Abstract
Transcranial direct current stimulation (tDCS) using intensities ≤ 2 mA on physical and cognitive outcomes has been extensively investigated. Studies comparing the effects of different intensities of tDCS have yielded mixed results and little is known about how higher intensities (> 2 mA) affect outcomes. This study examined the effects of tDCS at 2 mA and 4 mA on leg muscle fatigability. This was a double-blind, randomized, sham-controlled study. Sixteen healthy young adults underwent tDCS at three randomly ordered intensities (sham, 2 mA, 4 mA). Leg muscle fatigability of both legs was assessed via isokinetic fatigue testing (40 maximal reps, 120°/s). Torque- and work-derived fatigue indices (FI-T and FI-W, respectively), as well as total work performed (TW), were calculated. FI-T of the right knee extensors indicated increased fatigability in 2 mA and 4 mA compared with sham (p = 0.01, d = 0.73 and p < 0.001, d = 1.61, respectively). FI-W of the right knee extensors also indicated increased fatigability in 2 mA and 4 mA compared to sham (p = 0.01, d = 0.57 and p < 0.001, d = 1.12, respectively) and 4 mA compared with 2 mA (p = 0.034, d = 0.37). tDCS intensity did not affect TW performed. The 2 mA and 4 mA tDCS intensities increased the fatigability of the right knee extensors in young, healthy participants, potentially from altered motor unit recruitment/discharge rate or cortical hyperexcitability. Despite this increase in fatigability, the TW performed in both these conditions was not different from sham.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The principle underpinning the neuromodulatory technique transcranial direct current stimulation (tDCS) to affect brain function is that tDCS modulates the excitability of neurons by changing the resting membrane potential (Bindman et al. 1964; Purpura and McMurtry 1965). tDCS can elicit changes in excitability that outlast the period of stimulation when applied for several minutes during and before a task (Nitsche and Paulus 2000, 2001, 2011). In recent years, it has been suggested that tDCS may be a potential treatment for neurological and psychiatric disorders (Kuo et al. 2014; Liew et al. 2014; Player et al. 2014). Currently, the majority (~ 96%) of tDCS sessions have been limited to intensities of 2 mA or less (Bikson et al. 2016). Thus, this standard of 2 mA has become an unofficial ‘upper limit’ in tDCS research. However, because tDCS technologies and methodologies have improved, exploration of higher intensities is both plausible and recommended (Rothwell 2012). Indeed, some are already exploring tDCS at intensities as high as 4 mA (Chhatbar et al. 2017, 2018; Khadka et al. 2019; Trapp et al. 2019). However, of these higher intensity investigations that included a motor performance, all of them had small sample sizes (≤ 3), and most were primarily concerned with assessing tolerability and not motor performance changes (Chhatbar et al. 2017, 2018; Trapp et al. 2019). Examining a broader range of stimulation parameters is necessary, because recent evidence suggests that a higher current density (which is a function of electrode size and intensity) may not always result in greater increases in excitability (Bastani and Jaberzadeh 2013a; Chew et al. 2015; Kidgell et al. 2013). Furthermore, a recent review also highlighted the variable effects of tDCS on motor performances, especially in association with increasing stimulation intensity (Esmaeilpour et al. 2018).
A few studies have compared the effect of different tDCS intensities on cortical excitability (Ammann et al. 2017; Chew et al. 2015; Ho et al. 2016; Kidgell et al. 2013; Murray et al. 2015), working memory (Papazova et al. 2018; Stephens et al. 2017), and schizophrenia (Andrade 2013; Hoy et al. 2014; Papazova et al. 2018), with mixed results. For example, Ammann et al. (2017) showed that tDCS at 2 mA, but not 1 mA, significantly increased cortical excitability, whereas the findings of Ho et al. (2016) indicated that 2 mA tDCS did not necessarily result in increased excitability than 1 mA tDCS in healthy participants. The effects of different intensities on motor performances are also sparse. Nevertheless, Liu et al. (2018) found a significant improvement of 2 mA over 1 mA tDCS on performance measures of professional rowers, although this was without a comparison to sham. Together, these studies indicate an uncertain effect of increasing intensity to increase the beneficial effects of tDCS. As mentioned above, the uncertain dose–response effect of increasing intensity to enhance neurophysiological and behavioral outcomes was also the conclusion of a recent review (Esmaeilpour et al. 2018). Therefore, there is a need to understand whether increasing current intensity (i.e., > 2 mA) over a given brain area enhances behavioral and neurophysiological outcomes, like fatigability.
Fatigue is a commonly assessed outcome measure in tDCS studies, and is defined as “the decrease in physical and/or mental performance that results from changes in central, psychological, and/or peripheral factors” (Rudroff et al. 2016). These changes depend on the task being performed, the environmental conditions in which it is performed, and the physical and mental capacity of the individual (Rudroff et al. 2016). Specifically, performance fatigability can be defined as the magnitude or rate of change in a performance criterion relative to a reference value over a given time of task performance. On the other hand, perceptions of fatigue can be defined as a subjective sensation of weariness, increasing sense of effort, mismatch between effort expended and actual performance, or exhaustion (Kluger et al. 2013). Improving perceived and performance fatigue in healthy participants and people with neurological disorders, such as multiple sclerosis, has been an important topic for several years (Finsterer and Mahjoub 2014; Lefaucheur et al. 2017). Non-medication treatments (e.g., resistance exercise, yoga, and occupational therapy) and prescription medications (e.g., amantadine, modafinil, and methylphenidate) have yielded small effects, or effects that are insignificant at the individual level (Finsterer and Mahjoub 2014). Thus, alternative treatments for fatigue, like tDCS, have been investigated in healthy participants (see Angius et al. 2018b for a review) and in people with neurological impairments (Cancelli et al. 2018; Ferrucci et al. 2014; Lefaucheur et al. 2017; Tecchio et al. 2014). Furthermore, as both the Angius et al. (2018b) and the Esmaeilpour et al.’s (2018) reviews highlight performance outcomes in tDCS research (e.g., fatigue), especially with different intensities and intensities beyond the 2 mA standard, which can be unpredictable.
The mechanisms underscoring the effects of excitatory tDCS on performance fatigue could be related to an increased cortical excitability within the primary motor cortex (M1), which could lead to hypothetical reductions in supraspinal fatigue and ratings of perceived exertion (Alix-Fages et al. 2019; Angius et al. 2017). Increased corticospinal excitability, together with alterations in motor unit recruitment strategies (Krishnan et al. 2014), could theoretically explain the observed improvements in performance fatigue after or during unilateral or bilateral tDCS (Abdelmoula et al. 2016; Alix-Fages et al. 2019; Angius et al. 2016, 2018a; Cogiamanian et al. 2007; Lattari et al. 2018; Okano et al. 2015; Oki et al. 2016; Radel et al. 2017; Williams et al. 2013). However, there are also reports of no effects of tDCS on fatigue (Flood et al. 2017; Hameau et al. 2018; Kan et al. 2013; Muthalib et al. 2013; Vitor-Costa et al. 2015). Most of these previous studies have investigated fatigue at submaximal performances, often with isometric contractions (Alix-Fages et al. 2019). However, isometric testing has poor applicability to real-world, dynamic performances (Kollock et al. 2015) and isokinetic testing may better reflect functional performance (Sliwowski et al. 2018). Interestingly, only one has reported the effects of tDCS on isokinetic performance fatigability of the knee extensor muscles (Ciccone et al. 2019), and the authors found no improvements in performance fatigability with 2 mA tDCS over the temporal lobe. The results of fatigue investigations may also be ambiguous, because fatigue is often not clearly defined or is used as an outcome without meaningful measurements in clinical and research settings (Rudroff et al. 2016). Furthermore, the lack of standardized tDCS protocols makes it difficult to compare fatigue studies. Nevertheless, most have used (1) isometric contractions, (2) tDCS administered prior to the task, and (3) intensities less than 2 mA. Thus, the effects of tDCS on isokinetic performance fatigue, especially at intensities > 2 mA, are uncertain.
Therefore, the purpose of this study was to determine the effects of 2 mA and 4 mA tDCS on leg muscle fatigability in healthy young adults, using objective performance fatigability data (peak torque and total work; Gleeson and Mercer 1992), obtained from an isokinetic system. It was hypothesized that M1 tDCS would decrease leg muscle fatigability and that the 4 mA intensity would yield greater decreases in fatigability than the 2 mA intensity.
Materials and methods
Participants
Sixteen physically active young adults were recruited (females = 9; mean ± SD, age = 24.5 ± 3.8 years, height = 170 ± 11.7 cm, weight = 71.1 ± 14.4 kg). Inclusion criteria included: (1) young adult (18–30 years); (2) right-side dominant; (3) performing at least 30 min of moderate intensity physical activity on 3 days/week for the previous 3 months; (4) no chronic neurological, psychiatric, or medical conditions; and (5) no psychoactive medications. The exclusion criteria were: (1) pregnancy; (2) holes or fissures in the skull; (3) metal objects or devices implanted in the skull (e.g., metal plate). All participants were naïve to tDCS stimulation. This study was approved by the University of Iowa’s Institutional Review Board and all participants provided written informed consent before beginning participation.
Experimental protocol
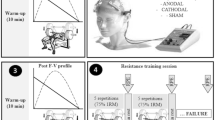
This was a double-blind, randomized sham-controlled study design. Participants completed four laboratory visits, with a 5–8 day separation between visits. At the first session, right-side dominance was objectively determined with an isokinetic maximal strength test of the knee extensors and flexors of both legs (see “Isokinetic strength test”) and the participants performed a familiarization trial of the isokinetic fatigue test (FT; see “Isokinetic fatigue test”). Only participants that had right-side dominance were recruited to avoid the potential for functional brain morphology differences between right- and left-dominant people (Jang et al. 2017). After strength testing, the FT was performed on each leg, with a short (< 2 min) interval between the right leg FT (R-FT) and the left leg FT (L-FT). For consistency between participants and subsequent tDCS sessions, the R-FT was always completed first during all four sessions. For the remaining visits (i.e., two, three, and four), one of three tDCS sessions (sham, 2 mA, and 4 mA; see “tDCS sessions”) were randomly administered and R-FT and L-FT were completed in the same order as the first session (i.e., R-FT first).
Isokinetic strength test
All isokinetic tests (i.e., strength and fatigue) were administered with an isokinetic dynamometer (HUMAC NORM, CSMi, Stoughton, MA). The strength test for the right leg commenced with a submaximal warm-up of the knee extensors and flexors (15 concentric/concentric repetitions, 60°/s). After a ≥ 30 s rest interval, the participants performed five sets of one maximal effort knee extension and flexion (concentric/concentric, 60°/s; Montenegro et al. 2015), with a ≥ 30 s rest between sets. After ~ 2 min, the left leg strength test was performed in the same manner as the right. For dominance verification, the highest peak torque from the five maximal efforts was retained. In addition, the test administrators provided verbal encouragement to help the participants achieve maximal efforts.
Isokinetic fatigue test
The FT involved 40 continuous maximal contractions [concentric/concentric 120°/s (Saenz et al. 2010)] of the knee extensors and knee flexors. Similar muscle fatigue protocols have previously been used in healthy and neurological populations (Ciccone et al. 2019; Hameau et al. 2018; Lambert et al. 2001; Mackey et al. 2018; Thorstensson and Karlsson 1976). In sessions during which tDCS was administered (i.e., visits two, three, and four), the participants undertook the same 15 repetition warm-up as described above. After the warm-up, and at the designated time during tDCS (see “tDCS sessions” below), the R-FT followed immediately by the L-FT was performed. Because some have suggested that tDCS might improve the performance of limbs ipsilateral to stimulation (Schambra et al. 2011), the FT was performed with both legs. The total time to complete both FTs was 4.5–5 min, including the transition time between right and left legs. To maximize participant effort, the work achieved in each contraction was visually displayed (i.e., as a series of bars) and verbal encouragement was provided.
tDCS sessions
tDCS was administered with a battery powered 1X1 tDCS Low-Intensity Stimulator (Model 1300A, Soterix Medical Inc., New York, NY). Two carbon electrodes were inserted into 5 × 7 cm EASYpad sponges (35 cm2 surface area; Soterix Medical Inc., New York, NY). Current density was 0.06 mA/cm2 and 0.11 mA/cm2 for the 2 mA and 4 mA intensities, respectively. Each sponge was saturated with 10–15 ml of 0.9% NaCL saline and held in place using an EASYstrap (Soterix Medical Inc., New York, NY). The active electrode was located over C3, using the 10–20 EEG placement convention (Klem et al. 1999), and the return electrode was localized over the supraorbital area on the contralateral side (Fig. 1). This montage, which targeted the dominant M1, was chosen to maximize motor performance (Schambra et al. 2011). It is noted that because size of the electrodes was relatively large (5 cm × 7 cm), the edge of the active electrode either abutted or covered the center of the skull (Cz), similar to previous studies targeting unilateral M1 leg areas (Jayaram and Stinear 2009; Foerster et al. 2018). Thus, the leg areas of the dominant M1, which are located in the longitudinal fissure (Foerster et al. 2018), were also covered by the electrodes. Active tDCS conditions (i.e., 2 mA and 4 mA) began with a 30 s ramp-up, after which the intensity remained at the specified level for 20 min before a 30 s ramp-down to 0 mA. For sham tDCS, the stimulator performed a 30 s ramp-up to 2 mA and a 30 s ramp-down to 0 mA, after which the intensity stayed at 0 mA for 20 min. After the 20 min stimulation time, the stimulator again performed a 30 s ramp-up to 2 mA and a 30 s ramp-down to 0 mA.
The tDCS device included a ‘PRE-STIM TICKLE’ function, which activated a 1 mA stimulation for ~ 30 s. This function was performed before each tDCS session to investigate the contact quality between the active and return electrodes, and helped to ensure that the electrodes were adequately soaked and securely placed; adjustments to sponge saturation and scalp contact were made as necessary. During the first tDCS session, the location of the active and return electrodes on the EASYstrap were recorded for each participant and the electrodes were secured in the same place for subsequent sessions. Stimulation was always delivered with the participant seated in the dynamometer chair. At minute 15, the participants performed R-FT and then L-FT. As mentioned above, the total time to complete both FTs was 4.5–5 min. Even though most have administered tDCS before a task (Alix-Fages et al. 2019), this may activate neuronal populations in a non-specific way (Nitsche et al. 2008). However, there is also some evidence, particularly from motor and cognitive learning studies (Ammann et al. 2016; Martin et al. 2014; Stagg et al. 2011, 2013), that performing tDCS during a task, which could theoretically enhance endogenous signals activated during task execution, may be more beneficial. Thus, tDCS stimulation (active or sham) was performed both before and during the FT of the right and left legs.
At the end of each tDCS session, the participants were asked to describe any sensations experienced [e.g., itching, tingling, burning, etc. (Aparicio et al. 2016)] and to rate sensation severity on a 10-point Likert-type scale (1 = lowest, 10 = highest). The participants were also asked to guess which tDCS intensity which they experienced (sham, 2 mA, 4 mA). Feedback about the guesses was not provided until the end of the final session.
Data analysis
To investigate the effects of different intensities of tDCS on leg muscle fatigue, two fatigue indices for the knee extensors and flexors of both legs were calculated: one for torque production (FI-T; percent decline in torque production from the beginning to the end of the FT) and one for work performed (FI-W; percent decline in work performed from the beginning to the end of the FT). The peak torque for each repetition of each FT was used to calculate FI-T as follows: FI-T = ([mean of the first five repetitions − mean of the last five repetitions]/mean of the first five repetitions) × 100 (Ciccone et al. 2019; Hameau et al. 2018; Mackey et al. 2018; Thorstensson and Karlsson 1976). The peak work of each repetition was used to calculate FT-W as follows: (total work performed in the last half/total work performed in the first half) × 100 (Lambert et al. 2001). In addition, the total work (TW) performed during the FT (i.e., sum of the work performed in each repetition) was calculated for fatigue indices that were significantly different between tDCS conditions. However, because several participants were unable to achieve a maximum effort at the beginning of the FT, the first two repetitions were considered adaptation and were not included in either FI calculation or the TW calculation. Sensation reports and blinding accuracy of tDCS were determined by averaging the severity of similar sensation descriptions and calculating the percent of correct tDCS condition guesses, respectively.
Statistical analysis
Because differences between the knee extensors and knee flexors were expected a priori (Gur et al. 1999), and the focus of this study was to determine the effects of different intensities of tDCS on leg muscle fatigability, a stimulation (sham vs. 2 mA vs. 4 mA) repeated-measures ANOVA of the FI-T and FI-W data was performed for each leg muscle group (right extensor, left extensors, right flexors, and left flexors). Additionally, any participant that had an FI-T ≤ 0% (i.e., torque production higher at the end compared to the beginning of the FT) was not included in the statistical analysis. This exclusion helped to avoid bias from less-motivated participants. Pairwise post hoc comparisons (paired t tests) and effect sizes (Cohen’s d) were calculated to clarify any significant tDCS differences. Also, TW was assessed using paired t tests on tDCS conditions that had significant FI-T and FI-W pairs. Because this analysis and study were exploratory in nature, significance was accepted at p < 0.05, uncorrected. In the case of a violation of the sphericity assumption for a repeated-measures ANOVA, Greenhouse–Geisser corrections were used. The assumption of homogeneity of variance was tested using Leven’s test of equal variances and adjustments to the degrees of freedom were made as necessary. Statistical analyses were performed using SPSS 25 (IBM Corp, Armonk, NY, USA).
Results
All participants completed all study visits and none had FI results below the bias correction cutoff (FI-T ≤ 0%). The assumptions for the statistical tests were sufficiently met and no corrections were made. Data are reported as mean ± SD in the text and mean ± SEM in the figures. The results of the repeated-measure ANOVA for FI-T indicated a significant effect of stimulation for the right knee extensors (p < 0.001), but not for the right knee flexors (p = 0.80), the left knee extensors (p = 0.57), or the left knee flexors (p = 0.11). Figure 2 shows the results of the post hoc testing, and revealed that both the 2 mA (54.4 ± 8.2%) and the 4 mA (56.4 ± 7.4%) tDCS conditions had significantly higher FI-T (i.e., more fatigability) compared with sham (50.4 ± 6.7%; p = 0.01, d = 0.73 and p < 0.001, d = 1.61, respectively), but 2 mA was not different from 4 mA (p = 0.143, d = 0.39).
For FT-W, the repeated-measures ANOVA indicated a significant effect of stimulation, again for the right knee extensors (p < 0.001), with the pairwise testing (Fig. 3), indicating that 2 mA (62.6 ± 5.1%) and 4 mA (59.1 ± 7.4%) conditions had significantly smaller FI-W (i.e., more fatigability) than sham (56.7 ± 5.4%; p = 0.01, d = 0.57 and p < 0.001, d = 1.12, respectively) and that the 4 mA condition was significantly smaller than the 2 mA condition (p = 0.034, d = 0.37). The results of the repeated-measures ANOVA for TW did not reveal any significant differences between any of the tDCS conditions (sham = 3642.6 ± 1331.2 J; 2 mA = 3539.6 ± 1292.5 J; 4 mA = 3622.9 ± 1235.6 J; Fig. 4).
The tolerability and blinding results of the different tDCS conditions are shown in Table 1. Most of the participants reported feeling similar sensations across all three conditions (i.e., tingling, itching, and burning), accompanied by a slight increase in severity. The most intense of these common sensations, burning, occurred in the 4 mA condition, but was still considered moderate. Notably, there were rare (i.e., n = 1–3), but novel sensations reported in the 2 mA and the 4 mA, most of which were mild—moderate. However, there were a few moderate—severe sensations in each condition (sham: poking = 6; 2 mA: prickling = 5.5, spike = 6; 4 mA: pressure = 7, and headache = 8).
Discussion
The purpose of this study was to investigate the effects of standard intensity tDCS (2 mA) and higher intensity tDCS (4 mA) on leg muscle fatigability in young, healthy participants. Contrary to our hypothesis, the results of the FI analyses indicated increased fatigability of the right knee extensors in the 2 mA and the 4 mA tDCS conditions compared with sham (Figs. 1, 2) and between the 2 and 4 mA conditions (Fig. 2). Interestingly, the total work performed by this muscle group was the same in all three conditions (Fig. 3). Taken together, these results indicate that, despite a significant increase in the fatigability of the right knee extensors from tDCS stimulation, the participants performed an equal amount of work in each condition. The stimulation tolerability reports also revealed that the severity of the sensations felt during sham, 2 mA, and 4 mA stimulation were very similar for most participants (Table 1). A few participants reported moderate—severe sensations (i.e., > 6), but these were rare and occurred in each tDCS condition, including sham. However, it is noted that all of the participants reported that these sensations were only present during the stimulation period, and even the most severe sensations (pressure = 7 and headache = 8, both reported in the 4 mA condition) resolved within minutes after stimulation ended. Nevertheless, future studies using stimulation intensities > 2 mA warrant considerate application and consistent communication with participants before and during higher intensity tDCS application. For the stimulation blinding, approximately two-third of participants correctly guessed the sham and 2 mA conditions, while slightly less than half correctly guess the 4 mA condition (43.8%). However, an equal number of participants incorrectly guessed 2 mA (43.8%) for the 4 mA condition. Thus, only 12.5% of participants incorrectly guessed sham for the 4 mA condition, which may indicate that participant blinding at higher intensities may not be as feasible as more moderate intensities (i.e., ≤ 2 mA) (Fertonani et al. 2015; Kessler et al. 2012).
Previous studies showed a decrease in fatigability (Angius et al. 2016; Cogiamanian et al. 2007) and increased total work (Sales et al. 2016) from tDCS. However, the fatiguing protocols and measurements of these studies differ from the present study in potentially important ways. For example, Angius et al. (2016) and Cogiamanian et al. (2007) used a submaximal isometric contraction and measured the time to fatigue, and Sales et al. (2016) found increased TW with tDCS, but only used five maximal isokinetic contractions. The present fatigue task included repetitive maximal isokinetic contractions and measured TW over 38 contractions. Thus, the differences in effects of tDCS on muscle fatigue and TW might be task dependent and warrants further investigation.
It is well accepted that excitatory tDCS increases cortical excitability (Dissanayaka et al. 2017; Nitsche and Paulus 2000), and influences a range of physiologic and cognitive outcomes (Berryhill and Martin 2018; Sanchez-Kuhn et al. 2017). Increased cortical excitability could heighten the neural drive to the muscles and increase motor unit (MU) recruitment and/or rate of discharge (Krishnan et al. 2014). Thus, enhanced neural drive could have altered the orderly recruitment of MU [i.e., smallest to largest, (Henneman 1957)] and the larger MUs could have been recruited earlier (Krishnan et al. 2014), resulting in an increased fatigability of the muscle.
It has also been suggested that current flow orientation also affects cortical excitability from tDCS (Rawji et al. 2018) and individual differences in cortical alignment and connectivity (Lee et al. 2019) might alter the purported excitatory and inhibitory effects of the active and return electrodes, respectively. Similarly, because little is known about higher intensity stimulation (> 2 mA), it is also possible that the return electrode is be excitatory at higher intensities, which may affect performance. Furthermore, the size of the stimulating electrodes (which influences current density and the brain areas stimulated) may also play an important role in localizing stimulation effects (Bastani and Jaberzadeh 2013b). The large electrodes in our study (35 cm2) may have stimulated nearby cortical functional areas, which may have inhibitory effects on the motor cortex. Again, as suggested by Bastani and Jaberzadeh (2013b), smaller electrodes may result in more spatially focused stimulation and may increase the efficacy of tDCS for the induction of effective corticospinal excitability.
In addition, if it is assumed that the baseline cortical excitability of young, healthy participants is purposefully homeostatic (Chai et al. 2019), and then, increasing excitability could interfere with the efficient genesis and delivery of desired brain signals. In other words, tDCS may induce hyperexcitability in healthy participants and lead to undesired neuronal activity that may hamper endogenous signals. This idea is also supported by the findings of Bastani and Jaberzadeh (2013a), who found that low-intensity/density tDCS (0.3 mA, current density = 0.013 mA/cm2) was excitatory, while relatively higher intensities/densities (0.7 mA and 1.4 mA; current density = 0.029 mA/cm2 and 0.058 mA/cm2, respectively) were inhibitory in healthy participants. The authors postulated that lower intensity/density stimulation might result in excitatory activation via Ca2+ channels without activating GABA and NMDA receptors, which are voltage-dependent (Mellor and Randall 1998). Similarly, Fujiyama et al. (2017) found significant skill learning improvements from inhibitory ‘priming’ of M1 before excitatory stimulation of M1, compared with M1 excitatory stimulation alone, during a force-learning task in young and older healthy adults. Inhibiting M1 (reducing excitability) may have depressed the baseline cortical activity in these participants and the subsequent excitatory stimulation restored or only slightly increased excitability beyond baseline; thus, the net effect may have been similar to the previously mentioned low-intensity condition of Bastani and Jaberzadeh (2013a). Taken together, these results (including those of the present study) may question the suitability of young, healthy participants for exploring the motor performance effects of higher intensity tDCS (≥ 2 mA); however, more definitive investigation is certainly necessary to justify this suggestion. Nevertheless, exploration of higher intensity stimulation in populations with lower baseline excitability resulting from injury or disease (e.g., multiple sclerosis, Parkinson’s disease, or stroke) is certainly warranted to determine if increasing tDCS intensity further increases benefits (Sanchez-Kuhn et al. 2017).
Similarly, the timing of anodal stimulation (before or during) may differently affect leg muscle fatigability. Administering tDCS before a motor performance might increase cortical excitability in a non-specific way (Nitsche et al. 2008), whereas performing tDCS during the desired task might enhance task-specific cortical signals. However, changing excitability homeostasis, which is purported to alter performance, might be modulated by administering the simulation before task performance (i.e., homeostatic metaplasticity; Ziemann and Siebner 2008). In contrast, non-homeostatic excitability changes, called “gating,” might occur when tDCS is administered during the task (i.e., online; Ziemann and Siebner 2008) and online gating may be better suited for improving motor learning (Ammann et al. 2016). In this study, tDCS was delivered both before and during the fatigue task, but the increased fatigability most likely reflects non-homeostatic (online gating) tDCS effects. Thus, it is possible that accessing the homeostatic metaplastic effects of tDCS, by administering stimulation before the task, might yield different results.
There are a few limitations of note for this study. First, the relatively small number of participants may decrease the generalizability of the results. In addition, cortical excitability was not tested, and thus, the effects of higher intensity tDCS (4 mA) on the excitability of this sample are uncertain. Similarly, as mentioned above, the anode was centered over C3 and the leg areas of M1 are located in the longitudinal fissure (Foerster et al. 2018). Although the size of the electrode was sufficiently large to border or overlap Cz in all participants, indicating that M1 leg areas were covered and potentially stimulated, the different sizes of the participants’ heads might have resulted in inconsistent stimulation of the leg areas between participants. Studies targeting unilateral leg areas might benefit from using smaller electrodes and targeting C1/C2, or purposefully bordering/covering Cz to more reliably target the desired sections of M1. In addition, muscle activity of the knee extensors and flexors was not collected and the physiologic underpinnings of the results are ambiguous. The applicability of single-joint testing to functional activities may also be questionable (Kollock et al. 2015) and suggests caution in interpreting the results to real-world, multi-joint activities. Furthermore, the integrity of participant blinding in repeated-measures tDCS investigations (when participants experience more than one condition/intensity) represents a consistent limitation to studies in this field (O’Connell et al. 2012). This could be especially true for higher intensity (> 2 mA) tDCS. Sensation blinding might also be assessed by determining sensations experienced at different times during the stimulation period or by changing the ‘target’ stimulation in the sham condition. In this study, the ramp-up and ramp-down intensity for the sham condition was 2 mA. However, blinding integrity might have been enhanced by setting the intensity of the sham condition to the highest intensity used in the study (4 mA), or even exceeding the highest intensity (e.g., 5 mA). Such finding might prove beneficial for future repeated-measures tDCS studies and warrants investigation. Finally, this study applied tDCS both before and during the FT, but the optimal time of tDCS application is has not been determined. Some have suggested that tDCS may be best delivered during a task (Nitsche et al. 2008), but other evidence may recommend a time period of several minutes after stimulation (60–90 min) before peak cortical excitability occurs (Batsikadze et al. 2013).
Future work should explore the effects of tDCS at higher intensities (> 2 mA) and more closely determine the intensity dose–response. Understanding this association is especially important, because a recent review concluded that the evidence of increasing tDCS intensity to enhance outcomes was inconclusive (Esmaeilpour et al. 2018). There are also indications that increasing stimulation time and/or intensity may shift the intended tDCS effects from excitatory to inhibitory (Batsikadze et al. 2013; Monte-Silva et al. 2013). Future investigations should also include clinical populations that suffer from reduced cortical activity/excitability (e.g., multiple sclerosis, Parkinson’s disease, and stroke) and might, therefore, experience greater benefits from higher intensity tDCS. In addition, transcranial magnetic stimulation (TMS) and electromyography (EMG) should also be included to test changes in cortical excitability and the physiological effects (i.e., muscle activity) of altered brain excitability. Similarly, functional neuroimaging to better elucidate the effects of different intensities of tDCS on brain activity is also needed. Finally, the best timing of high-intensity tDCS stimulation (e.g., during and before) is a key component to improving tDCS applicability and should be efficiently investigated.
Conclusion
The 2 mA and 4 mA tDCS intensities significantly increased the fatigability of the right knee extensors in young, healthy participants, potentially from altered motor unit recruitment/discharge rate or cortical hyperexcitability. However, despite this increase in fatigability, the TW performed in both conditions was not different from sham. The 4 mA tDCS stimulation was generally well tolerated by the participants, and most sensation reports and severities were similar between the tDCS conditions. The few reports of moderate–severe sensations, with the most severe in the 4 mA condition, were transient and quickly resolved after stimulation had ended. Higher intensity stimulation, especially in clinical populations, should be systematically and considerately investigated.
References
Abdelmoula A, Baudry S, Duchateau J (2016) Anodal transcranial direct current stimulation enhances time to task failure of a submaximal contraction of elbow flexors without changing corticospinal excitability. Neuroscience 322:94–103. https://doi.org/10.1016/j.neuroscience.2016.02.025
Alix-Fages C et al (2019) Short-term effects of anodal transcranial direct current stimulation on endurance and maximal force production. A systematic review and meta-analysis. J Clin Med. https://doi.org/10.3390/jcm8040536
Ammann C, Spampinato D, Marquez-Ruiz J (2016) Modulating motor learning through transcranial direct-current stimulation: an integrative view. Front Psychol 7:1981. https://doi.org/10.3389/fpsyg.2016.01981
Ammann C, Lindquist MA, Celnik PA (2017) Response variability of different anodal transcranial direct current stimulation intensities across multiple sessions. Brain Stimulat 10:757–763. https://doi.org/10.1016/j.brs.2017.04.003
Andrade C (2013) Once- to twice-daily, 3-year domiciliary maintenance transcranial direct current stimulation for severe, disabling, clozapine-refractory continuous auditory hallucinations in schizophrenia. J ECT 29:239–242. https://doi.org/10.1097/YCT.0b013e3182843866
Angius L, Pageaux B, Hopker J, Marcora SM, Mauger AR (2016) Transcranial direct current stimulation improves isometric time to exhaustion of the knee extensors. Neuroscience 339:363–375. https://doi.org/10.1016/j.neuroscience.2016.10.028
Angius L, Hopker J, Mauger AR (2017) The ergogenic effects of transcranial direct current stimulation on exercise performance. Front Physiol 8:90. https://doi.org/10.3389/fphys.2017.00090
Angius L, Mauger AR, Hopker J, Pascual-Leone A, Santarnecchi E, Marcora SM (2018a) Bilateral extracephalic transcranial direct current stimulation improves endurance performance in healthy individuals. Brain Stimulat 11:108–117. https://doi.org/10.1016/j.brs.2017.09.017
Angius L, Pascual-Leone A, Santarnecchi E (2018b) Brain stimulation and physical performance. Prog Brain Res 240:317–339. https://doi.org/10.1016/bs.pbr.2018.07.010
Aparicio LVM, Guarienti F, Razza LB, Carvalho AF, Fregni F, Brunoni AR (2016) A systematic review on the acceptability and tolerability of transcranial direct current stimulation treatment in neuropsychiatry trials. Brain Stimulat 9:671–681. https://doi.org/10.1016/j.brs.2016.05.004
Bastani A, Jaberzadeh S (2013a) Differential modulation of corticospinal excitability by different current densities of anodal transcranial direct current stimulation. PLoS ONE 8:e72254. https://doi.org/10.1371/journal.pone.0072254
Bastani A, Jaberzadeh S (2013b) The a-tDCS differential modulation of corticospinal excitability: the effects of electrode size Brain stimulation 6:932–937. https://doi.org/10.1016/j.brs.2013.04.005
Batsikadze G, Moliadze V, Paulus W, Kuo MF, Nitsche MA (2013) Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J Physiol 591:1987–2000. https://doi.org/10.1113/jphysiol.2012.249730
Berryhill ME, Martin D (2018) Cognitive effects of transcranial direct current stimulation in healthy and clinical populations: an overview. J ECT 34:e25–e35. https://doi.org/10.1097/YCT.0000000000000534
Bikson M et al (2016) Safety of transcranial direct current stimulation: evidence based update. Brain Stimulat 9:641–661. https://doi.org/10.1016/j.brs.2016.06.004
Bindman LJ, Lippold OC, Redfearn JW (1964) The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. J Physiol 172:369–382. https://doi.org/10.1113/jphysiol.1964.sp007425
Cancelli A et al (2018) Personalized, bilateral whole-body somatosensory cortex stimulation to relieve fatigue in multiple sclerosis. Multiple Sclerosis (Houndmills, Basingstoke, England) 24:1366–1374. https://doi.org/10.1177/1352458517720528
Chai Z, Ma C, Jin X (2019) Cortical stimulation for treatment of neurological disorders of hyperexcitability: a role of homeostatic plasticity. Neural Regen Res 14:34–38. https://doi.org/10.4103/1673-5374.243696
Chew T, Ho KA, Loo CK (2015) Inter- and intra-individual variability in response to transcranial direct current stimulation (tDCS) at varying current intensities. Brain Stimulat 8:1130–1137. https://doi.org/10.1016/j.brs.2015.07.031
Chhatbar PY, Chen R, Deardorff R, Dellenbach B, Kautz SA, George MS, Feng W (2017) Safety and tolerability of transcranial direct current stimulation to stroke patients—a phase I current escalation study. Brain Stimulat 10:553–559. https://doi.org/10.1016/j.brs.2017.02.007
Chhatbar PY et al (2018) Evidence of transcranial direct current stimulation-generated electric fields at subthalamic level in human brain in vivo. Brain Stimulat 11:727–733. https://doi.org/10.1016/j.brs.2018.03.006
Ciccone AB, Deckert JA, Schlabs CR, Tilden MJ, Herda TJ, Gallagher PM, Weir JP (2019) Transcranial direct current stimulation of the temporal lobe does not affect high-intensity work capacity. J Strength Cond Res 33:2074–2086. https://doi.org/10.1519/jsc.0000000000002561
Cogiamanian F, Marceglia S, Ardolino G, Barbieri S, Priori A (2007) Improved isometric force endurance after transcranial direct current stimulation over the human motor cortical areas. Eur J Neurosci 26:242–249. https://doi.org/10.1111/j.1460-9568.2007.05633.x
Dissanayaka T, Zoghi M, Farrell M, Egan GF, Jaberzadeh S (2017) Does transcranial electrical stimulation enhance corticospinal excitability of the motor cortex in healthy individuals? A systematic review and meta-analysis. Eur J Neurosci 46:1968–1990. https://doi.org/10.1111/ejn.13640
Esmaeilpour Z, Marangolo P, Hampstead BM, Bestmann S, Galletta E, Knotkova H, Bikson M (2018) Incomplete evidence that increasing current intensity of tDCS boosts outcomes. Brain Stimulat 11:310–321. https://doi.org/10.1016/j.brs.2017.12.002
Ferrucci R et al (2014) Transcranial direct current stimulation (tDCS) for fatigue in multiple sclerosis. NeuroRehabilitation 34:121–127. https://doi.org/10.3233/NRE-131019
Fertonani A, Ferrari C, Miniussi C (2015) What do you feel if I apply transcranial electric stimulation? Safety, sensations and secondary induced effects. Clin Neurophysiol 126:2181–2188. https://doi.org/10.1016/j.clinph.2015.03.015
Finsterer J, Mahjoub SZ (2014) Fatigue in healthy and diseased individuals. Am J Hosp Palliat Care 31:562–575. https://doi.org/10.1177/1049909113494748
Flood A, Waddington G, Keegan RJ, Thompson KG, Cathcart S (2017) The effects of elevated pain inhibition on endurance exercise performance. PeerJ 5:e3028. https://doi.org/10.7717/peerj.3028
Foerster ÁS, Rezaee Z, Paulus W, Nitsche MA, Dutta A (2018) Effects of cathode location and the size of anode on anodal transcranial direct current stimulation over the leg motor area in healthy humans. Front Neurosci 12:443–443. https://doi.org/10.3389/fnins.2018.00443
Fujiyama H et al (2017) Preconditioning tDCS facilitates subsequent tDCS effect on skill acquisition in older adults. Neurobiol Aging 51:31–42. https://doi.org/10.1016/j.neurobiolaging.2016.11.012
Gleeson NP, Mercer TH (1992) Reproducibility of isokinetic leg strength and endurance characteristics of adult men and women. Eur J Appl Physiol Occup Physiol 65:221–228. https://doi.org/10.1007/bf00705085
Gur H, Akova B, Punduk Z, Kucukoglu S (1999) Effects of age on the reciprocal peak torque ratios during knee muscle contractions in elite soccer players. Scand J Med Sci Sports 9:81–87
Hameau S, Bensmail D, Roche N, Zory R (2018) Adaptations of fatigue and fatigability after a short intensive, combined rehabilitation program in patients with multiple sclerosis. J Rehabilit Med 50:59–66. https://doi.org/10.2340/16501977-2277
Henneman E (1957) Relation between size of neurons and their susceptibility to discharge. Science 126:1345–1347. https://doi.org/10.1126/science.126.3287.1345
Ho KA et al (2016) The effect of transcranial direct current stimulation (tDCS) electrode size and current intensity on motor cortical excitability: evidence from single and repeated sessions. Brain Stimulat 9:1–7. https://doi.org/10.1016/j.brs.2015.08.003
Hoy KE, Arnold SL, Emonson MR, Daskalakis ZJ, Fitzgerald PB (2014) An investigation into the effects of tDCS dose on cognitive performance over time in patients with schizophrenia. Schizophr Res 155:96–100. https://doi.org/10.1016/j.schres.2014.03.006
Jang H, Lee JY, Lee KI, Park KM (2017) Are there differences in brain morphology according to handedness? Brain Behav 7:e00730. https://doi.org/10.1002/brb3.730
Jayaram G, Stinear JW (2009) The effects of transcranial stimulation on paretic lower limb motor excitability during walking. J Clin Neurophysiol 26:272–279. https://doi.org/10.1097/WNP.0b013e3181af1d41
Kan B, Dundas JE, Nosaka K (2013) Effect of transcranial direct current stimulation on elbow flexor maximal voluntary isometric strength and endurance. Appl Physiol Nutr Metab 38:734–739. https://doi.org/10.1139/apnm-2012-0412
Kessler SK, Turkeltaub PE, Benson JG, Hamilton RH (2012) Differences in the experience of active and sham transcranial direct current stimulation. Brain Stimulat 5:155–162. https://doi.org/10.1016/j.brs.2011.02.007
Khadka N et al (2019) Adaptive current tDCS up to 4 mA. Brain Stimul. https://doi.org/10.1016/j.brs.2019.07.027
Kidgell DJ et al (2013) Different current intensities of anodal transcranial direct current stimulation do not differentially modulate motor cortex plasticity. Neural Plast 2013:603502. https://doi.org/10.1155/2013/603502
Klem GH, Luders HO, Jasper HH, Elger C (1999) The 10–20 electrode system of the International Federation. Int Feder Clin Neurophysiol Electroencephalogr Clin Neurophysiol Suppl 52:3–6
Kluger BM, Krupp LB, Enoka RM (2013) Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology 80:409–416. https://doi.org/10.1212/WNL.0b013e31827f07be
Kollock R, Van Lunen BL, Ringleb SI, Onate JA (2015) Measures of functional performance and their association with hip and thigh strength. J Athl Train 50:14–22. https://doi.org/10.4085/1062-6050-49.3.49
Krishnan C, Ranganathan R, Kantak SS, Dhaher YY, Rymer WZ (2014) Anodal transcranial direct current stimulation alters elbow flexor muscle recruitment strategies. Brain Stimulat 7:443–450. https://doi.org/10.1016/j.brs.2014.01.057
Kuo MF, Paulus W, Nitsche MA (2014) Therapeutic effects of non-invasive brain stimulation with direct currents (tDCS) in neuropsychiatric diseases. Neuroimage 85(Pt 3):948–960. https://doi.org/10.1016/j.neuroimage.2013.05.117
Lambert CP, Archer RL, Evans WJ (2001) Muscle strength and fatigue during isokinetic exercise in individuals with multiple sclerosis. Med Sci Sports Exerc 33:1613–1619
Lattari E, de Oliveira BS, Oliveira BRR, de Mello Pedreiro RC, Machado S, Neto GAM (2018) Effects of transcranial direct current stimulation on time limit and ratings of perceived exertion in physically active women. Neurosci Lett 662:12–16. https://doi.org/10.1016/j.neulet.2017.10.007
Lee J et al (2019) Different brain connectivity between responders and nonresponders to dual-mode noninvasive brain stimulation over bilateral primary motor cortices in stroke patients. Neural Plast 2019:3826495. https://doi.org/10.1155/2019/3826495
Lefaucheur JP, Chalah MA, Mhalla A, Palm U, Ayache SS, Mylius V (2017) The treatment of fatigue by non-invasive brain stimulation. Neurophysiol Clin Clin Neurophysiol 47:173–184. https://doi.org/10.1016/j.neucli.2017.03.003
Liew SL, Santarnecchi E, Buch ER, Cohen LG (2014) Non-invasive brain stimulation in neurorehabilitation: local and distant effects for motor recovery. Front Hum Neurosci 8:378. https://doi.org/10.3389/fnhum.2014.00378
Liu X et al (2018) Increased interhemispheric synchrony underlying the improved athletic performance of rowing athletes by transcranial direct current stimulation. Brain Imaging Behav. https://doi.org/10.1007/s11682-018-9948-3
Mackey CS, Thiele RM, Conchola EC, DeFreitas JM (2018) Comparison of fatigue responses and rapid force characteristics between explosive- and traditional-resistance-trained males. Eur J Appl Physiol 118:1539–1546. https://doi.org/10.1007/s00421-018-3883-2
Martin DM, Liu R, Alonzo A, Green M, Loo CK (2014) Use of transcranial direct current stimulation (tDCS) to enhance cognitive training: effect of timing of stimulation. Exp Brain Res 232:3345–3351. https://doi.org/10.1007/s00221-014-4022-x
Mellor JR, Randall AD (1998) Voltage-dependent deactivation and desensitization of GABA responses in cultured murine cerebellar granule cells. J Physiol 506(Pt 2):377–390. https://doi.org/10.1111/j.1469-7793.1998.377bw.x
Montenegro R, Okano A, Gurgel J, Porto F, Cunha F, Massaferri R, Farinatti P (2015) Motor cortex tDCS does not improve strength performance in healthy subjects. Motriz Revista de Educação Física 21:185–193
Monte-Silva K, Kuo MF, Hessenthaler S, Fresnoza S, Liebetanz D, Paulus W, Nitsche MA (2013) Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimulat 6:424–432. https://doi.org/10.1016/j.brs.2012.04.011
Murray LM, Edwards DJ, Ruffini G, Labar D, Stampas A, Pascual-Leone A, Cortes M (2015) Intensity dependent effects of transcranial direct current stimulation on corticospinal excitability in chronic spinal cord injury. Arch Phys Med Rehabil 96:S114–121. https://doi.org/10.1016/j.apmr.2014.11.004
Muthalib M, Kan B, Nosaka K, Perrey S (2013) Effects of transcranial direct current stimulation of the motor cortex on prefrontal cortex activation during a neuromuscular fatigue task: an fNIRS study. Adv Exp Med Biol 789:73–79. https://doi.org/10.1007/978-1-4614-7411-1_11
Nitsche MA, Paulus W (2000) Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 527(Pt 3):633–639. https://doi.org/10.1111/j.1469-7793.2000.t01-1-00633.x
Nitsche MA, Paulus W (2001) Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 57:1899–1901. https://doi.org/10.1212/wnl.57.10.1899
Nitsche MA, Paulus W (2011) Transcranial direct current stimulation—update 2011. Restor Neurol Neurosci 29:463–492. https://doi.org/10.3233/rnn-2011-0618
Nitsche MA et al (2008) Transcranial direct current stimulation: state of the art. Brain Stimulat 1:206–223. https://doi.org/10.1016/j.brs.2008.06.004
Oconnell NE, Cossar J, Marston L, Wand BM, Bunce D, Moseley GL, De Souza LH (2012) Rethinking clinical trials of transcranial direct current stimulation: participant and assessor blinding is inadequate at intensities of 2mA. PLoS ONE 7:e47514–e47514. https://doi.org/10.1371/journal.pone.0047514
Okano AH et al (2015) Brain stimulation modulates the autonomic nervous system, rating of perceived exertion and performance during maximal exercise. Br J Sports Med 49:1213–1218. https://doi.org/10.1136/bjsports-2012-091658
Oki K, Mahato NK, Nakazawa M, Amano S, France CR, Russ DW, Clark BC (2016) Preliminary evidence that excitatory transcranial direct current stimulation extends time to task failure of a sustained submaximal muscular contraction in older adults. J Gerontol A Biol Sci Med Sci 71:1109–1112. https://doi.org/10.1093/gerona/glw011
Papazova I et al (2018) Improving working memory in schizophrenia: effects of 1mA and 2mA transcranial direct current stimulation to the left DLPFC. Schizophr Res 202:203–209. https://doi.org/10.1016/j.schres.2018.06.032
Player MJ et al (2014) Increase in PAS-induced neuroplasticity after a treatment course of transcranial direct current stimulation for depression. J Affect Disord 167:140–147. https://doi.org/10.1016/j.jad.2014.05.063
Purpura DP, McMurtry JG (1965) Intracellular activities and evoked potential changes during polarization of motor cortex. J Neurophysiol 28:166–185. https://doi.org/10.1152/jn.1965.28.1.166
Radel R, Tempest G, Denis G, Besson P, Zory R (2017) Extending the limits of force endurance: stimulation of the motor or the frontal cortex? Cortex J Devot Study Nerv Syst Behav 97:96–108. https://doi.org/10.1016/j.cortex.2017.09.026
Rawji V et al (2018) tDCS changes in motor excitability are specific to orientation of current flow. Brain Stimul 11:289–298. https://doi.org/10.1016/j.brs.2017.11.001
Rothwell JC (2012) Clinical applications of noninvasive electrical stimulation: problems and potential. Clin EEG Neurosci 43:209–214. https://doi.org/10.1177/1550059412444973
Rudroff T, Kindred JH, Ketelhut NB (2016) Fatigue in multiple sclerosis: misconceptions and future research directions. Front Neurol 7:122. https://doi.org/10.3389/fneur.2016.00122
Saenz A et al (2010) Knee isokinetic test-retest: a multicentre knee isokinetic test-retest study of a fatigue protocol. Eur J Phys Rehabilit Med 46:81–88
Sales MM, De Sousa CV, Browne RAV, Fontes EB, Olher RDRV, Ernesto C, Simões HG (2016) Transcranial direct current stimulation improves muscle isokinetic performance of young trained individuals. Med Sport 69:163–172
Sanchez-Kuhn A, Perez-Fernandez C, Canovas R, Flores P, Sanchez-Santed F (2017) Transcranial direct current stimulation as a motor neurorehabilitation tool: an empirical review. Biomed Eng Online 16:76. https://doi.org/10.1186/s12938-017-0361-8
Schambra HM, Abe M, Luckenbaugh DA, Reis J, Krakauer JW, Cohen LG (2011) Probing for hemispheric specialization for motor skill learning: a transcranial direct current stimulation study. J Neurophysiol 106:652–661. https://doi.org/10.1152/jn.00210.2011
Sliwowski R, Grygorowicz M, Wieczorek A, Jadczak L (2018) The relationship between jumping performance, isokinetic strength and dynamic postural control in elite youth soccer players. J Sports Med Phys Fit 58:1226–1233. https://doi.org/10.23736/s0022-4707.17.07289-9
Stagg CJ, Jayaram G, Pastor D, Kincses ZT, Matthews PM, Johansen-Berg H (2011) Polarity and timing-dependent effects of transcranial direct current stimulation in explicit motor learning. Neuropsychologia 49:800–804. https://doi.org/10.1016/j.neuropsychologia.2011.02.009
Stagg CJ, Lin RL, Mezue M, Segerdahl A, Kong Y, Xie J, Tracey I (2013) Widespread modulation of cerebral perfusion induced during and after transcranial direct current stimulation applied to the left dorsolateral prefrontal cortex. J Neurosci 33:11425–11431. https://doi.org/10.1523/jneurosci.3887-12.2013
Stephens JA, Jones KT, Berryhill ME (2017) Task demands, tDCS intensity, and the COMT val(158)met polymorphism impact tDCS-linked working memory training gains. Sci Rep 7:13463. https://doi.org/10.1038/s41598-017-14030-7
Tecchio F et al (2014) Multiple sclerosis fatigue relief by bilateral somatosensory cortex neuromodulation. J Neurol 261:1552–1558. https://doi.org/10.1007/s00415-014-7377-9
Thorstensson A, Karlsson J (1976) Fatiguability and fibre composition of human skeletal muscle. Acta Physiol Scand 98:318–322. https://doi.org/10.1111/j.1748-1716.1976.tb10316.x
Trapp NT, Xiong W, Gott BM, Espejo GD, Bikson M, Conway CR (2019) Proceedings# 51: 4 mA adaptive transcranial direct current stimulation for treatment-resistant depression: early demonstration of feasibility with a 20-session course. Brain Stimulat Basic Transl Clin Res Neuromodul 12:e124–e125
Vitor-Costa M, Okuno NM, Bortolotti H, Bertollo M, Boggio PS, Fregni F, Altimari LR (2015) Improving cycling performance: transcranial direct current stimulation increases time to exhaustion in cycling. PLoS ONE 10:e0144916. https://doi.org/10.1371/journal.pone.0144916
Williams PS, Hoffman RL, Clark BC (2013) Preliminary evidence that anodal transcranial direct current stimulation enhances time to task failure of a sustained submaximal contraction. PLoS ONE 8:e81418. https://doi.org/10.1371/journal.pone.0081418
Ziemann U, Siebner HR (2008) Modifying motor learning through gating and homeostatic metaplasticity. Brain Stimulat 1:60–66. https://doi.org/10.1016/j.brs.2007.08.003
Acknowledgements
We thank the study participants for their effort and time. In addition, we thank Emily Jester, Veronica Smith, and Delaney McDowell for their assistance in data collection. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Communicated by Winston D Byblow.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Workman, C.D., Kamholz, J. & Rudroff, T. Increased leg muscle fatigability during 2 mA and 4 mA transcranial direct current stimulation over the left motor cortex. Exp Brain Res 238, 333–343 (2020). https://doi.org/10.1007/s00221-019-05721-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-019-05721-w