Abstract
Purpose
Currently, cortical voluntary activation (VATMS) is assessed by superimposing transcranial magnetic stimulation (TMS) on a maximal voluntary contraction (MVC), 75% MVC and 50% MVC, each contraction being interspersed with 5–10 s of relaxation. Here, we assessed whether this traditional approach (TRADI) underestimates central fatigue due to this short recovery compared to a continuous method (CONTI).
Methods
VATMS, motor-evoked potential (MEP), and cortical silent period (CSP) of the vastus lateralis were determined in 12 young healthy adults before and after a 2-min sustained MVC of knee extensors in two randomly assigned sessions. In TRADI, evaluations comprised a 7-s rest between the three contractions (100, 75, and 50% MVC) and evaluation following the 2-min sustained MVC started after a minimal rest (3–4 s). In CONTI, evaluations were performed with no rest allowed between the three levels of contraction, and evaluation after the 2-min sustained MVC commenced without any rest.
Results
MVC was equally depressed at the end of the 2 min in both conditions. Post 2-min sustained MVC, VATMS change was greater in CONTI than in TRADI (−29 (15)% [−42, −17] vs. −9 (4)% [−13, −5], respectively, P < 0.001). Differences were also observed between TRADI and CONTI for MEP and CSP immediately after the fatiguing exercise. All differences between the two methods disappeared after 2 min of recovery.
Conclusion
After a 2-min sustained MVC, a few seconds of recovery change the amount of measured VATMS and associated parameters of central fatigue. The continuous method should be preferred to determine deficits in voluntary activation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuromuscular fatigue has been defined as any exercise-induced decline in maximal force or power produced by a muscle (Bigland-Ritchie and Woods 1984; Gandevia 2001). Fatigue is generally quantified as a decrement in maximal isometric strength that develops soon after exercise onset. The underlying mechanisms may originate at any level of the motor pathway and are usually divided into central and peripheral sites of failure. The former refers to the decline in voluntary activation or neural drive of the central nervous system (Goodall et al. 2012a), while the latter concerns alterations at or distal to the neuromuscular junction.
Peripheral fatigue is classically identified as a decrease in twitch or tetanic force evoked by peripheral nerve stimulation on relaxed muscles (Millet et al. 2011). The most common method to characterize central fatigue is maximal voluntary activation (VA) calculated by twitch interpolation (Merton 1953). This technique consists of superimposing peripheral electrical stimulation during a maximal voluntary contraction (MVC), which results in a superimposed twitch (SIT). The greater the SIT relative to a reference stimulation on a relaxed muscle, the lower the VA. This observed activation deficit may be due to the inability of the motor units to fire quickly enough or to a failure in their spatial recruitment (Gandevia 2001). Any decrease in VA with exercise characterizes the presence of central fatigue, which may originate anywhere at or above the site of the stimulation. Consequently, it is not possible to distinguish the changes happening within the central nervous system. The use of other techniques is, therefore, helpful to identify the different failure sites proximal to the neuromuscular junction, which may be spinal and/or supraspinal.
Transcranial magnetic stimulation (TMS) has grown in popularity due to its clinical significance in the diagnosis [e.g., idiopathic generalized epilepsy (Reutens et al. 1993)] and possible treatment of diseases [e.g., depression (Triggs et al. 1999) and Parkinson’s disease (Siebner et al. 1999)]. In addition, single and double pulse TMS (e.g., motor-evoked potential, MEP; cortical silent period, CSP; short and long intracortical inhibitions) is extensively used to understand and characterize fatigue etiology in sport (Goodall et al. 2012b) and medical conditions such as multiple sclerosis (Thickbroom et al. 2006), cancer-related fatigue (Cai et al. 2014), and chronic fatigue syndrome (Sacco et al. 1999). In the voluntary activation calculation through TMS (VATMS), the superimposed stimulus is evoked by magnetic stimulation applied directly over the motor cortex. If a SIT is observed during a MVC, it means that a reserve of force, which was not used voluntarily, was still available. Thus, motor cortical output was unable to drive all motor units maximally before stimulation (Taylor et al. 2005), which may suggest that part of the central failure could be supraspinal (Gandevia et al. 1996). Nonetheless, a decline in VATMS after exercise does not exclusively indicate the presence of supraspinal fatigue, as fatigue at the spinal level may still occur.
Since TMS is a relatively recent technique, most of the experiments used peripheral stimulation to assess VA. Nonetheless, VATMS and its changes with fatigue have been shown to be reliable for the upper (Todd et al. 2004) and lower (Goodall et al. 2009; Sidhu et al. 2009a) limbs. Since corticospinal excitability is enhanced during voluntary contraction (Todd et al. 2003), the reference twitch needs to be estimated. To do so, a linear regression between SIT and voluntary force is drawn to obtain an estimated resting twitch (ERT) by extrapolation (Todd et al. 2003). For a linear relationship between TMS and voluntary force to reliably estimate the resting twitch, TMS should be evoked at contractions above 50% MVC (Goodall et al. 2009, 2012b). Hence, the most currently used method encompasses a superimposed TMS during an MVC and two submaximal contractions (75% MVC and 50% MVC), interspersed with a short period (5–10 s) between contractions.
When investigated for a few seconds and up to several minutes after repeated or sustained contractions, VATMS has been observed to decline in upper (Gandevia et al. 1996; Hunter et al. 2008; Szubski et al. 2007) and lower (Bachasson et al. 2016; Goodall et al. 2009; Gruet et al. 2014; Mileva et al. 2012) limbs. However, considering the rapid recovery of neuromuscular function that occurs within the first 2 min after exercise (Froyd et al. 2013), the rate of fatigue, and notably central fatigue, which appears to recover more quickly than peripheral fatigue, may have been underestimated in these studies. For instance, Gruet et al. (2014) reported a full recovery of knee extensors VATMS 96 s after high-intensity quadriceps contractions to task-failure, whereas peak twitch (Pt) only recovered partially 276 s after exercise. As a consequence, existing evidence may not reflect the true central fatigue induced by exercise.
Therefore, the purpose of the current study was to compare the traditional method (TRADI) of VATMS assessment with a continuous method (CONTI), where neuromuscular function was evaluated with no rest between contractions and immediately at the end of exercise. We hypothesized that VATMS deficit would be smaller in TRADI due to the rapid recovery of central fatigue, confirming the need to use a CONTI method to assess the actual VA depression induced by exercise. Since peripheral fatigue recovery is slower, we also hypothesized that the methodology would either have a minimal or non-existent effect on it.
Materials and methods
Subjects
Twelve healthy active men [mean (SD), age 23 (3) years, height 181 (6) cm, and mass 74 (8) kg] participated in the study. Written informed consent was obtained from all subjects prior to their participation. This study was approved by the ethics board at the University of Calgary (REB 14-1625) and conformed to the standards from the latest edition of the Declaration of Helsinki. All subjects were in good physical condition with no signs or symptoms of neurological, cardiovascular, circulatory or orthopedic disorders, or contraindications to TMS. Subjects refrained from any type of physical exercise at least 2 days before testing and did not drink any beverages with caffeine on test days.
Experimental protocol
To compare the rate of central fatigue using the CONTI and TRADI methods, VATMS was determined before and after a 2-min sustained MVC of knee extensors in two randomly assigned sessions, performed on the same day, with a wash-out period of 1.5 h. Corticospinal excitability (through MEP) and intracortical inhibition (through CSP) were also measured with TMS. Femoral nerve electrical stimulation (FNES) was applied to assess neuromuscular transmission (through muscle compound action potential, M-wave) and contractile properties (through Pt). Electromyography activity (EMG) of vastus lateralis (VL), rectus femoris (RF), and biceps femoris (BF) muscles was monitored throughout the sessions.
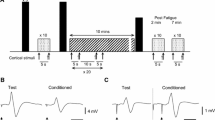
Figure 1a illustrates the experimental protocol, which consisted, after the baseline measurements, in two times the following with a 1.5-h wash-out period: neuromuscular function (NMF) tests before (PRE), immediately after (POST) and 2–4–6 min after (R2–R4–R6, respectively) a 2-min sustained MVC (fatiguing task). The baseline measurements (Fig. 1b) comprised (a) the determination of stimulation intensity to elicit the highest M-wave and Pt response in the unfatigued state (this intensity was further used for stimulation after the fatiguing protocol); (b) a warm-up composed of 20 submaximal contractions with progressive increases in force followed by two 3–4-s MVCs interspersed with 1 min to set the percentage MVC used for optimal TMS site and intensity determination; (c) the determination of optimal TMS site; and (d) the determination of optimal TMS intensity. The NMF tests performed before the fatiguing task (PRE) were performed twice before each 2-min sustained MVC. Figure 1c shows the NMF tests methodology to assess central (VATMS, MEP, and CSP) and peripheral (Pt, M-wave) fatigue. The CONTI method comprised a single voluntary contraction including an initial ~3–4-s MVC followed by a voluntary reduction in force output to match 75% MVC and then 50% MVC with no rest period in-between. To allow for performance of this continuous voluntary contraction without any rest between the 3 levels of force (i.e., MVC, 75 and 50% MVC), one experimenter monitored the instantaneous values of force produced during MVC (LabChart software was continuously providing the highest value of force achieved). Then, after the TMS had been delivered during the MVC, the scale of the force channel of LabChart was reset to the maximum value of force produced before TMS delivery. Since before the start of the experiment, two red twines (target line) were affixed on the screen corresponding to 75 and 50% of the maximum force value displayed in the scale of the force channel, resetting this maximum value ensured that the upper twine corresponded to 75% and the lower twine to 50% of the maximum value represented in the channel. This reset was displayed in less than 2 s after TMS, while the subject was either relaxing between contractions (TRADI) or still contracting at the previous torque level (CONTI). This procedure enabled the performance of the NMF test right after the 2-min sustained MVC without any rest in CONTI.
a Experimental protocol, which consisted of baseline measurements and two times the following testing (interspersed by a 1.5-h wash-out period): neuromuscular function (NMF) tests before (PRE), immediately after (POST) and 2–4–6 min (R2–R4–R6) after a 2-min sustained MVC (fatiguing task). b Baseline measurements, which comprised: (a) electrically evoked responses to elicit the highest M-wave and twitch response in the unfatigued state; (b) warm-up followed by 2 × 3–4-s MVCs; (c) determination of optimal TMS site; and (d) determination of optimal TMS intensity. The black arrow indicates femoral nerve electrical stimulation (FNES), whereas the dotted arrow represents the transcranial magnetic stimulation (TMS). c The NMF tests methodology to assess central and peripheral fatigue. The continuous method (CONTI) comprised one unique voluntary contraction, while the traditional method (TRADI) consisted of three ~3–4-s voluntary contractions (MVC, 75% MVC, and 50% MVC) interspersed by ~7-s relaxation periods
The TRADI method consisted of three ~3–4-s voluntary contractions: one MVC, one 75% MVC, and one 50% MVC, each contraction being interspersed by ~7-s relaxation periods. In TRADI, a 3–4-s rest was also allowed between exercise termination and fatigue evaluation. This was not the case in CONTI. During voluntary contractions, TMS was only delivered when the subjects attained and maintained the desired force level. Immediately after TMS delivery, subjects were asked to re-contract at the same force level as fast as possible.
Dependent variables
Force measurements
Subjects sat on a custom-built quadriceps chair with the right hip angle set at 90° and knee joint angle set at 100° of flexion (full knee extension = 180°). A noncompliant strap connected to a force transducer was attached 3–5 cm above the subject’s lateral malleoli. Force data were acquired using a PowerLab data acquisition system (16/30—ML880/P, ADInstruments, Bella Vista, Australia) at a sampling rate of 2 kHz. The subjects were firmly secured to the chair with noncompliant straps to minimize body movement. The target force to meet during each submaximal contraction of the TMS testing procedure and real-time visual feedback of the force produced was provided to the subject via custom software and macros (LabChart 7, ADInstruments) on a wide computer screen throughout the experiment. Strong verbal encouragement was given during brief and prolonged MVCs.
Electromyographic recordings
The EMG signals of the right VL, RF and BF were recorded using bipolar silver chloride surface electrodes of 10-mm diameter (Type 0601000402, Contrôle Graphique Medical) during the voluntary contractions and electrical/magnetic stimuli. The recording electrodes were secured lengthwise to the skin over the muscle belly, with an inter-electrode distance of 25 mm. The positions of the electrodes were marked on the skin in case replacement was necessary. The reference electrode was attached to the patella. Low impedance (Z < 5 kΩ) at the skin–electrode surface was obtained by abrading the skin with fine sand paper and cleaning with alcohol. EMG data were recorded with a PowerLab system (16/30—ML880/P, ADInstruments) with a sampling frequency of 2 kHz. The EMG signal was amplified with octal bio-amplifier (Octal Bioamp, ML138, ADInstruments) with a bandwidth frequency ranging from 5 to 500 Hz (input impedance = 200 MΩ, common mode rejection ratio = 85 dB, gain = 1000), transmitted to the PC and analyzed with the LabChart 7 software (ADInstruments).
Femoral nerve electrical stimulation
Electrical stimulation was delivered percutaneously to the femoral nerve via a self-adhesive electrode (10-mm diameter, Ag–AgCl, Type 0601000402, Contrôle Graphique Medical, Brie-Comte-Robert, France). The anode, a 10 × 5 cm self-adhesive stimulation electrode (Medicompex SA, Ecublens, Switzerland), was located in the gluteal fold. A constant current stimulator (Digitimer DS7A, Hertfordshire, United Kingdom) was used to deliver a square-wave stimulus of 1-ms duration with maximal voltage of 400 V. The optimal stimulus intensity was determined from knee extensors response producing both maximal Pt and maximal M-wave amplitude. The stimulating intensity [151 (67) mA] was supramaximal (i.e., 120% of optimal intensity) and held constant throughout the protocol. Supramaximal FNES was delivered: (1) 2 s after the MVC in relaxed muscle in TRADI or 2 s after last 50% MVC in relaxed muscle in CONTI and (2) during the 50% MVC (see Fig. 1c).
Transcranial magnetic stimulation
A magnetic stimulator (Magstim 2002, The Magstim Company, Dyfed, United Kingdom) was used to stimulate the motor cortex area of the right leg. Single TMS pulses of 1-ms duration were delivered via a concave double-cone coil (diameter 110 mm, maximum output 1.4 T) positioned over the vertex of the scalp and held tangentially to the skull. To determine optimal TMS site, the coil was positioned to preferentially activate the left motor cortex (contralateral to the right leg) and elicit the largest MEP in the VL with only a small MEP in the BF (<10% of VL MEP) during isometric knee extension at 10% MVC with a stimulus intensity of 50% of maximal stimulator power output (Sidhu et al. 2009a). The optimal stimulus site was marked on a breathable swimming cap, which was worn on the head throughout the protocol to ensure reproducibility of the stimulus conditions for each subject. To determine optimal stimulation intensity, TMS was elicited during brief (~5 s) submaximal (20% MVC) isometric knee extensions at 30, 40, 50, 60, 70, and 80% of maximal stimulator power output in random order. Four consecutive contractions were performed at each stimulus intensity with 10 s of rest between contractions. The stimulus intensity [68 (10)% of maximal stimulator power output] that elicited the largest right VL MEPs and SIT with small MEP of the right BF (<10% of VL MEP) was considered optimal and employed throughout the protocol, as previously suggested (Rupp et al. 2012). If a plateau was not reached, higher intensities (i.e., 90 and 100%) were investigated.
Data analysis
Mechanical responses
Peak forces measured during MVC were calculated as the maximal values obtained before the stimuli. At PRE, the highest of the two MVCs was chosen for further analysis. To calculate global force quantity (intensity × duration) produced throughout the 2-min sustained MVC (Rozand et al. 2014), force time integral (N s) was calculated and summed over eight periods of 15 s along the 2 min. Pt was also determined.
M-wave
M-wave peak-to-peak amplitudes, durations, and areas were calculated from VL EMG responses to single FNES in the relaxed muscle (M-wave) and at 50% MVC (Msup, Fig. 1c).
TMS parameters
Motor-evoked potential areas of quadriceps muscles during submaximal (MEP50 and MEP75 for 50 and 75% MVC, respectively) and maximal contractions (MEP100) were normalized to Msup (Sidhu et al. 2012). The duration of the CSP during submaximal (CSP50 and CSP75 for 50 and 75% MVC, respectively) and maximal contractions (CSP100) was determined manually as the interval from the stimulus to the return of continuous EMG activity (Sidhu et al. 2009b). VATMS was quantified by measurement of the superimposed force responses to TMS. At PRE, only the highest VATMS value from the two NMF tests was used for further analysis. The SIT amplitude evoked during contractions at 100, 75, and 50% MVC was calculated, and the y-intercept of the linear regression between the mean SITs and voluntary force was used to quantify ERT (Todd et al. 2003). For both TRADI and CONTI, VATMS (%) was then calculated using the following equation:
The reliability of this method for the determination of VATMS has been previously described for the knee extensors (Goodall et al. 2009; Sidhu et al. 2009a). 6 VATMS out of a total of 120 assessments had to be ruled out, as 5 showed a poor linear regression between SIT and voluntary force and 1 POST NMF test (in TRADI condition) was not performed by a subject.
EMG during 2-min sustained MVC
During the 2-min sustained MVC, the EMG activity of VL, RF, and BF was calculated as the average root-mean-square (RMS) over the same 15-s periods, as for force analysis (see above).
Statistical analyses
Data are presented in the text as mean (SD) and 95% confidence interval [CI]. Data are shown in the figures and tables as mean (SD). The statistical analyses were performed with Statistica (version 8, Tulsa, USA). Normality of distribution and homogeneity of variances of the main variables were checked using a Shapiro–Wilk normality test and the Levene’s test, respectively. A two-way ANOVA (condition × time) with repeated measures was performed for the two conditions (CONTI and TRADI) and five timepoints of each condition (PRE, POST, R2, R4, and R6) or nine timepoints when investigating torque/EMG kinetics during the 2-min MVCs (MVC PRE, 0–15, 15–30, 30–45, 45–60, 60–75, 75–90, 90–105, and 105–120 s). Because VATMS was not normally distributed at PRE, this parameter was analyzed with a two-way ANOVA with repeated measures performed on the change from baseline measurements, i.e., condition (CONTI, TRADI) × time (ΔPRE-POST, ΔPRE-R2, ΔPRE-R4, and ΔPRE-R6). Where the ANOVA revealed significant main effects or interactions, the data were further explored using pair-wise comparisons with a Bonferroni correction. For all statistical analyses, an alpha level of 0.05 was used as the cutoff for significance.
Results
Fatiguing protocol
Figure 2 shows the typical force trace and EMG VL during the TRADI and CONTI evaluation of one subject. Force and EMG VL values were not different between TRADI and CONTI during the 2-min sustained MVC (Fig. 3). Force was significantly depressed at the end of the 2-min MVC (P < 0.001) to a similar extent in both conditions (−75 (5)% [−78, −73] vs. −74 (7)% [−77, −70] in TRADI and CONTI, respectively). VL and RF EMG were depressed at the end of the 2-min MVC, with no differences between conditions. In addition, force quantity produced during the 2-min MVC showed no differences between TRADI and CONTI (28.7 (4.5) kN s [26.1, 31.2] vs. 28.0 (4.6) kN s [25.4, 30.6], respectively, NS).
Typical force trace and VL EMG during TRADI (a) and CONTI (b) evaluation before the fatiguing task. The bottom part of the panel shows the MEP and CSP at MVC, 75% MVC and 50% MVC. Dotted lines represent TMS. The solid lines at the top represent FNES, whereas at the bottom they represent the end of the CSP
Maximal voluntary force
Maximal voluntary contraction values are reported in Fig. 4a. At PRE, MVCs were similar in both conditions. MVC changes from PRE to POST were significantly greater in CONTI compared to TRADI (−79 (6)% [−83, −76] vs. −45 (10)% [−51, −40], respectively, P < 0.001). At R2, R4, and R6, the MVCs were not different between conditions. Despite a partial recovery, MVC at R6 was still lower than at baseline both in TRADI (−15 (8)% [−19, −10], P < 0.001) and in CONTI (−15 (7)% [−19, −11], P < 0.001).
a Maximal voluntary contraction force (MVC) before (PRE), at the end of the 2-min sustained MVC (POST), and 2 min (R2), 4 min (R4), and 6 min (R6) after exercise. N = 12. b Maximal voluntary activation by TMS (VATMS) before (PRE), at the end of the 2-min sustained MVC (POST), and 2 min (R2), 4 min (R4), and 6 min (R6) after exercise. N = 8. Dashed line indicates wash-out period of 1.5 h. Data are mean (SD) in TRADI and CONTI conditions. Significantly different from PRE of the same condition: *P < 0.05, ***P < 0.001; significantly different from the same timepoint in TRADI: ††† P < 0.001
Peripheral fatigue
Mechanical and EMG responses to electrical stimulation are shown in Table 1. Pt did not differ between conditions at PRE. At POST, Pt was significantly depressed (P < 0.001) to a similar extend in both conditions (−62 (15)% [−71, −53] vs. −63 (18)% [−73, −52] in TRADI and CONTI, respectively). Values at R6 indicated a partial and similar recovery in TRADI and CONTI. The M-wave area was not different between conditions at PRE. There was a significant effect of time (P < 0.001), i.e., M-wave area increased similarly in both TRADI and CONTI at POST (17 (9)% [11, 22] vs. 17 (11)% [10, 24], respectively, P < 0.001), R2 (20 (6)% [16, 24] vs. 11 (11)% [5, 18], respectively, P < 0.001), R4 (8 (21)% [−4, 21] vs. 7 (8)% [3, 12], respectively, P = 0.001), and R6 (10 (8)% [5, 15] vs. 5 (9)% [0, 10], respectively, P = 0.009).
Voluntary activation
VATMS at PRE was not different between conditions (Fig. 4b). At POST, VATMS decreased significantly in both conditions, but the decline was significantly greater in CONTI compared to TRADI (−29 (15)% [−40, −19] vs. −9 (4)% [−12, −6], respectively, P < 0.001). At R2, VATMS was not significantly different compared to PRE anymore for both conditions.
Corticospinal excitability and intracortical inhibition
Figure 5 shows the changes in MEP during fatigue and recovery. At PRE, MEP100, MEP75, and MEP50 did not differ between CONTI and TRADI. At POST, MEP100 showed a significant increase in CONTI only (115 (63)% [76, 154], P < 0.001) and this parameter was significantly greater in CONTI than in TRADI at this timepoint (P = 0.004). From R2, MEP100 values were not different from PRE, whatever the conditions. MEP75 remained unchanged throughout the protocol for both conditions. There was a significant effect of time (P < 0.001) for MEP50, as it decreased similarly in both conditions at POST (−10 (24)% [−25, 4] vs. −33 (19)% [−45, −21] in TRADI and CONTI, respectively). At R2, MEP50 had completely recovered in both conditions.
Vastus lateralis motor-evoked potential areas (MEP) assessed by TMS and normalized to Msup areas. Data are presented before (PRE), at the end of the 2-min sustained MVC (POST), and 2 min (R2), 4 min (R4), and 6 min (R6) after exercise. a–c MEPs obtained during MVC (MEP100) and during submaximal contraction at 75% MVC (MEP75) and 50% MVC (MEP50), respectively. N = 10. Dashed line indicates wash-out period of 1.5 h. Data are mean (SD) for TRADI and CONTI conditions. Significantly different from PRE of the same condition: ***P < 0.001; significantly different from the same timepoint in TRADI: †† P < 0.01; significantly different from PRE (main effect of time): ‡‡‡ P < 0.001
Changes in CSP are shown in Table 1. At PRE, CSP100, CSP75, and CSP50 did not differ between CONTI and TRADI. CSP100 was significantly greater at POST in CONTI only (22 (18)% [11, 32], P = 0.003). CSP75 analysis showed an effect of time. Although no significant changes were observed compared to PRE, CSP75 was significantly longer at POST compared to R4 (3 (13)% [−5, 11] vs. 8 (17)% [−2, 18] in TRADI and CONTI, respectively, P = 0.017) and R6 (8 (11)% [2, 15] vs. 7 (13)% [0, 15] in TRADI and CONTI, respectively, P = 0.002). There was a significant time effect for CSP50, as it was significantly longer at POST compared to PRE in both conditions (7 (10)% [1, 12] vs. 15 (10)% [9, 21] in TRADI and CONTI, respectively, P = 0.002).
Discussion
The objective of this paper was to determine whether the methodology to evaluate VATMS would influence the rate of central fatigue and corticospinal properties. Our study design is the first to permit the comparison between the conventional method, which starts the neuromuscular evaluation a few seconds after exercise termination and allows a short relaxation between contractions, with a continuous method, which comprised fatigue assessment at exercise completion and with no recovery period between contractions. The main results are that 3–4 s between the end of exercise and neuromuscular evaluation, together with 7 s of rest between contractions, leads to a 20% underestimation of the extent of central fatigue assessed by VATMS. Furthermore, intracortical inhibition and corticospinal excitability changes were also underestimated when short relaxation times were allowed. Therefore, this study highlights the need for measuring central parameters immediately after exercise completion to adequately appreciate fatigue amplitude and etiology. The proposed method to evaluate central fatigue has implications not only in the field of sport but also in the clinical setting, where quantifying and characterizing fatigue is relevant (Cai et al. 2014; Sacco et al. 1999; Thickbroom et al. 2006).
Maximal voluntary force changes and peripheral fatigue
The present study showed a ~75% decline in force at the end of the 2-min sustained MVC, regardless of the condition. These findings are in accordance with previous literature, as 100-s and 2-min sustained MVCs of the knee extensors have been shown to induce force decrements of about 63–79% (Doix et al. 2013; Goodall et al. 2009; Kennedy et al. 2014; Lloyd et al. 2015; Martin and Rattey 2007; Place et al. 2007; Rattey et al. 2006; Siegler and Marshall 2015).
Although there was a similar force drop during the 2-min sustained MVC, the fatiguing protocol induced a decline in MVC at POST of about 79% in CONTI and 45% in TRADI. Previous literature has documented a decrement in knee extensors MVC ranging from 10 to 33% at the POST 2-min measurement (Doix et al. 2013; Goodall et al. 2009; Kennedy et al. 2014; Lloyd et al. 2015; Martin and Rattey 2007; Place et al. 2007; Rattey et al. 2006). The lower decline in these studies is likely due to a delay in neuromuscular assessment after fatigue, which ranged from a few seconds up to 30 s (Martin and Rattey 2007). Our study corroborates this idea, as a 3–4-s rest period led to a 34% recovery in force. Neuromuscular function evaluation should, therefore, be performed as close to exercise completion as possible to avoid fatigue underestimation and fatigue etiology misinterpretations, as discussed below.
As opposed to MVC, Pt was reduced after exercise to a similar extent in both conditions. The 63% decline observed in the present study was greater compared to the decrease of 26% in Pt reported previously (Goodall et al. 2009). At the end of the fatiguing protocol, Goodall et al. (2009) performed 3 MVCs with peak twitch assessment 2 s after each MVC. The rest (even if minimal) between the end of the fatigue protocol and the first MVC, between each MVC and peripheral stimulation, and between peripheral stimulation and the following MVC might have led to some additional recovery. In addition, the authors averaged the three peak twitch values. Due to the rest between assessments, the second and third evoked responses already benefited from some recovery (probably more than 15–20 s), which might have significantly affected the average obtained from the three assessments. Therefore, the rest between assessments and the use of the average of the three peak twitch values may help explain the lower peak twitch depression in Goodall et al. (2009) compared to our study.
The lack of difference in Pt depression between TRADI and CONTI suggests that it may take longer to recover from peripheral fatigue. In fact, Pt was still depressed 6 min after exercise in both conditions.
Cortical voluntary activation and corticospinal excitability changes
Since the linear association between SIT and voluntary force was preserved even after fatigue, we were able to extrapolate the ERT (Todd et al. 2003) in both methods. Goodall et al. (2009) documented a 17% VATMS decline after the same knee extensor fatiguing protocol. To our knowledge, only one study (Sidhu et al. 2009b) used a CONTI method to assess neuromuscular fatigue. However, in this study, the continuous method was used after a 30-s MVC, which was itself conducted after cycling exercise. Thus, it is not possible to differentiate the role of methodology vs. the role of the type of fatiguing exercise in VATMS reduction. The present study is the first one to demonstrate that a few seconds of relaxation were enough to observe a pronounced difference in VATMS between CONTI and TRADI.
Differences between conditions were not limited to VATMS. Indeed, MEP100 was increased at POST in CONTI only, with no differences anymore at R2. The increased MEP100 in CONTI is in agreement with previous literature in the upper body for sustained MVC, since MEP100 in these experiments was performed without rest (Gandevia et al. 1996; Kennedy et al. 2014; Szubski et al. 2007). Our findings are also consistent with previous works on the knee extensors, as enhanced MEP100 was reported at task failure after a repeated isometric submaximal contraction protocol (Gruet et al. 2014). Gruet et al. (2014) also reported a very rapid recovery, as MEP100 was not different from baseline 96 s after task failure. This quick recovery is further supported by our study, as the TRADI condition did not show any significant MEP100 depression at POST. Thus, it seems that after sustained maximal contractions, only a few seconds are sufficient for TMS-induced changes in corticospinal excitability during MVC to be reversed.
This is the first study documenting changes in MEP at various force levels after a 2-min sustained MVC of the knee extensors. Strikingly, MEP50 was depressed at POST in CONTI and TRADI. To our knowledge, only Todd et al. (2003) assessed MEP changes at the same relative forces after a sustained contraction of the elbow flexors. In their study, however, the sustained contraction terminated when the subjects were no longer able to maintain 60% of the initial MVC. Although MEP50 was significantly smaller with fatigue, MEP100 and MEP75 remained unaltered. These findings are, at least partly, in agreement with the changes observed in our study. Indeed, it seems that a longer recovery time is required for MEP50 to return back to baseline, as there was a similar depression in both TRADI and CONTI. Todd et al. (2003) further support this idea, as the depression in MEP50 was still evident 8 s after exercise termination. In our study, MEP100 did not show any differences at POST in TRADI, suggesting a quicker recovery than MEP50. Thus, Todd et al. (2003) may have failed to report any changes at MEP100 due to the 8-s rest allowed after exercise completion.
Before exercise, MEP amplitude usually reaches its peak near 50% MVC, decreasing thereafter in stronger contractions up to 100% MVC. Since motoneuron discharge rate increases from 50% up to 100% MVC, it is more likely that upon TMS delivery more motoneurons are in the refractory period at 100% MVC than at 50% MVC. Thus, motoneurons are less responsive to TMS at maximal voluntary contractions, which explain why MEP is smaller at 100% MVC than at 50% MVC (Todd et al. 2016).
As a result of the slowing of motoneuron discharge rate during a 2-min MVC (Bigland-Ritchie et al. 1983), it is plausible to admit that, after exercise, discharge frequency during an MVC is closer to that of a 50% MVC before exercise. Therefore, after exercise, TMS is likely to elicit a greater response at 100% MVC, because less motoneurons are in the refractory period, which may explain the exercise-induced increase in MEP100 observed in CONTI.
Motoneuron discharge rate at 50% MVC after a 2-min MVC is likely to be similar to the discharge rate of contractions <50% MVC before exercise. The lower corticospinal excitability at these discharge rates with lower spatial recruitment at these torque levels may explain the decrease in MEP50 after exercise.
Although the pronounced exercise-induced changes in absolute force might have affected motor unit recruitment (both spatial and temporal) at submaximal and maximal force intensities, which in turn might have induced different responses in MEP100 and MEP50, this remains speculative and needs to be addressed in future research.
Intracortical inhibitions
The 2-min sustained MVC induced a lengthening in CSP50 in both conditions, while CSP100 was increased solely in CONTI. At the end of a sustained contraction, intracortical inhibitions during MVC have been shown to be enhanced both in the upper (Gandevia et al. 1996; Szubski et al. 2007; Taylor et al. 1996) and lower limbs (Gruet et al. 2014; Mileva et al. 2012; Sidhu et al. 2009b). Since CSP100 returned to baseline at POST in TRADI, it appears that 3–4 s are sufficient to see a full recovery in CSP100. Conversely, the lengthened CSP100 6 s after exercise in Gruet et al. (2014) would suggest a more long-lasting CSP100 increase. The use of a repeated isometric submaximal contraction protocol by Gruet et al. (2014) differed from the sustained MVC used in our study, which may explain the contrasting results in CSP100.
Most studies assessed intracortical inhibition during maximal contractions only. Nonetheless, longer CSP50 have been previously reported in knee extensors (Gruet et al. 2014; Sidhu et al. 2009b) and ankle flexors (Mileva et al. 2012) after fatiguing tasks. Since in Gruet et al. (2014), these changes were only noticeable right at the end of the fatiguing protocol (i.e., the first post measurement revealed a total recovery of intracortical inhibition), a 6-s relaxation appeared to be enough to observe a full recovery of intracortical inhibitions in that study. However, we observed a CSP50 depression both in CONTI and in TRADI. Further research is thus required to investigate if fatigue-induced changes in CSP depend on the intensity of contraction (maximal vs. submaximal) as well as on motor task intensity and/or modality (i.e., sustained vs. intermittent).
The concomitant analysis of MEPs and CSPs helps to explain the etiology of VATMS deficit. In the CONTI session, VATMS, MEP, and CSP changes occurred at POST, with a complete recovery of all parameters in R2. However, the TRADI condition revealed an attenuated but still significant VATMS decline at POST, with changes only in MEP50 and CSP50, which confirms that central fatigue does not necessarily imply modified corticospinal excitability and inhibition (Gandevia 2001). Consequently, central mechanisms other than changes in intracortical inhibitions and excitability of motor cortical cells with corticospinal projections might explain central fatigue. Such a hypothesis has been previously suggested, as when the elbow flexors were held ischemic following a sustained MVC, MEPs recovered, while VATMS did not (Gandevia et al. 1996; Taylor et al. 2000).
In addition to the impact of the methodology used on central fatigue quantification, the different timings of NMF assessment at POST (i.e., 3–4 s after 2-min MVC in TRADI vs. no rest in CONTI) might have also contributed to the differences observed in central fatigue. The fact that the MVC was significantly different at POST between TRADI and CONTI provides a good indication that neuromuscular function can significantly recover within 3–4 s, reinforcing the need to reduce any period of recovery when measuring fatigue.
Conclusion
The method widely used to quantify neuromuscular changes following a fatiguing protocol comprises electrical and magnetic stimulation at submaximal and maximal intensities, interspersed with a few seconds of recovery. In addition, most literature that reported central and peripheral fatigue allowed a period of rest between exercise completion and fatigue evaluation, which may range from a few seconds up to 5 min. The present study showed that the methodology greatly influences fatigue quantification, as the traditional method led to fatigue underestimation of maximal voluntary activation decline and associated parameters. Thus, the results from previous research that used this methodology might not reflect the actual central fatigue caused by the fatiguing protocols and prevent the right analysis of fatigue etiology. This is especially important in protocols that use maximal sustained contractions, as in this case, the recovery of the neuromuscular system is very quick. The differences between the two methods are not expected to be as high for other types of fatiguing protocols. The continuous method used in the present study should be preferred to evaluate central fatigue induced by exercise in sport and in the clinical setting.
Abbreviations
- BF:
-
Biceps femoris
- CI:
-
Confidence interval
- CONTI:
-
Continuous method
- CSP50–CSP75–CSP100 :
-
Cortical silent period at 50–75–100% MVC
- EMG:
-
Electromyography
- ERT:
-
Estimated resting twitch
- FNES:
-
Femoral nerve electrical stimulation
- MEP50–MEP75–MEP100 :
-
Motor-evoked potential at 50–75–100% MVC
- M-wave:
-
Muscle compound action potential on the relaxed muscle
- Msup:
-
Muscle compound action potential measured during voluntary contraction (50% MVC)
- MVC:
-
Maximal voluntary contraction
- NMF:
-
Neuromuscular function
- POST:
-
At the end of the 2-min sustained MVC
- PRE:
-
Before the 2-min sustained MVC
- Pt:
-
Peak twitch
- R2–R4–R6:
-
2–4–6 min after the end of the 2-min sustained MVC
- RF:
-
Rectus femoris
- RMS:
-
Root-mean square
- SIT:
-
Superimposed twitch
- VA:
-
Voluntary activation
- VATMS :
-
Cortical voluntary activation
- VL:
-
Vastus lateralis
- TMS:
-
Transcranial magnetic stimulation
- TRADI:
-
Traditional method
References
Bachasson D, Temesi J, Gruet M et al (2016) Transcranial magnetic stimulation intensity affects exercise-induced changes in corticomotoneuronal excitability and inhibition and voluntary activation. Neuroscience 314:125–133. doi:10.1016/j.neuroscience.2015.11.056
Bigland-Ritchie B, Woods JJ (1984) Changes in muscle contractile properties and neural control during human muscular fatigue. Muscle Nerve 7:691–699. doi:10.1002/mus.880070902
Bigland-Ritchie B, Johansson RS, Lippold OCJ et al (1983) Changes in motoneurone firing rates during sustained maximal voluntary contractions. J Physiol 340:335–346. doi:10.1113/jphysiol.1983.sp014765
Cai B, Allexandre D, Rajagopalan V et al (2014) Evidence of significant central fatigue in patients with cancer-related fatigue during repetitive elbow flexions till perceived exhaustion. PLoS One 9:e115370. doi:10.1371/journal.pone.0115370
Doix ACM, Lefèvre F, Colson SS (2013) Time course of the cross-over effect of fatigue on the contralateral muscle after unilateral exercise. PLoS One 8:1–8. doi:10.1371/journal.pone.0064910
Froyd C, Millet GY, Noakes TD (2013) The development of peripheral fatigue and short-term recovery during self-paced high-intensity exercise. J Physiol 591:1339–1346. doi:10.1113/jphysiol.2012.245316
Gandevia SC (2001) Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81:1726–1771
Gandevia SC, Allen GM, Butler JE, Taylor JL (1996) Supraspinal factors in human muscle fatigue: evidence for suboptimal output from the motor cortex. J Physiol 490(2):529–536
Goodall S, Romer LM, Ross EZ (2009) Voluntary activation of human knee extensors measured using transcranial magnetic stimulation. Exp Physiol 94:995–1004. doi:10.1113/expphysiol.2009.047902
Goodall S, González-Alonso J, Ali L et al (2012a) Supraspinal fatigue after normoxic and hypoxic exercise in humans. J Physiol 590:2767–2782. doi:10.1113/jphysiol.2012.228890
Goodall S, Howatson G, Romer L, Ross E (2012b) Transcranial magnetic stimulation in sport science: a commentary. Eur J Sport Sci 14:S332–S340. doi:10.1080/17461391.2012.704079
Gruet M, Temesi J, Rupp T et al (2014) Dynamics of corticospinal changes during and after a high-intensity quadriceps exercise. Exp Physiol 8:1–27. doi:10.1113/expphysiol.2014.078840
Hunter SK, Todd G, Butler JE et al (2008) Recovery from supraspinal fatigue is slowed in old adults after fatiguing maximal isometric contractions. J Appl Physiol 105:1199–1209. doi:10.1152/japplphysiol.01246.2007
Kennedy DS, McNeil CJ, Gandevia SC, Taylor JL (2014) Fatigue-related firing of distal muscle nociceptors reduces voluntary activation of proximal muscles of the same limb. J Appl Physiol 116:385–394. doi:10.1152/japplphysiol.01166.2013
Lloyd A, Hodder S, Havenith G (2015) The interaction between peripheral and central fatigue at different muscle temperatures during sustained isometric contractions. Am J Physiol Regul Integr Comp Physiol 309:R410–R420. doi:10.1152/ajpregu.00061.2015
Martin PG, Rattey J (2007) Central fatigue explains sex differences in muscle fatigue and contralateral cross-over effects of maximal contractions. Pflugers Arch Eur J Physiol 454:957–969. doi:10.1007/s00424-007-0243-1
Merton PA (1953) Voluntary strength and fatigue. J Physiol 123:553–564
Mileva KN, Sumners DP, Bowtell JL (2012) Decline in voluntary activation contributes to reduced maximal performance of fatigued human lower limb muscles. Eur J Appl Physiol 112:3959–3970. doi:10.1007/s00421-012-2381-1
Millet GY, Martin V, Martin A, Vergès S (2011) Electrical stimulation for testing neuromuscular function: from sport to pathology. Eur J Appl Physiol 111:2489–2500. doi:10.1007/s00421-011-1996-y
Place N, Maffiuletti NA, Martin A, Lepers R (2007) Assessment of the reliability of central and peripheral fatigue after sustained maximal voluntary contraction of the quadriceps muscle. Muscle Nerve 35:486–495. doi:10.1002/mus.20714
Rattey J, Martin PG, Kay D et al (2006) Contralateral muscle fatigue in human quadriceps muscle: evidence for a centrally mediated fatigue response and cross-over effect. Pflugers Arch Eur J Physiol 452:199–207. doi:10.1007/s00424-005-0027-4
Reutens DC, Berkovic SF, Macdonell RAL (1993) Magnetic stimulation of the brain in generalized epilepsy: reversal of cortical hyperexcitability by anticonvulsants. Ann Neurol 34:351–355. doi:10.1002/ana.410340308
Rozand V, Cattagni T, Theurel J et al (2014) Neuromuscular fatigue following isometric contractions with similar torque time integral. Int J Sports Med. doi:10.1055/s-0034-1375614
Rupp T, Jubeau M, Wuyam B et al (2012) Time-dependent effect of acute hypoxia on corticospinal excitability in healthy humans. J Neurophysiol 108:1270–1277. doi:10.1152/jn.01162.2011
Sacco P, Hope PAJ, Thickbroom GW et al (1999) Corticomotor excitability and perception of effort during sustained exercise in the chronic fatigue syndrome. Clin Neurophysiol 110:1883–1891. doi:10.1016/S1388-2457(99)00144-3
Sidhu SK, Bentley DJ, Carroll TJ (2009a) Cortical voluntary activation of the human knee extensors can be reliably estimated using transcranial magnetic stimulation. Muscle Nerve 39:186–196. doi:10.1002/mus.21064
Sidhu SK, Bentley DJ, Carroll TJ (2009b) Locomotor exercise induces long-lasting impairments in the capacity of the human motor cortex to voluntarily activate knee extensor muscles. J Appl Physiol 106:556–565. doi:10.1152/japplphysiol.90911.2008
Sidhu SK, Cresswell AG, Carroll TJ (2012) Motor cortex excitability does not increase during sustained cycling exercise to volitional exhaustion. J Appl Physiol 113:401–409. doi:10.1152/japplphysiol.00486.2012
Siebner HR, Mentschel C, Auer C, Conrad B (1999) Repetitive transcranial magnetic stimulation has a beneficial effect on bradykinesia in Parkinson’s disease. NeuroReport 10:589–594. doi:10.1097/00001756-199902250-00027
Siegler JC, Marshall P (2015) The effect of metabolic alkalosis on central and peripheral mechanisms associated with exercise induced muscle fatigue in humans. Exp Physiol 100:519–530. doi:10.1113/EP085054
Szubski C, Burtscher M, Loscher WN (2007) Neuromuscular fatigue during sustained contractions performed in short-term hypoxia. Med Sci Sport Exerc 39:948–954. doi:10.1249/mss.0b013e3180479918
Taylor JL, Butler JE, Allen GM, Gandevia SC (1996) Changes in motor cortical excitability during human muscle fatigue. J Physiol 490(Pt 2):519–528
Taylor JL, Petersen N, Butler JE, Gandevia SC (2000) Ischaemia after exercise does not reduce responses of human motoneurones to cortical or corticospinal tract stimulation. J Physiol 525(Pt 3):793–801. doi:10.1111/j.1469-7793.2000.00793.x
Taylor JL, Todd G, Gandevia SC (2005) Evidence for a supraspinal contribution to human muscle fatigue. Proc Aust Physiol Soc 36:83–89. doi:10.1111/j.1440-1681.2006.04363.x
Thickbroom GW, Sacco P, Kermode AG et al (2006) Central motor drive and perception of effort during fatigue in multiple sclerosis. J Neurol 253:1048–1053. doi:10.1007/s00415-006-0159-2
Todd G, Taylor JL, Gandevia SC (2003) Measurement of voluntary activation of fresh and fatigued human muscles using transcranial magnetic stimulation. J Physiol 551:661–671. doi:10.1113/jphysiol.2003.044099
Todd G, Taylor JL, Gandevia SC (2004) Reproducible measurement of voluntary activation of human elbow flexors with motor cortical stimulation. J Appl Physiol 97:236–242. doi:10.1152/japplphysiol.01336.2003
Todd G, Taylor JL, Gandevia SC (2016) Measurement of voluntary activation based on transcranial magnetic stimulation over the motor cortex. J Appl Physiol 121:678–686. doi:10.1152/japplphysiol.00293.2016
Triggs WJ, McCoy KJM, Greer R et al (1999) Effects of left frontal transcranial magnetic stimulation on depressed mood, cognition, and corticomotor threshold. Biol Psychiatry 45:1440–1446. doi:10.1016/S0006-3223(99)00031-1
Acknowledgements
We would like to thank Dr. John Temesi for his valuable comments and revision of the manuscript, and Douglas Doyle-Baker for the English revision.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by a doctoral research grant from the University Savoie Mont Blanc.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures were performed in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Communicated by Nicolas Place.
Rights and permissions
About this article
Cite this article
Mira, J., Lapole, T., Souron, R. et al. Cortical voluntary activation testing methodology impacts central fatigue. Eur J Appl Physiol 117, 1845–1857 (2017). https://doi.org/10.1007/s00421-017-3678-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-017-3678-x