Hill et al. 2018, 2019b). In physically active men, 8 weeks of LLBFR leg extension resistance training performed at 20% of one-repetition maximum increased muscle cross-sectional area by 6.3% and increased one-repetition maximum by 40.1% (Laurentino et al. 2012). Furthermore, among previously untrained older adults, 6 weeks of LLBFR leg extension resistance training performed at 30% of one-repetition maximum increased muscle cross-sectional area by 4.3% and one-repetition maximum by 12–24% (Cook et al. 2017). Therefore, in conjunction with previous investigations (Laurentino et al. 2012; Cook et al. 2017; Hill et al. 2018, 2019b), the present findings indicated that LLBFR resistance training was an effective training modality to increase muscle size and muscle strength for a variety of muscle groups and populations.
This was the first study to describe the patterns of responses for muscle thickness, concentric peak torque, MVIC torque, and echo intensity and to examine the individual responses relative to the MD for each of these parameters. Specifically, for the composite data, there were quadratic increases for muscle thickness, concentric peak torque, and MVIC torque as a result of LLBFR resistance training (Fig. 1a, b, c, respectively). For the individual relationships, the increases in muscle thickness were primarily quadratic (five of 10 subjects), but linear (six to seven of 10 subjects) for concentric peak torque and MVIC torque (Table 2). Furthermore, muscle thickness increased predominately during the very early phase (across laboratory visits 1–7) and began to plateau during the latter half (laboratory visits 8–13) of the training intervention (Fig. 2a). Conversely, concentric peak torque and MVIC torque exhibited smaller increases during the very early phase of the intervention, but increased more rapidly during the latter half (Figs. 3a, 4a). Together, these findings suggested that increases in muscle size preceded increases in muscle strength.
The individual relationships were consistent with the individual absolute responses for muscle thickness and torque responses. Specifically, for muscle thickness, seven of 10 subjects exceeded the MD during the very early phase (laboratory visits 1–7) of the training intervention compared to three of 10 subjects that exceeded MD for concentric peak torque and MVIC torque during this time period (Table 2). Furthermore, real increases in muscle thickness preceded real increases in concentric peak torque for seven of 10 subjects and MVIC torque for six of 10 subjects. It is plausible, therefore, that the increases in muscle thickness contributed to the increases in concentric peak torque and MVIC torque as a result of LLBFR resistance training, which has been dissociated from neural adaptations (Loenneke et al. 2012; Colomer-Poveda et al. 2017; Hill et al. 2018, 2019a). The association between muscle size and muscle strength, however, has been debated (Hornsby et al. 2018; Loenneke et al. 2019). For example, detraining-induced decreases in muscle size did not result in muscle strength loss (Kubota et al. 2008). Additionally, the relative contributions that changes in muscle size facilitate muscle strength is less than previously thought (Erskine et al. 2014) and difficult to delineate (Buckner et al. 2016). However, LLBFR-induced increases in muscle strength have been associated with muscle hypertrophy and increased cellular expression promoting muscle growth (Bjørnsen et al. 2019). Furthermore, we (Hill et al. 2018, 2019a) have recently demonstrated that LLBFR-induced increases in muscle strength were associated with increases in muscle size, but not neural adaptations. Therefore, in conjunction with previous investigations (Hill et al. 2018, 2019a; Bjørnsen et al. 2019), our current findings indicated that LLBFR forearm flexion–extension resistance training elicited real increases in muscle size during the very early phase of training that preceded real increases in muscle strength that occurred later on.
In the present study, for the composite responses there were linear increases in echo intensity for the LLBFR and control groups (Fig. 1c). Echo intensity has been used to identify very early phase changes in muscle edema as a result of muscle damage (Damas et al. 2016; Dankel et al. 2020). Echo intensity has also been used to quantify muscle quality (Merrigan et al. 2018). For example, it has been suggested that increases in echo intensity may reflect connective tissue infiltration or increased adiposity as a result of aging, inactivity, or disease (Pillen et al. 2006; Arts et al. 2010; Wilhelm et al. 2014). Our findings indicated that for the composite relationships, there were linear increases in echo intensity across the 13 laboratory visits, but this was unlikely related to muscle edema or reduced muscle quality. Instead, the lack of individual changes in the absolute responses relative to the MD coupled with primarily non-significant individual relationships may indicate that the composite data for echo intensity is not a valid indicator of individual responses (Table 2). Furthermore, for eight of 10 subjects within the LLBFR resistance training group there were no real changes in echo intensity, while one subject exceeded the MD for a short period of time (laboratory visits 3–5) and one subject exceeded the MD on the 9th laboratory visit that remained elevated (Table 2). For these two subjects, however, the increases in muscle thickness were observed after the initial increase in echo intensity or prior to the sustained increase in echo intensity, respectively. Additionally, we (Hill et al. 2019c) and others (Fujita et al. 2008; Curty et al. 2018) have recently reported that BFR resistance training was not associated with muscle damage and, alternatively, may attenuate the development of delayed onset muscle soreness. Therefore, these data suggested that the observed increases in muscle thickness for the LLBFR group were not associated with muscle edema and changes in echo intensity should be examined on a subject-by-subject basis. Collectively, there were no meaningful relationships for echo intensity across the 13 laboratory visits and no real changes in echo intensity for 17 of 20 subjects.
Study limitations
As a limitation, the muscle thickness measurements were assessed from only one location from the triceps brachii muscle that may not reflect location-specific muscle hypertrophy. Additionally, muscle strength was assessed using concentric peak torque assessed and 120° s−1 and MVIC torque which may not reflect mode- or velocity-specific adaptations as a result of the resistance training intervention. In the present study, however, the purpose was to examine the patterns of responses and time-course of change in muscle size and strength. Future studies may consider examining these relationships using different muscle size and strength assessments.
Summary
The results of the present study indicated that 4 weeks of LLBFR forearm flexion–extension resistance training increased muscle thickness, concentric peak torque, and MVIC torque, but had no effects on echo intensity. For the composite data, there were quadratic increases for muscle thickness, concentric peak torque, and MVIC torque as a result of LLBFR resistance training. For the individual relationships, the increases in muscle thickness were primarily quadratic, but linear for concentric peak torque and MVIC torque. For muscle thickness, seven of 10 subjects exceeded the MD during the very early phase (laboratory visits 1–7) of the training intervention compared to three of 10 subjects that exceeded MD for concentric peak torque and MVIC torque during this same time period. There were linear increases in the composite echo intensity relationships for the LLBFR and control groups, but this was unlikely related to muscle edema or reduced muscle quality. That is, for eight of 10 subjects within the LLBFR resistance training group there were no real changes in echo intensity, while one subject exceeded the MD for a short period of time (laboratory visits 3–5) and one subject exceeded the MD on the 9th laboratory visit that remained elevated. Therefore, these data suggested that the observed increases in muscle thickness for the LLBFR group were not associated with muscle edema and changes in echo intensity should be examined on a subject-by-subject basis.
Abbreviations
- EMG:
-
Electromyography
- LLBFR:
-
Low-load venous blood flow restriction
- BFR:
-
Blood flow restriction
- MVIC:
-
Maximal voluntary isometric contraction
- ICC:
-
Intraclass correlation coefficients
- MD:
-
Minimal difference
- SEM:
-
Standard error of measurement
References
Arts IMP, Pillen S, Schelhaas HJ et al (2010) Normal values for quantitative muscle ultrasonography in adults. Muscle Nerve 41:32–41. https://doi.org/10.1002/mus.21458
Bjørnsen T, Wernbom M, Kirketeig A et al (2019) Type 1 muscle fiber hypertrophy after blood flow-restricted training in powerlifters. Med Sci Sports Exerc 51:288–298. https://doi.org/10.1249/MSS.0000000000001775
Buckner SL, Dankel SJ, Mattocks KT et al (2016) The problem of muscle hypertrophy: revisited. Muscle Nerve 54:1012–1014. https://doi.org/10.1002/mus.25420
Buckner SL, Dankel SJ, Mattocks KT et al (2017) Differentiating swelling and hypertrophy through indirect assessment of muscle damage in untrained men following repeated bouts of resistance exercise. Eur J Appl Physiol 117:213–224. https://doi.org/10.1007/s00421-016-3521-9
Colomer-Poveda D, Romero-Arenas S, Vera-Ibáñez A et al (2017) Effects of 4 weeks of low-load unilateral resistance training, with and without blood flow restriction, on strength, thickness, V wave, and H reflex of the soleus muscle in men. Eur J Appl Physiol 117:1339–1347. https://doi.org/10.1007/s00421-017-3622-0
Cook SB, LaRoche DP, Villa MR et al (2017) Blood flow restricted resistance training in older adults at risk of mobility limitations. Exp Gerontol 99:138–145. https://doi.org/10.1016/j.exger.2017.10.004
Counts BR, Dankel SJ, Barnett BE, Kim D, Mouser JG, Allen KM, Thiebaud RS, Abe T, Bemben MG, Loenneke JP (2016) Influence of relative blood flow restriction pressure on muscle activation and muscle adaptation. Muscle Nerve 53(3):438–445
Curty VM, Melo AB, Caldas LC et al (2018) Blood flow restriction attenuates eccentric exercise-induced muscle damage without perceptual and cardiovascular overload. Clin Physiol Funct Imaging 38:468–476. https://doi.org/10.1111/cpf.12439
Damas F, Phillips SM, Lixandrão ME et al (2016) Early resistance training-induced increases in muscle cross-sectional area are concomitant with edema-induced muscle swelling. Eur J Appl Physiol 116:49–56. https://doi.org/10.1007/s00421-015-3243-4
Dankel SJ, Abe T, Spitz RW et al (2020) Impact of acute fluid retention on ultrasound echo intensity. J Clin Densitom 23:149–150. https://doi.org/10.1016/j.jocd.2019.02.008
DeFreitas JM, Beck TW, Stock MS et al (2011) An examination of the time course of training-induced skeletal muscle hypertrophy. Eur J Appl Physiol 111:2785–2790. https://doi.org/10.1007/s00421-011-1905-4
Erskine RM, Fletcher G, Folland JP (2014) The contribution of muscle hypertrophy to strength changes following resistance training. Eur J Appl Physiol 114:1239–1249. https://doi.org/10.1007/s00421-014-2855-4
Fujita T, Brechue WF, Kurita K et al (2008) Increased muscle volume and strength following 6 days of low-intensity resistance training with restricted muscle blood flow. Int J KAATSU Train Res 4:1–8. https://doi.org/10.3806/ijktr.4.1
Hill EC, Housh TJ, Keller JL et al (2018) Early phase adaptations in muscle strength and hypertrophy as a result of low-intensity blood flow restriction resistance training. Eur J Appl Physiol 118:1831–1843. https://doi.org/10.1007/s00421-018-3918-8
Hill EC, Housh TJ, Keller JL et al (2019a) Low-load blood flow restriction elicits greater concentric strength than non-blood flow restriction resistance training but similar isometric strength and muscle size. Eur J Appl Physiol. https://doi.org/10.1007/s00421-019-04287-3
Hill EC, Housh TJ, Keller JL et al (2019b) The validity of the EMG and MMG techniques to examine muscle hypertrophy. Physiol Meas 40:025009. https://doi.org/10.1088/1361-6579/ab057e
Hill EC, Housh TJ, Smith CM et al (2019c) Eccentric and concentric blood flow restriction resistance training on indices of delayed onset muscle soreness in untrained women. Eur J Appl Physiol 119:2363–2373. https://doi.org/10.1007/s00421-019-04220-8
Hornsby WG, Gentles JA, Haff GG et al (2018) What is the impact of muscle hypertrophy on strength and sport performance? Strength Cond J 40:99–111. https://doi.org/10.1519/SSC.0000000000000432
Jenkins NDM, Housh TJ, Buckner SL et al (2016) Neuromuscular adaptations after 2 and 4 weeks of 80% versus 30% 1 repetition maximum resistance training to failure. J Strength Cond Res 30:2174–2185. https://doi.org/10.1519/JSC.0000000000001308
Jenkins NDM, Miramonti AA, Hill EC et al (2017) Greater neural adaptations following high- vs. low-load resistance training. Front Physiol 8:331. https://doi.org/10.3389/fphys.2017.00331
Kubota A, Sakuraba K, Sawaki K et al (2008) Prevention of disuse muscular weakness by restriction of blood flow. Med Sci Sports Exerc 40:529–534. https://doi.org/10.1249/MSS.0b013e31815ddac6
Laurentino GC, Ugrinowitsch C, Roschel H et al (2012) Strength training with blood flow restriction diminishes myostatin gene expression. Med Sci Sports Exerc 44:406–412. https://doi.org/10.1249/MSS.0b013e318233b4bc
Loenneke JP, Wilson JM, Marín PJ et al (2012) Low intensity blood flow restriction training: a meta-analysis. Eur J Appl Physiol 112:1849–1859. https://doi.org/10.1007/s00421-011-2167-x
Loenneke JP, Thiebaud RS, Fahs CA, Rossow LM, Abe T, Bemben MG (2013) Blood flow restriction does not result in prolonged decrements in torque. Eur J Appl Physiol 113(4):923–931
Loenneke JP, Kim D, Fahs CA, Thiebaud RS, Abe T, Larson RD, Bemben DA, Bemben MG (2017) The influence of exercise load with and without different levels of blood flow restriction on acute changes in muscle thickness and lactate. Clin Physiol Funct Imaging 37(6):734–740
Loenneke JP, Dankel SJ, Bell ZW et al (2019) Is muscle growth a mechanism for increasing strength? Med Hypotheses 125:51–56. https://doi.org/10.1016/j.mehy.2019.02.030
Merrigan JJ, White JB, Hu YE et al (2018) Differences in elbow extensor muscle characteristics between resistance-trained men and women. Eur J Appl Physiol 118:2359–2366. https://doi.org/10.1007/s00421-018-3962-4
Moritani T, deVries HA (1979) Neural factors versus hypertrophy in the time course of muscle strength gain. Am J Phys Med 58:115–130
Patterson SD, Hughes L, Warmington S et al (2019) Blood flow restriction exercise position stand: considerations of methodology, application, and safety. Front Physiol 10:533. https://doi.org/10.3389/fphys.2019.00533
Pillen S, van Keimpema M, Nievelstein RAJ et al (2006) Skeletal muscle ultrasonography: visual versus quantitative evaluation. Ultrasound Med Biol 32:1315–1321. https://doi.org/10.1016/j.ultrasmedbio.2006.05.028
Sale DG (1988) Neural adaptation to resistance training. Med Sci Sports Exerc 20:S135-145. https://doi.org/10.1249/00005768-198810001-00009
Thiebaud RS, Yasuda T, Loenneke JP, Abe T (2013) Effects of low-intensity concentric and eccentric exercise combined with blood flow restriction on indices of exercise-induced muscle damage. Interv Med Appl Sci 5(2):53–59
Weir JP (2005) Quantifying test–retest reliability using the intraclass correlation coefficient and the SEM. J Strength Cond Res 19:231–240. https://doi.org/10.1519/15184.1
Wilhelm EN, Rech A, Minozzo F et al (2014) Relationship between quadriceps femoris echo intensity, muscle power, and functional capacity of older men. Age 36:9625. https://doi.org/10.1007/s11357-014-9625-4
Yasuda T, Ogasawara R, Sakamaki M et al (2011) Combined effects of low-intensity blood flow restriction training and high-intensity resistance training on muscle strength and size. Eur J Appl Physiol 111:2525–2533. https://doi.org/10.1007/s00421-011-1873-8
Yasuda T, Loenneke JP, Thiebaud RS, Abe T (2012) Effects of blood flow restricted low-intensity concentric or eccentric training on muscle size and strength. PLoS One 7(12):e52843
Acknowledgements
We thank the participants for their participation throughout the duration of the study.
Funding
This research was supported by the NASA Nebraska Space Grant (Grant no. NNX15AI09H).
Author information
Authors and Affiliations
Contributions
ECH and TJH: were substantial contributors to study concept and design; ECH: carried out data acquisition, analysis, interpretation, and was the primary author; ECH, JLK, CMS, JPA, RJS, GOJ, and JTC: helped with subject recruitment. All authors approved the final version of this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest, financial or otherwise, are declared by the authors. All data are presented honestly, without fabrication, falsification, or data manipulation.
Additional information
Communicated by William J. Kraemer.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hill, E.C., Housh, T.J., Keller, J.L. et al. Patterns of responses and time-course of changes in muscle size and strength during low-load blood flow restriction resistance training in women. Eur J Appl Physiol 121, 1473–1485 (2021). https://doi.org/10.1007/s00421-021-04627-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-021-04627-2