Abstract
The main aim was to analyse the impact of an official match on hormonal and redox status, muscle damage and inflammation and neuromuscular function. Seven high-level male soccer players from the same team performed an official match and data were collected 72 h before, 24, 48 and 72 h post-match. Plasma testosterone/cortisol ratio (T/C), creatine kinase (CK), superoxide dismutase (SOD), glutathione peroxidase (GPX) and reductase (GR) activities, myoglobin (Mb), C-reactive protein (CRP), uric acid (UA), protein sulfhydryls (–SH), malondialdehyde (MDA) concentrations and total antioxidant status (TAS) were measured. Sprint, jump and change of direction performance, and maximal isokinetic knee extension and flexion were obtained as neuromuscular functional parameters. Cortisol increased and T/C decreased until 48 h recovery (P < 0.05). Mb, CRP and –SH (P < 0.05) increased at 24 h and CK, TAS, SOD and MDA (P < 0.05) increased up to 48 h recovery. GR increased and GPX decreased at 24 h recovery (P < 0.05). Jump performance decreased 24 h post-match (P < 0.05), but no significant alterations in sprint, change of direction and muscle strength were observed. In conclusion, an official match resulted in changes in plasma biomarkers until 48 h of recovery period, without major impact on performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soccer has been the topic of intense research over the last decade. The competitive demands of soccer may inflict strains on various physiological systems, including the musculoskeletal, nervous, immune, and metabolic systems, to the point where post-exercise recovery strategies become influential in the player’s preparation for the next match (Reilly and Ekblom 2005). During the soccer season, players’ performance is not only determined by appropriate conditioning, but also by the ability to recover and regenerate after exposure to multiple stress stimuli (e.g., training and competition) (Kraemer et al. 2004). Decline in physical performance (Ascensao et al. 2008; Ispirlidis et al. 2008; Magalhaes et al. 2010; Rampinini et al. 2011), increase in muscle damage markers (Andersson et al. 2008; Ascensao et al. 2008; Ispirlidis et al. 2008; Magalhaes et al. 2010), a robust pro- and anti-inflammatory cytokine response (Andersson et al. 2010a; Ispirlidis et al. 2008), and increased catabolic (Ispirlidis et al. 2008; Kraemer et al. 2004) and pro-oxidant states (Ascensao et al. 2008; Magalhaes et al. 2010) have been described immediately after “friendly” soccer matches and throughout the post-match recovery.
A considerable amount of studies have been using friendly match and soccer-specific protocols performed in laboratory as a model to reproduce the overall game physical demands; however, the data emerging from high-level competitive games are scarce. The analysis of high-level official soccer matches is interesting for both coaches and researchers as these truly represent the real context of a high-level competition. In fact, it is reasonable to assume that the physiological and psychological stress imposed by a friendly match does not mimic the real stress of an official soccer competition. Despite the scarcity of studies, there are evidences reporting that exercise intensity during soccer friendly matches (absolute values and percentage of maximum heart rate) may be lower than during official matches (Rodrigues et al. 2007). In addition, due to the increase in the frequency of match commitments in professional soccer, a clear knowledge of the impact and time course of the physiological changes induced by an official soccer match will help the designing and development of more effective strategies to accelerate recovery (Andersson et al. 2008).
When research protocols aim to study fatigue and recovery in sport, specific performance variables and biochemical and muscle-status markers should be included in the analysis to achieve a higher efficiency of the monitorization process (Bishop et al. 2008). In fact, performance measurements, hormonal and oxidative and muscle damage markers have been suggested as important stress biomarkers in the monitorization of fatigue and recovery (Bishop et al. 2008). Although data regarding associations of pro-oxidant redox status, inflammation, muscle damage and physical performance have been reported in high-level male and female and low-level male soccer players after friendly matches (Andersson et al. 2008; Ispirlidis et al. 2008; Rampinini et al. 2011), no studies have yet examined the same cohort of male professional players during the post-match recovery period that follows a high-level competitive official match. Given the higher training background of professional players and evidences from previous reports from elite female players (Andersson et al. 2008), high-level junior players (Rampinini et al. 2011) and adult elite players (Lago-Penas et al. 2011; Meister et al. 2011; Odetoyinbo et al. 2009) it is possible that performance impairments and biochemical responses may be blunted. Therefore, the purpose of the present study was to examine, for the first time, whether and to what extent, muscle damage, plasma oxidative stress and damage, hormonal changes, plasma inflammatory markers, as well as lower limb neuromuscular variables such as jump, 5 and 30 m sprint, change in direction performance and strength parameters, were altered in response to an official soccer match.
Methods
Subjects
A group of seven male professional outfield soccer players (age 22–31 years; height 172–191 cm; weight 71–95 kg; % body fat 7–10.7 %) from a team competing in the Portuguese Professional Soccer League participated in this study. The total match duration was 94 min and finished with a draw (2–2). From the ten outfield players that started the match, three players were excluded from the game (one was injured and two were substituted in the beginning of the second quarter of the second half) and therefore, just the seven players that played the full time were involved in the present study. The goalkeeper was also excluded due to the obvious physical loading differences when compared with the other players of distinct position roles. In accordance with the club policy, all soccer players underwent usual physical examination in the beginning and throughout the season (e.g., blood sample analyzes, rest ECG, lung X-ray). The experimental protocol was approved by the local board, and followed the Declaration of Helsinki of the World Medical Associations for research with humans. All participants were fully informed about the aims, experimental protocol and procedures, and provided written informed consent.
Experimental design
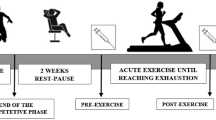
The studied official match was the last of the championship, to allow repetitive evaluations throughout the post-match recovery period. However, no performance or biochemical variables were obtained at the end of the match due to logistical problems. Blood samples and functional data, namely countermovement jump (CMJ), 5 (T5) and 30 (T30) meters sprint times, change of direction ability (COD t test) and leg extensor and flexor isokinetic concentric maximal strength were assessed at different time points: pre-match (96 h post the previous official match and 72 h before the studied match), and at 24, 48 and 72 h of the recovery period.
Being the last official match, the players were not involved in any programmed training activity and were asked to refrain from any form of physical exercise during the experimental period. Subjects were engaged in normal training routines during the 72 h before match, and abstained from exercise during the 72 h post-match, with exception of the functional evaluation tests.
Procedures
The game started at 4 pm and ended at 6 pm with temperature and relative humidity around 20 °C and 45–50 %, respectively. To match the circadian rhythms of the different variables, data collection (blood sample and functional data measurements) in all time points began at 6 pm. Procedures sequence was as follows: 1st: blood samples collection; 2nd: CMJ, 3rd: T5 and T30, 4th: COD and 5th: isokinetic strength. In the laboratory (6 pm), a resting blood sample was taken after subjects have been standing for at least 15 min, after which subjects consumed a light standardized meal and drink and rested for approximately 2 h (6–8 pm). The meal consisted of 1.7 g white bread and 0.3 g of low-fat spread; both values are per kilogram of body mass (Thompson et al. 2003). At 8 pm, CMJ, sprint ability and COD tests were performed in indoor facilities. Before the tests, all the players performed a 10–15 min warm-up consisting of light jogging, specific mobility exercises and stretching routine, and 10 m sprints. Players completed two trials of each test interspersed by 1 min recovery period and the best result was considered for analysis. Each subject was allowed to a minimum of 5 min rest between different tests to ensure adequate recovery. Subjects were already familiarized with the different physical tests because they performed this test battery during several pre-season and in-season visits to our laboratory for physical fitness monitoring. Players were instructed to maintain normal routines for daily food and water intake, and followed the same dietary recommendations defined by the medical staff. In addition, during the days of physical tests, players were instructed to refrain from drinking beverages containing caffeine and/or alcohol and from consuming food during the 2 h before testing (4–6 pm).
Physical performance tests
Counter-movement jump (CMJ)
The CMJ was performed using a platform, Ergojump (Digitime 1000, Digest Finland) according to Bosco et al. (1983), whereby the highest vertical jump (cm) and the longest flying time (s) were registered. The best of the two trials was used for data analyses.
Sprint ability
Sprint measurements were carried out using telemetric photoelectric cells (Brower Timing System, IRD-T175, Utah, USA) mounted on tripods positioned approximately 0.75 m above the floor and situated 3 m apart facing each other on either side of the starting line (0 m), at 5 and at 30 m. The players stood 0.3 m behind the starting line, started at their own discretion, being time activated when players cross the first pair of photocells, and they ran as fast as they could to complete 30 m distance. The best of two trials was used for data analyses.
Change of direction (COD)
Change of direction (COD) ability was evaluated using an adapted version of the agility test (t test) (Semenick 1990). T test does not include a perceptual or decision-making component and can be used to measure change in direction ability. Subjects began with both feet 0.3 m behind the starting point A. At their own discretion, each subject sprinted forward 9.14 m (10 yd) to point B and touched the base of the cone with the right hand. Thereafter, sprinted to the left 4.57 m (5 yd) and touched the base of a cone (C) with the left hand. Subjects then sprinted to the right 9.14 m (10 yd) and touched the base of a cone (D) with the right hand. At that moment sprinted to the left 4.57 m back to point B and touched the base of a cone with the left hand. They turn 270° and then ran to point A, passing the finishing line. Two test trials were performed, and times were recorded to the nearest 100 of a second and the best trial was used for data analysis. As described for sprint ability, measurements were carried out using telemetric photoelectric cells (Brower Timing System, IRD-T175, UT, USA). The players stood 0.3 m behind the starting line, being time activated when they passed the electronic sensors, and the clock stopped the instant players crossed again the Point A.
Strength
Maximal gravity corrected concentric peak torque of quadriceps (Q) and hamstring (H) and the ratio between concentric H and Q peak torque values (H/Q) expressed in percentage were measured during isokinetic knee joint movement (Biodex System 2, NY, USA) of the dominant and non-dominant leg at the angular velocity of 90° s−1 (1.57 rad s−1). After individual self-report, the dominant leg was determined by a routine visual inspection in a simple target-kicking test requiring accuracy. Before muscle function measurements, subjects perform a standardized warm-up consisting of 5 min period on a cycle ergometer (Monark E-824) with a fixed load corresponding to 2 % of body weight. Players were then seated on the dynamometer chair at 85° inclination (external angle from the horizontal) with stabilization straps and the knee to be tested was positioned at 90° of flexion (0° = fully extended knee). The subjects were instructed to kick and also to bend the tested leg as hard and as fast as they could through a complete range of motion (from 90° to 0°). All subjects also performed a specific sub-maximal warm-up protocol on the Biodex device to familiarize with the isokinetic device and test procedure. Three maximal repetitions at angular velocity 90° s−1 (1.57 rad s−1) were therefore carried out. The coefficients of variation (CV) were 5.1, 3.5, 4.8, 4.1 and 4.3 %, for CMJ, sprint, t test, knee extensors and knee flexors, respectively.
Blood sampling and preparations
All the venous blood samples were taken by conventional clinical procedures using EDTA as anticoagulant. Nevertheless, no tourniquet was used to minimize potentially oxidative stress induced by an ischemia–reperfusion maneuver.
The freshly withdraw blood (~10 ml) was immediately centrifuged at 3,000 rpm during 10 min for careful removal of the plasma. Plasma was separated into several aliquots and rapidly frozen at −80 °C for later biochemical analysis.
Biochemical assays
Plasma testosterone (T) and cortisol (C) were measured immune-enzymatically using commercial test kits VIDAS® testosterone (REF. 30418) and VIDAS® cortisol S (REF. 30451). The CV were 6.4 and 3.8 %, respectively.
Plasma creatine kinase (CK) activity was determined spectrophotometrically using a commercial test kit (ABX A11A01632, Montpellier, FR). Plasma myoglobin concentration was assessed using a commercial test kit (myoglobin bioMerieux 30446, Carnaxide, PT). C-reactive protein (CRP) was measured using an enzyme-linked immune sorbent assay system (ELISA-PENTRA 400, Horiba ABX, Montpellier, FR). The CV were 3.8, 4.5 and 5 %, respectively.
Total antioxidant status (TAS) was measured spectrophotometrically using a commercial kit (Randox NX2332 Crumlin, UK). Uric acid (UA) was determined by an enzymatic method using a commercial kit (Horiba ABX A11A01670, Montpellier, France). The CV were 3.6 and 2.9 %, respectively.
Regarding enzyme activities in plasma, superoxide dismutase (SOD) activity was measured spectrophotometrically at 550 nm using a commercial Ransod kit from Randox (catalogue no. SD 125, Crumlin, UK). The activity of glutathione peroxidase (GPX) was assayed by a spectrophotometric technique at 340 nm using a commercial Ransel kit from Randox (catalogue no. RS 505). The activity of glutathione reductase (GR) was measured with a spectrophotometric procedure at 340 nm using a commercial GR kit from Randox (catalogue no. GR 2368). The CV was 4.4, 5.7, and 4.7%, respectively.
Plasma MDA was assayed according to Rohn et al. (1993) with some modifications and measured by the formation of thiobarbituric acid reactive substances at 535 nm. Plasma SH was spectrophotometrically evaluated at 414 nm according to Hu (1990). Protein content was spectrophotometrically assayed using bovine serum albumin as standard according to Lowry et al. (1951). Samples were analyzed in duplicate and the mean of the two values was used for statistical analysis. The CV was 6.1 and 6.8 %, respectively.
Statistical analysis
Mean and standard error mean (SEM) were calculated. The normality was tested with the Shapiro–Wilks test. After this assumption, an one-way analysis of variance (ANOVA) with repeated measures was used to establish whether any of the subsequent test results were significantly different from pre-match results. The coefficient of variation of the physical tests was estimated using a test–retest procedure with a random subsample of five players. To achieve this purpose, players returned to the laboratory 8 days after the match (i.e., 96 h after the last evaluation) and performed the physical tests in the same order. All data analysis was performed using SPSS 18.0 package. A significance level of 0.05 was chosen.
Results
The results of performance in the different muscle power-related tests are present in Table 1. CMJ performance was significantly reduced from pre-match to 24 h (36–45 cm; P < 0.05) of the post-match recovery period; however, sprint and COD ability did not significantly change during the post-match recovery period.
The changes in isokinetic strength parameters before (pre), 24, 48 and 72 h post-match are present in Table 2. The H/Q ND was significantly higher at 24 h post-match (54–61 %; P < 0.05) compare to pre-match. No changes were observed in the other evaluated parameters.
No significant alterations were observed in plasma T (Table 3) concentration during the recovery period; however, plasma C concentration significantly increased at 24 h (51–73 ng ml−1; P < 0.05) and 48 h (54–108 ng ml−1; P < 0.05) as compared to baseline. The T/C ratio decreased significantly at 24 h (6.5–12.4 %; P < 0.05; Table 3) and 48 h (4.2–14 %; P < 0.05) as compared to pre-match values.
As can be observed in Table 3, plasma Mb content was significantly higher at 24 h (28–52 μg L−1; P < 0.05) than in pre-match. Plasma CK activity increased at 24 h (844–1277 U L−1; P < 0.05) and 48 h (330–795 U L−1; P < 0.05) as compared to pre-match.
A significant increase in plasma CRP content was observed at 24 h post-match (1.1–4 mg L−1; P < 0.05; Table 3) as compared to baseline.
Data concerning the variation of the different redox state parameters throughout the post-match recovery period are described in Table 4. Plasma TAS significantly increased at 24 h (1.06–1.39 nmol L−1; P < 0.05) and 48 h (1.06–1.27 nmol L−1; P < 0.05) as compared to pre-match. No significant alterations were observed in plasma UA content during the recovery period. Plasma SOD activity increased at 24 h (754–815 U L−1; P < 0.05) and 48 h after the match (727–760 U L−1; P < 0.05). Plasma GPX activity decreased at 24 h post-match (839–1048 U L−1; P < 0.05). The activity of GR (P < 0.05) and –SH content (P < 0.05) in plasma was significantly higher at 24 h (66.5–78.6 U L−1 and from 1.6 to 2.5 μmol g−1, respectively) of the recovery period than in pre-match. A significantly higher level of plasma MDA was analysed at 24 h (13.5–29 μmol g−1; P < 0.05) and 48 h of the recovery period (16.9–18.6 μmol g−1; P < 0.05) as compared to baseline.
Discussion
Data demonstrate, for the first time, that an official soccer match of male elite players induces a hormonal catabolic environment during the recovery period. In addition, increased levels of oxidative stress and muscle damage throughout 48 h of the recovery period were observed. Although match induced certain biochemical alterations, the performance of these high-level adult soccer players was affected only to a smaller extent and until 24 h of recovery period, at least in the examined neuromuscular parameters, i.e., jump performance significantly decreased 24 h post-match, but no significant alterations in sprint, change in direction and muscle strength measures were observed during the recovery period.
The different physical parameters assessed within studies investigating match fatigue and recovery (e.g., isokinetic vs. isometric strength and the different sprint distances), makes difficult to compare the baseline results between studies. Nevertheless, the professional players from our study have considerably higher isokinetic strength levels than those reported in other studies involving players of lower (Ascensao et al. 2008; Magalhaes et al. 2010) or similar level at the same (Magalhães et al. 2004) and even at lower angular velocities (60° s−1) (Malliou et al. 2003; Mercer et al. 1997). The higher levels of lower limb muscle strength observe at baseline in the players that participated in our study may have contributed, at least in part, to explain the observed differences with the previous studies examining match fatigue and recovery (Ascensao et al. 2008; Magalhaes et al. 2010). However, the difference was not evident regarding CMJ performance before the match when compared with players of similar (48 cm) (Ispirlidis et al. 2008) and lower level (ranging from 47 to 51 cm) (Fatouros et al. 2010; Magalhaes et al. 2010). Nevertheless, players' from the present study showed a higher CMJ performance than that reported from others high-level soccer players (Malliou et al. 2003; Meister et al. 2011; Ronnestad et al. 2011). Regarding 5 and 30 m sprint results, the observed baseline performances are comparable (Kollath and Quade 1993), better (Brocherie et al. 2005) and worse (Wisloff et al. 2004) than those reported in players of other professional leagues.
In the present study, CMJ was the only neuromuscular parameter impaired at 24 h recovery. Accordingly, Andersson et al. (2008) reported a longer impairment of CMJ, when compared with other neuromuscular parameters, after a friendly elite female soccer match. Data from the present study and others examining elite adult and junior players responses to match (Andersson et al. 2008; Krustrup et al. 2011; Odetoyinbo et al. 2009; Rampinini et al. 2011), and during periods of high match exposure (Lago-Penas et al. 2011; Meister et al. 2011) seem to suggest that professional players show small performance impairments and biochemical disturbances than those already described in players of lower level (Ascensao et al. 2008; Fatouros et al. 2010; Magalhaes et al. 2010). In accordance with our data, Rampinini et al. (2011) observed that high-level young players (U-20) partially recover performance at 24 h post-match and completely recovered at 48 h. In contrast, data with semi-professional players (Ascensao et al. 2008; Fatouros et al. 2010; Magalhaes et al. 2010), reported a larger range of performance decrements (e.g., strength, jump and sprint performance) and longer recovery periods (48–72 h). Furthermore, Odetoyinbo et al. (2009) observed that game-physical performance of professional players was not affected when two matches were played interspersed by 2 days of recovery. In addition, Meister et al. (2011) observed that a 3 week period of high match exposure in elite football players did not affect laboratory, psychometric and performance parameters. The higher training background of professional players may explain the faster recovery and lower performance impairments. In fact, muscle adapts to training regimens involving many SSC-related actions and is less impaired with repeated exposure (Reilly et al. 2008). In addition, the better physiological responses to match play (Edwards and Clark 2006) and to high-intensity intermittent exercise (HIT) of high standard players (Rampinini et al. 2010), may contribute, to explain at least in part, the lower performance impairments and faster recovery patterns that have been reported in players of higher standard (Andersson et al. 2008; Krustrup et al. 2011; Rampinini et al. 2011). However, it is important to note that although most of the functional measurements did not significantly decrease, some showed a trend to decrease (e.g., 5.7 % KED at 24 h and 5 % KEND at 48 h). These declines and cumulative deficits during periods of condensed competition may unable players to overcome the demands of training and competition.
Regarding biochemical markers, the official soccer match altered the catabolic/anabolic-related hormonal homeostasis towards a predominant catabolic response during the first 48 h of the recovery period. Ispirlidis et al. (2008) observed that a friendly soccer match induced significant increases in C concentration only immediately after. However, in line with our findings, the authors did not observe alterations in plasma T (free T) concentration throughout the 144 h of the recovery period (Ispirlidis et al. 2008).
The increase in plasma concentration/activity of certain intracellular proteins (e.g., Mb and CK) has been widely used as indirect markers of tissue damage (Brancaccio et al. 2007). Increased Mb contents immediately after (Ascensao et al. 2008; Magalhaes et al. 2010) and in CK activity throughout the 72–96 h of the recovery period of a friendly match (CK) with semi-professional (Ascensao et al. 2008; Fatouros et al. 2010; Magalhaes et al. 2010) and professional players (Ispirlidis et al. 2008), have been reported. These previous studies observed lower baselines values of CK (approximately ranging from 80 to 200 U L−1) than those observed in the present study (300.6 U L−1) and with other professional players (310 U L−1) (Rampinini et al. 2011). Considering that our baseline values were obtained 96 h after the previous official game of the regular championship, probably the higher pre-match CK values are associated with “residual” levels of muscle damage from previous regular training (McLellan et al. 2010). The longer responses (72–96 h post-match) and/or higher variations (from baseline to 24 and 48 h of recovery) that have been reported in semi-professional players (Ascensao et al. 2008; Fatouros et al. 2010; Magalhaes et al. 2010) (ranging from 400 to 800 %) compared with the present and other studies involving players of higher standard (85–201 %) (Andersson et al. 2008; Krustrup et al. 2011; Rampinini et al. 2011) can be related with the already described factors (e.g., training status in moment of the match). In fact, less trained subjects exhibited higher post-exercise CK increases [for refrs see (Brancaccio et al. 2007)]. These biochemical responses in addition to others (e.g., UA, lipid and protein oxidation markers) seem to corroborate the hypothesis that professional players show lower physiological disturbances than low-level players (Ascensao et al. 2008; Fatouros et al. 2010; Magalhaes et al. 2010). Nevertheless, given the high number of factors that are associated with plasma CK inter-individual variability (e.g., age, muscle mass, physical activity) both in rest and in response to exercise (low and high responders) (Brancaccio et al. 2007) some caution should be taken when comparing the magnitude of changes between studies.
Our data showed an increased in TAS at 24 and 48 h post-match. Together with other reports, data seem to support that a soccer match induces a pro-oxidant insult resulting in a compensatory response in plasma TAS immediately after and during the first 48 h of the recovery period (Andersson et al. 2010b; Fatouros et al. 2010; Magalhaes et al. 2010). Although we did not observe variations in UA, studies have shown that plasma UA levels increase immediately after a friendly match (Magalhaes et al. 2010) and during the recovery period (Fatouros et al. 2010; Ispirlidis et al. 2008).
Plasma SOD activity was significantly higher during the 48 h of the post-match, which is in line with studies involving untrained individuals and other athletic populations (Fisher-Wellman and Bloomer 2009). With respect to the antioxidant glutathione cycle-related enzymes, although we observed a decrease in GPX only at 24 h of recovery period, others (Fatouros et al. 2010), examining semi-professional players, observed an increased activity during the 24–72 h post-match recovery period. Regarding plasma GR activity we observed an up-regulation at 24 h post-match. Taking into account the critical physiological role of the balance between the first (SOD) and second step (GPX and/or catalase) antioxidant enzymes, the significant increase in plasma SOD/GPX ratio observed after the game, mainly due to a decreased in GPX activity as referred to previously, seems to favour H2O2 accumulation. This fact, could be indicative of a low plasma scavenging efficiency contributing to enhanced oxidative stress (Gaeta et al. 2002), which is consistent with the increased levels of MDA found in present study and others (Ascensao et al. 2008; Fatouros et al. 2010; Ispirlidis et al. 2008; Magalhaes et al. 2010).
Plasma-SH increased at 24 h of the recovery period suggesting decreased disulphide linkages (–S–S–) from both proteins and reduced glutathione. These findings contrast with the previous reports of semi-professional soccer players immediately after and through the first 48 h of the recovery period (Ascensao et al. 2008; Magalhaes et al. 2010). The moment of the soccer season might contribute to the different results between studies. In fact, some reports suggest that the antioxidant status (e.g., TAS) may change within the season (Teixeira et al. 2009) influencing the capacity of the antioxidant defence system and the ability to restore the redox balance. The influence of players training background (Martinovic et al. 2009) (e.g., years of practice) and standard (Nakagami et al. 2009) on results cannot be ruled out, since it has been associated with higher concentrations of –SH. Probably, the vigorous training and competition resulting in oxidative stress may lead to an upregulation in antioxidant defences and associated shift in redox balance favouring a more reducing environment, thereby protecting athlete from excessive oxidative damage during subsequent training sessions (Fisher-Wellman and Bloomer 2009).
In summary, a high-level official match led to increased catabolic hormonal environment, markers of muscle damage, inflammation and oxidative stress until 48 h of recovery period. Although match induced significant biochemical disturbances, the performance of these high-level adult soccer players was only affected to a smaller extent and until 24 h of recovery period, suggesting that high-level players possess a high capability to cope with the game demands as shown by the fast recovery pattern and lower performance impairments, at least in the examined neuromuscular parameters.
References
Andersson H, Raastad T, Nilsson J, Paulsen G, Garthe I, Kadi F (2008) Neuromuscular fatigue and recovery in elite female soccer: effects of active recovery. Med Sci Sports Exerc 40(2):372–380
Andersson H, Bohn SK, Raastad T, Paulsen G, Blomhoff R, Kadi F (2010a) Differences in the inflammatory plasma cytokine response following two elite female soccer games separated by a 72-h recovery. Scand J Med Sci Sports 20(5):740–747
Andersson H, Karlsen A, Blomhoff R, Raastad T, Kadi F (2010b) Plasma antioxidant responses and oxidative stress following a soccer game in elite female players. Scand J Med Sci Sports 20(4):600–608
Ascensao A, Rebelo A, Oliveira E, Marques F, Pereira L, Magalhaes J (2008) Biochemical impact of a soccer match—analysis of oxidative stress and muscle damage markers throughout recovery. Clin Biochem 41(10–11):841–851
Bishop PA, Jones E, Woods AK (2008) Recovery from training: a brief review. J Strength Cond Res 22(3):1015–1024
Bosco C, Komi PV, Tihanyi J, Fekete G, Apor P (1983) Mechanical power test and fiber composition of human leg extensor muscles. Eur J Appl Physiol Occup Physiol 51(1):129–135
Brancaccio P, Maffulli N, Limongelli FM (2007) Creatine kinase monitoring in sport medicine. Br Med Bull 81–82:209–230
Brocherie F, Morikawa T, Hayakawa N, Yasumatsu M (2005) Pre-season anaerobic performance of elite japanese soccer players. In: Reilly T, Araújo D, Cabri J (eds) Science and Football V. E and F. N. Spon, London, pp 144–153
Edwards AM, Clark NA (2006) Thermoregulatory observations in soccer match play: professional and recreational level applications using an intestinal pill system to measure core temperature. Br J Sports Med 40(2):133–138
Fatouros IG, Chatzinikolaou A, Douroudos II, Nikolaidis MG, Kyparos A, Margonis K, Michailidis Y, Vantarakis A, Taxildaris K, Katrabasas I, Mandalidis D, Kouretas D, Jamurtas AZ (2010) Time-course of changes in oxidative stress and antioxidant status responses following a soccer game. J Strength Cond Res 24(12):3278–3286
Fisher-Wellman K, Bloomer RJ (2009) Acute exercise and oxidative stress: a 30 year history. Dyn Med 8:1
Gaeta LM, Tozzi G, Pastore A, Federici G, Bertini E, Piemonte F (2002) Determination of superoxide dismutase and glutathione peroxidase activities in blood of healthy pediatric subjects. Clin Chim Acta 322(1–2):117–120
Hu M-L (1990) Measurement of protein thiol groups and GSH in plasma. In: Parker L (ed) Methods in enzymology. Academic Press, San Diego, pp 380–385
Ispirlidis I, Fatouros IG, Jamurtas AZ, Nikolaidis MG, Michailidis I, Douroudos I, Margonis K, Chatzinikolaou A, Kalistratos E, Katrabasas I, Alexiou V, Taxildaris K (2008) Time-course of changes in inflammatory and performance responses following a soccer game. Clin J Sport Med 18(5):423–431
Kollath E, Quade K (1993) Measurement of sprinting speed of professional and amateur soccer players. In: Reilly T, Clarys J, Stibbe A (eds) Science and football II. E and F. N. Spon, London, pp 31–36
Kraemer WJ, French DN, Paxton NJ, Hakkinen K, Volek JS, Sebastianelli WJ, Putukian M, Newton RU, Rubin MR, Gomez AL, Vescovi JD, Ratamess NA, Fleck SJ, Lynch JM, Knuttgen HG (2004) Changes in exercise performance and hormonal concentrations over a big ten soccer season in starters and nonstarters. J Strength Cond Res 18(1):121–128
Krustrup P, Ortenblad N, Nielsen J, Nybo L, Gunnarsson TP, Iaia FM, Madsen K, Stephens F, Greenhaff P, Bangsbo J (2011) Maximal voluntary contraction force, SR function and glycogen resynthesis during the first 72 h after a high-level competitive soccer game. Eur J Appl Physiol 111(12):2987–2995
Lago-Penas C, Rey E, Lago-Ballesteros J, Casais L, Dominguez E (2011) The influence of a congested calendar on physical performance in elite soccer. J Strength Cond Res 25(8):2111–2117
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Magalhaes J, Rebelo A, Oliveira E, Silva JR, Marques F, Ascensao A (2010) Impact of Loughborough Intermittent Shuttle Test versus soccer match on physiological, biochemical and neuromuscular parameters. Eur J Appl Physiol 108(1):39–48
Magalhães J, Oliveira J, Ascensao A, Soares J (2004) Concentric quadriceps and hamstrings isokinetic strength in volleyball and soccer players. J Sports Med Phys Fit 44(2):119–125
Malliou P, Ispirlidis I, Beneka A, Taxildaris K, Godolias G (2003) Vertical jump and knee extensors isokinetic performance in professional soccer players related to the phase of the training period. Isokinet Exerc Sci 11:165–169
Martinovic J, Dopsaj V, Dopsaj MJ, Kotur-Stevuljevic J, Vujovic A, Stefanovic A, Nesic G (2009) Long-term effects of oxidative stress in volleyball players. Int J Sports Med 30(12):851–856
McLellan CP, Lovell DI, Gass GC (2010) Creatine kinase and endocrine responses of elite players pre, during, and post rugby league match play. J Strength Cond Res 24(11):2908–2919
Meister S, Faude O, Ammann T, Schnittker R, Meyer T (2011) Indicators for high physical strain and overload in elite football players. Scand J Med Sci Sports 32(11):875–881
Mercer TH, Gleeson NP, Mitchell J (1997) Fitness profiles of professional soccer players before and after pre-season conditioning. In: Reilly T, Bangsbo J, Hughes M (eds) Science and Footbal III. E and FN Spon, London, pp 112–117
Nakagami Y, Takatsu H, Okamoto M, Terada Y, Maruta K (2009) Relationship between team performance levels and serum SH (sulfhydryl group) changes brought about by intensive athletic training summer camp. J Anal Bio Sci 32(5):427–433
Odetoyinbo K, Wooster B, Lane A (2009) The effect of a succession of matches on the activity profiles of professional soccer players. In: Reilly T, Korkusuz F (eds) Science and Football VI. The procedings of the Sixth World Congress on Science and Football, Routledge, pp 105–110
Rampinini E, Sassi A, Azzalin A, Castagna C, Menaspa P, Carlomagno D, Impellizzeri FM (2010) Physiological determinants of Yo–Yo intermittent recovery tests in male soccer players. Eur J Appl Physiol 108(2):401–409
Rampinini E, Bosio A, Ferraresi I, Petruolo A, Morelli A, Sassi A (2011) Match-Related Fatigue in Soccer Players. Med Sci Sports Exerc 43(11):2161–2170
Reilly T, Ekblom B (2005) The use of recovery methods post-exercise. J Sports Sci 23(6):619–627
Reilly T, Drust B, Clarke N (2008) Muscle fatigue during football match-play. Sports Med 38(5):357–367
Rodrigues V, Coelho DB, Mortimer L, Condessa L, Soares D, Silami-Garcia E (2007) Exercise intensity in training sessions and official games in soccer. J Sports Sci Med 1:57–61
Rohn T, Hinds T, Vincenzi F (1993) Ion transport ATPases as targets for free radical damage. Protection by an aminosteroid of the Ca2+ pump ATPase and Na+/K+ pump ATPase of human red blood cell membranes. Biochem Pharmacol 3:525–534
Ronnestad BR, Nymark BS, Raastad T (2011) Effects of in-season strength maintenance training frequency in professional soccer players. J Strength Cond Res 25(10):2653–2660
Semenick D (1990) The T-test. Natl Strength Cond Assoc J 12(1):36–37
Teixeira V, Valente H, Casal S, Pereira L, Marques F, Moreira P (2009) Antioxidant status, oxidative stress, and damage in elite kayakers after 1 year of training and competition in 2 seasons. Appl Physiol Nutr Metab 34(4):716–724
Thompson D, Williams C, Garcia-Roves P, McGregor SJ, McArdle F, Jackson MJ (2003) Post-exercise vitamin C supplementation and recovery from demanding exercise. Eur J Appl Physiol 89(3–4):393–400
Wisloff U, Castagna C, Helgerud J, Jones R, Hoff J (2004) Strong correlation of maximal squat strength with sprint performance and vertical jump height in elite soccer players. Br J Sports Med 38(3):285–288
Acknowledgments
We would like to thank to the Professional Soccer Club, to their technical staff, medical department and to the soccer players involved in the study for their committed participation. The excellent technical and practical assistance and skilful involvement of Eduardo Oliveira, Emanuel Passos and Inês Aleixo is appreciated.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
The authors declare that all the procedures of the experiments comply with the current laws of the country in which they were performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by William J. Kraemer.
Rights and permissions
About this article
Cite this article
Silva, J.R., Ascensão, A., Marques, F. et al. Neuromuscular function, hormonal and redox status and muscle damage of professional soccer players after a high-level competitive match. Eur J Appl Physiol 113, 2193–2201 (2013). https://doi.org/10.1007/s00421-013-2633-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-013-2633-8