Abstract
A carbohydrate (CHO) solution consumed before exposure to hypoxia has been reported to reduce arterial oxygen desaturation at rest. The purpose of this study was to determine whether this effect occurred during exercise and when the CHO load is part of a meal. Eleven male subjects (mean age 20.1 ± 1.8 years, BMI 24.3 ± 2.4 kg m2) consumed either a high-CHO (2,340 kJ, 70 % CHO, 12 % protein) or an isoenergetic high-protein (35 % CHO, 48 % protein) breakfast meal 60 min before being exposed to 15 min of hypoxia (FIO2 = 13.5 %) followed by 30 min of exercise in hypoxia (60 % of \( \dot{V}{\text{O}}_{{2{ \max }}} \)). Saturation of oxygen via a pulse oxymeter (SpO2), ventilatory parameters, substrate oxidation, interstitial glucose concentrations, and heart rate variability (HRV) were monitored continuously during the whole session. Results showed no effect from the type of meal on SpO2 at rest but a 3.1 ± 0.4 % reduction of desaturation during exercise (P < 0.005) compared to the high-protein version. This was associated with higher levels of ventilation (P < 0.05) and CO2 production (P < 0.01). Glucose oxidation was higher after the high-CHO than the high-protein breakfast over the whole session (+19.4 + 4.0 %, P < 0.0001), whereas the interstitial glucose levels were increased only at rest (P < 0.001). HRV indices were not different between conditions. In conclusion, a high-CHO meal consumed prior to moderate exercise in hypoxia condition reduced oxygen desaturation compared to a high-protein meal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Improving tolerance to hypoxia is a constant concern for people who must be exposed to high altitude and some recent results suggest that dietary choices may represent an efficient strategy. It has actually been shown that ingesting a carbohydrate (CHO) solution during an acute exposure to hypoxia increased arterial oxygen saturation (SaO2) (Lawless et al. 1999). More recently, it was reported that drinking a glucose solution 40 min prior to hypoxia exposure was followed by higher oxygen saturation measured by pulse oximetry (SpO2) (Golja et al. 2008; Klemenc and Golja 2011), CO2 production and ventilation (\( \dot{V}_{E} \)) (Golja et al. 2008) compared to an equal volume of water. These results suggest that ingested CHO may contribute to improved physiological tolerance to hypoxia through increased O2 transport and delivery to the tissues. CHO would enhance the production of CO2 (Ferrannini 1988) which in turn would stimulate \( \dot{V}_{E} \). However, this effect seems to last only during the metabolism of the ingested CHO (Golja et al. 2008) and would require spreading intake over the whole period of exposure.
It remains to be determined whether this response to hypoxia observed with a CHO solution may occur when CHO is consumed as part of a meal. An increase in oxygen alveolar (PAO2) or arterial pressures (PaO2) has been reported with a high-CHO diet in some older studies (Bickerman et al. 1945; Eckman et al. 1945). A high CHO meal was followed by increased CO2 production, PaO2 and SaO2 compared to a low-CHO meal in a more recent study (Hansen et al. 1972), but the difference in CHO between the meals was unrealistic (~93 and ~5 % of energy for the high and low-CHO meals, respectively). A smaller difference in the CHO content of the diet (68 vs. 45 %) actually failed to alter SpO2 (Swenson et al. 1997).
The importance of the oxygen supply is still enhanced when it is associated with exercise and the main problem therefore becomes the tolerance to exercise in hypoxia (Favret and Richalet 2007; Richalet et al. 2012). An increased reliance on CHO oxidation in acute hypoxia has been reported in some (Friedmann et al. 2004; Lundby and Van Hall 2002; Peronnet et al. 2006), but not all studies (Beidleman et al. 2002; Bouissou et al. 1987). It was shown that exogenous CHO was not used differently in hypoxia and in normoxia during exercise and did not contribute to this increased CHO oxidation (Peronnet et al. 2006). Interestingly, the hypoxia-induced activation of the sympathetic nervous system (SNS) (Favret and Richalet 2007), evaluated by heart rate variability (HRV), was reported to be increased by the prior ingestion of a glucose solution (Klemenc et al. 2008). Given the role of SNS activity on arterial oxygen through increased heart rate (HR) (Gonzalez et al. 1998), this may contribute to an explanation of the effect of CHO on oxygen saturation during hypoxia.
To date, no study has assessed whether the CHO content of a prior meal may alter oxygen saturation and \( \dot{V}_{E} \) during exercise performed in hypoxia. This was the aim of the present study. In France, breakfast usually contains very high level of CHO and therefore was taken as model for the high-CHO meal. Based on the high oxygen cost of protein metabolism and their failure to increase SNS activity (Welle et al. 1981) proteins were partly substituted for CHO in the low-CHO meal. The effect of a high-protein meal on the tolerance to hypoxia has never been assessed, but it was reported that rats exposed to hypoxia specifically reduced their protein intake (Morel et al. 2005) and that increasing the level of protein in their diet impaired their tolerance to hypoxia (Bigard et al. 1996).
To determine the kinetics of changes between the factors supposedly involved in the CHO-induced modifications in SpO2 and \( \dot{V}_{E} \), systemic glucose, substrate oxidation and sympathovagal balance were continuously recorded in parallel to respiratory parameters. Our hypothesis was that following the high-CHO meal, the magnitude of the hypoxia-induced decrease in SpO2 would be lower than following the high-protein meal, and that this would be associated with higher systemic glucose level and a shift toward the sympathetic component of the sympathovagal balance.
Subjects and methods
Subjects
Twelve healthy active male subjects were recruited among Sport and Physical Activity students on the campus of Paris 13 University for participation in the study. Data from only 11 subjects were analyzed due to technical problems in a session with one subject without possibility to plan a new trial. Inclusion criteria were that the volunteers be male, healthy, moderately active (less than 5 h of sports or intensive exercise per week) and ranging in age between 18 and 25 years. Smokers, subjects who drank alcohol more than occasionally, who had food allergies, who took medication or who had shown intolerance to hypoxia, were excluded from the study. Subjects’ characteristics are shown in Table 1. Their fat mass was assessed by an 8-electrode bioelectrical impedance analyzer (Tanita BC 418MA, Tanita Co. NL) and abdominal fat mass by ViScan (Tanita Co, NL). This number of subjects was based on previous studies showing differences in cardiac, respiratory and metabolic parameters during exercise following meals with different macronutrient compositions (Paul et al. 2003; Rowlands and Hopkins 2002; Whitley et al. 1998). The subjects were informed about the nature and the risks of the experimental procedure before their written consent was obtained. The protocol was conducted according to the standards set by the Declaration of Helsinki and was officially approved by the representatives of the regional board of the National Ethics Committee (Comité de Protection des Personnes no 10). Subjects were paid for participation.
Study design
The study followed a within-subject design with subjects completing each of two test conditions: one with a high-CHO (high-CHO), and one with a high-protein (high-PRO) meal. These conditions were tested in single blind and in a counterbalanced order, completed at a 1-week interval, on the same day of the week.
Preliminary testing
At least 3 days before the first trial, individual maximal oxygen consumption at a fraction of inspired oxygen (FIO2) of 13.5 % (\( {\text{Hx}}\dot{V}{\text{O}}_{{2{ \max }}} \)) corresponding to a simulated altitude of 3,500 m, was determined by a maximal workload test conducted on a bicycle ergometer (Ergoselect 100P, Ergoline, Bitz, Deutschland). Subjects breathed through a mouthpiece (Hans Rudolph, Kansas City, MO, USA) connected to a pneumotachograph calibrated manually with a 3-L syringe before each session (Sensormedics, Viasys Healthcare, Höchberg, Germany) then to a 3-way valve (Hans Rudolph, Kansas City, MO, USA) which ensures separate ways between inspired air and expired air. Expired gases were collected continuously into a metabograph (Vmax Encore, Viasys healthcare, Palm Springs, CA, USA) to measure \( \dot{V}{\text{O}}_{2} \) and \( \dot{V}{\text{CO}}_{2} \). These analyzers were calibrated (values and response time) by using two calibration gas mixture (16 % O2–4 % CO2 and 26 % O2–0 % CO2). Altitude was simulated by breathing a normobaric hypoxic gas mixture using a system diluting and mixing ambient air with nitrogen (Altitrainer 200®, SMTEC, Nyon, Switzerland). FIO2 was constantly controlled via the metabograph and heart rate (HR) was determined from a 12-electrode Cardiosoft electrocardiogram used to monitor cardiac function during the test. SpO2 was estimated by a multi-wavelength pulse oximeter (Biox 3740, Ohmeda, Louisville, CO, USA) via a probe fixed on the earlobe after local vasodilation by capsaicin cream. This technical procedure has been found to have a high level of concordance with arterial blood samples in normoxia or in hypoxia, either at rest or during exercise (Mollard et al. 2010). During this preliminary testing and all trials, ambient air temperature was maintained between 19 and 22 °C by air conditioning. The same equipment was used for all measurements throughout the study. Subjects were first exposed to hypoxia at rest during 5 min, then began a 5-min warm-up at 60 W and workload was then increased by 20 W min−1. The \( {\text{Hx}}\dot{V}{\text{O}}_{{2{ \max }}} \) was considered to be reached when both of the following criteria were met: (1) less than 2 mL kg−1 min−1 \( \dot{V}{\text{O}}_{2} \) increase despite workload increase; (2) respiratory energy ratio (RER) > 1.15. Mechanical power at \( \dot{V}{\text{O}}_{2\max } \) (\( P{\text{-Hx}}\dot{V}{\text{O}}_{{2{ \max }}} \)) was considered to be the power of the last completed stage at the \( {\text{Hx}}\dot{V}{\text{O}}_{{2{ \max }}} \). Based on the results of the measurement of the \( {\text{Hx}}\dot{V}{\text{O}}_{{2{ \max }}} \) in each subject, the cycling power corresponding to 60 % of \( {\text{Hx}}\dot{V}{\text{O}}_{{2{ \max }}} \) was determined individually in order to be maintained throughout the exercise session. During all the experimental sessions, subjects wore only shoes and a short appropriate for practicing sport.
Procedure
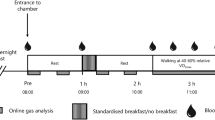
The procedure is described in Fig. 1. After an overnight fast, subjects came to the laboratory at 08:30. A glucose monitoring system sensor and a heart rate monitor, as described below, were placed on them. At 08:45, a first 15-min measurement of ventilatory parameters was completed in normoxia (NX1) while subjects sat quietly on a comfortable chair. According to the randomization, they were then served a high-CHO or a high-protein breakfast that they had to eat in its entirety. The duration of this meal was limited to 15 min. At 09:45, a series of 4 phases was initiated. The first phase was a second measurement of ventilatory parameters in normoxia (NX2) during 15 min. Then, the FIO2 was progressively decreased over 10 min to reach the target of 13.5 %, followed by a 15-min measurement of ventilatory parameters in hypoxia (HX), i.e., the second phase. At 10:25, subjects began to cycle for a 10-min progressive warm-up to reach about 40 % \( P{\text{-Hx}}\dot{V}{\text{O}}_{{2{ \max }}} \) in hypoxia representing about 60 % of \( {\text{Hx}}\dot{V}{\text{O}}_{{2{ \max }}} \). At 10:35, a 30-min measurement of ventilatory parameters during the steady-state exercise in hypoxia was done. Since it has been reported (Golja et al. 2008) that the effect of a glucose load on tolerance to hypoxia was weakening over a 30-min period, we decided to separate the exercise period in two consecutive 15-min phases: HX-EX1 and HX-EX2. Between HX-EX1 and HX-EX2, a first lactate measurement was completed. At the end of HX-EX2, subjects rated the Borg scale and the Lake Louise questionnaire, and a second lactate measurement was completed 5 min after the end of exercise. In the absence of data about the accurate kinetics of lactate at the end of an exercise practiced in hypoxia, and based on previously described immediate post-exercise increase in blood lactate (Billat 1996; Goodwin et al. 2007) this delay was considered as the most appropriate. The heart rate monitor and the Freestyle Navigator were then removed and subjects were allowed to leave the laboratory until the next trial planned the following week at 08:00.
Food
Subjects were asked to maintain stable eating and occupational habits over the 2 days preceding each trial. The day prior to a trial, they ate dinner at home at approximately the same time. The dinner was provided to subjects and consisted of lasagna (6.1 kJ g−1 with 39, 45 and 16 % from CHO, fat and protein, respectively). They were allowed to add some bread, a dessert and beverages to this main course, but were instructed to maintain these dinner meals similar across sessions and to report their intake on a diary. After dinner, they were required not to eat or drink anything but water until the next morning.
On the trial days, breakfasts consisted of the same food items in both conditions, i.e., a hot chocolate-flavored milk bowl with cereal and fig-flavoured cereal bars. One version was high in CHO, whereas the other was high in protein (Table 2). The energy load was the same for all subjects and similar between versions. Food items were kept as identical as possible across versions in order to reduce biases associated with varying orosensory characteristics. Palatability scale was rated after the consumption of breakfast. The scale consisted of a 100 mm horizontal line, preceded by “Do you like this meal?” and anchored with “not at all” at the left end and “extremely” at the right end. Scores were obtained by measuring the distance in millimeters from the left end of the line to the subject’s mark.
Ventilatory parameters
During measurements in normoxia or hypoxia and at rest or exercise, subjects wore the same equipment as during the \( \dot{V}{\text{O}}_{{2{ \max }}} \) test. \( \dot{V}{\text{O}}_{{2{ \max }}} \), \( \dot{V}{\text{CO}}_{2} \), RER and \( \dot{V}_{E} \) were measured each minute and SpO2 was estimated at the same frequency.
Glucose measurement
A continuous glucose monitoring system (Freestyle Navigator®, Abbott, Alameda, CA, USA) was used for assessing systemic glucose at a frequency of one reading per minute. It consisted of an electrochemical sensor with glucose oxidase immobilized into an electrode. The sensor was inserted under the skin at the back of the left arm and interstitial glucose was measured every minute after a 60-min initialization. As required by the manufacturer, two calibrations were performed at 09:30 and 10:30 on capillary blood taken from the fingertip and analyzed by the built-in blood glucometer of the receiver. Interstitial glucose monitored by this device has been reported to be a valid surrogate of the plasma glucose level (Clarke et al. 2005; Rebrin and Steil 2000). Its sensitivity, expressed in mean absolute relative difference (ARD) is 13.4 % (Geoffrey et al. 2011). However, a lag between plasma and interstitial glucose levels is constantly reported, the former preceding the latter by 4–10 min (Boyne et al. 2003). With the Navigator, this mean lag value has been calculated at 12.5 min (Kovatchev et al. 2009). Moreover, the effect of exercise on this delay is not known. Personal data suggest that this lag is dramatically reduced.
Energy expenditure, substrate oxidation and lactate measurements
Energy expenditure and substrate oxidation were derived from oxygen consumption and gas exchange as measured by the metabograph (see above). At rest, energy expenditure (EE) was calculated by the energy equivalent of O2 derived from the Weir equation (Weir 1949) and substrate oxidation was calculated using the Péronnet and Massicotte equations (Peronnet and Massicotte 1991) neglecting the contribution of protein to EE, which is small and difficult to measure during short exercises sessions. During exercise, the Jeukendrup and Wallis equation (Jeukendrup and Wallis 2005) for moderate to high-intensity exercise (50–75 % \( \dot{V}{\text{O}}_{{2{ \max }}} \)) was used for CHO oxidation and EE. This stoichiometric equation is more appropriate to exercise since it takes into account the fact that only 20 % of the glucose oxidized is derived from plasma, 80 % being provided by glycogen. Allowing a delay of 5 min to reach stability, only the last 10 min of each 15-min measure was used for analyses.
Lactate levels were measured on a capillary blood sample taken from the fingertip using Lactate Pro LT-1710 (Arkray, Kyoto, Japan). This device has an intra- and inter-analyser comparisons of 0.5 mM and 0.4 mM, respectively (Tanner et al. 2010), a coefficient of variation ranging between 2.8 and 5.0 % (Baldari et al. 2009) and an intra-analyzer correlation of 0.99 (Pyne et al. 2000). At blood concentrations below 4 mM, Lactate Pro was reported to be especially accurate and the correlation with a laboratory-based analyzer was r = 0.91 (Tanner et al. 2010).
Assessment of the autonomic nervous system
Autonomous modulation was evaluated by the frequency domain analysis of HRV. The R–R intervals were recorded by a heart rate monitor (T6, Suunto, Finland) and stored for analysis. This device shows a good agreement with ECG for measuring HRV (Weippert et al. 2010). The R–R interval sequences were then visually inspected, and the data considered as artifactual were manually replaced by interpolated or extrapolated data. The frequency of abnormal beats due to the R–R recording device used was less than 1 %. Then, suitable series of 512 R–R intervals were chosen for analysis during each 15-min measurement. This procedure yielded indices of HRV corresponding with metabolic and respiratory measurement. The HRV analyses were performed with the HRV Analysis Software 1.1 for Windows (The Biomedical Signal Analysis Group, Department of Applied Physics, University of Kuopio, Finland) (Niskanen et al. 2004). Fast Fourier transform spectra were calculated from this 512 R–R interval. Total power in the frequency range (0–0.40 Hz) was divided into low-frequency (LF, 0.04–0.15 Hz) and high-frequency powers (HF, 0.15–0.60 Hz). Oscillations occurring within the LF region have been attributed to both the vagus and cardiac sympathetic nerves (Pagani et al. 1986). HF oscillations are thought to be mediated almost entirely by the vagus nerve and thus represent parasympathetic modulation (Task Force 1996). To quantitatively evaluate the sympathovagal balance, the use of normalized units (nu) for the HF component has been recommended (Montano et al. 1994). The LF to HF ratio (LF/HF) is used to investigate the sympathetic modulation of HRV (Eckberg 1997). Our participants breathed spontaneously since it allows physiological cardio-ventilatory coupling (Larsen et al. 2010) and reproducibility has been shown to be similar between spontaneous and paced breath techniques with intraclass correlation coefficients between 0.65 and 0.88 (Pinna et al. 2007; Sinnreich et al. 1998). LF (ms2), HF (ms2) and LF/HF have even been reported to be more reproducible in spontaneous than in paced breath respiration (Pitzalis et al. 1996). During moderate exercise, HRV demonstrated a good to excellent degree of reproducibility with an intraclass correlation coefficient between 0.58 and 0.85 (McNarry and Lewis 2012).
Subjective parameters of tolerance
Tolerance to hypoxia was assessed using the Lake Louise acute mountain sickness scoring system (Sutton et al. 1991) featuring 8 symptoms (headaches, gastrointestinal symptoms, fatigue/weakness, dizziness/lightheadedness, breathing difficulties, lack of coordination, cough and mental confusion) on 4-point scales with answers ranging from “not at all” to “severe”. Although this questionnaire was established to assess acute mountain sickness after ascending to altitude, its items were considered as relevant to the tolerance to acute exposure to hypoxia and to detect differences according to the level of SpO2. Tolerance to exercise was assessed by the rating of perceived exertion on a 6–20 point Borg scale (Borg 1970). These scales were rated immediately following the exercise session in hypoxia.
Data analysis
Incremental area under the curve (AUC) of the interstitial glucose was calculated by the trapezoidal method from the start of the breakfast and over the whole session. Data analyses were conducted using SYSTAT Software (version 10.1, SPSS, Chicago, IL). Interstitial glucose profiles, SpO2 and \( \dot{V}_{E} \) profiles were analyzed using analyses of variance (ANOVA) for repeated measures with breakfast conditions (high-PRO and high-CHO) and phases (NX1, NX2, HX, HX-EX1 and HX-EX2) as within-subject factors. Interstitial glucose data were analyzed with lags between 0 and 15 min. Since no differences in statistical results were found, raw results without lags are reported and illustrated. When an effect was significant, appropriate comparisons by Scheffe’s tests were conducted in each phase. Statistical significance was set at P < 0.05. All results are expressed in mean ± SD.
Results
Palatability
The high-CHO version of the breakfast was rated a little more palatable than the high-protein version but this difference did not reach statistical significance (66 ± 11 vs. 52 ± 20 mm, respectively, P = 0.08).
Parameters associated with exercise in hypoxia
Exercise in hypoxia was performed at 92.7 ± 7.9 W corresponding to an intensity of 37.7 ± 0.8 % of \( P{\text{-Hx}}\dot{V}{\text{O}}_{{2{ \max }}} \) in both conditions. Oxygen uptake was 26.1 ± 3.3 and 25.8 ± 2.9 mL min−1 kg−1 in the high-CHO and in the high-PRO conditions, respectively. These \( \dot{V}{\text{O}}_{2} \) corresponded to an intensity of 58.3 ± 6.3 and 57.6 ± 5.7 % of \( {\text{Hx}}\dot{V}{\text{O}}_{{2{ \max }}} \), respectively.
Ventilatory parameters
Profiles of the ventilatory parameters and statistical significances between conditions are illustrated in Fig. 2. An interaction between the breakfast condition and phases was observed for \( \dot{V}{\text{O}}_{2} \,\left( {P = 0.01} \right) \), \( \dot{V}{\text{CO}}_{2} \left( {P < 0.01} \right) \),\( \dot{V}_{E} \,\left( {P < 0.01} \right) \), and SpO2 (P < 0.05). Comparisons between NX2 and NX1 showed that the meal increased \( \dot{V}{\text{O}}_{ 2} \), \( \dot{V}{\text{CO}}_{ 2} \) and \( \dot{V}_{E} \) in both conditions (all P < 0.01).
Ventilatory parameters (mean ± SD) during the first and second phases of normoxia (NX1 and NX2, respectively), hypoxia at rest (HX) and the first and second phases of exercise in hypoxia (HX-EX1 and HX-EX2, respectively). *Different between conditions;  different from the previous phase for both conditions; ⊥different from the previous phase only for high-PRO condition (all P < 0.05)
different from the previous phase for both conditions; ⊥different from the previous phase only for high-PRO condition (all P < 0.05)
Hypoxia increased \( \dot{V}{\text{CO}}_{ 2} \) and \( \dot{V}_{E} \) and decreased SpO2 in both conditions (all P < 0.05), but increased \( \dot{V}{\text{O}}_{2} \) in the high-PRO condition only (P < 0.01). Consistently, \( \dot{V}{\text{O}}_{2} \) was lower in the high-CHO than in the high-PRO condition (P < 0.05) during HX.
Exercise increased \( \dot{V}{\text{O}}_{2} \), \( \dot{V}{\text{CO}}_{2} \) and \( \dot{V}_{E} \) and decreased SpO2 during HX-EX1 in both conditions (all P < 0.0001). However, \( \dot{V}{\text{CO}}_{2} \), \( \dot{V}_{E} \) and SpO2 were higher in the high-CHO than in the high-PRO condition (all P < 0.05). During HX-EX2, \( \dot{V}{\text{O}}_{2} \) and \( \dot{V}_{E} \) still increased compared to HX-EX1 in both conditions (all P < 0.05). Last, \( \dot{V}{\text{CO}}_{2} \) increased (P < 0.05) and SpO2 decreased (P < 0.005) only in the high-PRO condition. Comparisons between conditions showed that during HX-EX2, \( \dot{V}{\text{CO}}_{2} \) was higher in the high-CHO than in the high-PRO condition (both P < 0.05), whereas these differences just failed to reach significance for SpO2 and \( \dot{V}_{E} \) (P = 0.08 and P = 0.09, respectively).
Interstitial glucose
Interstitial glucose profiles and AUCs in each breakfast condition are shown in Fig. 3. Glucose was not different between conditions at the onset of breakfast in the high-CHO (5.07 ± 0.45 mmol L−1) and high-PRO conditions (5.13 ± 0.42 mmol L−1). Whatever the tested lag, an effect of the breakfast composition (P < 0.005) and an interaction with the phases (P < 0.001) were observed. Comparisons showed that interstitial glucose levels were higher in the high-CHO than in the high-PRO condition during NX2 (P < 0.001) and HX (P < 0.001), but were not different between conditions during the exercise phases. Consistently, the glucose decline between the HX and HX-EX1 phases was greater in the high-CHO than in the high-PRO condition (P < 0.005). Over the session, the high-CHO breakfast elicited a larger glucose response (in AUC) than its high-protein version (P < 0.01).
Interstitial glucose levels (mean ± SD) during the first and second phases of normoxia (NX1 and NX2, respectively), hypoxia at rest (HX) and the first and second phases of exercise in hypoxia (HX-EX1 and HX-EX2, respectively) and (top right) incremental area under the curve (AUC) from NX2 to HX-EX2. *Different from the high-PRO breakfast condition (P < 0.05)
Heart rate variability indices
There was an effect of the phase for HR (P < 0.001), TP (P < 0.001), LFms2 (P < 0.001), HFms2 (P < 0.001), HFnu (P = 0.01) and LF/HF (P < 0.01), but no interaction with the breakfast condition (Table 3). Comparisons showed that HR was higher in NX2 than in NX1 (P < 0.005) and that HR and LF/HF were higher and HFnu lower in HX than in NX2 (all P < 0.05). During exercise, HR was higher and TP, LFms2 and HFms2 indices were lower in HX-EX1 than in HX (all P < 0.005). Finally, HR and HFnu were higher and TP, LFms2 and LF/HF were lower in HX-EX2 than in HX-EX1 (all P < 0.01).
Metabolic parameters
Results for metabolic parameters during each phase and statistical significances between conditions are illustrated in Fig. 4. An effect of the phases was observed for all variables (all P < 0.0001), but an effect of the breakfast condition only for RER (P < 0.05), fat oxidation (P < 0.05) and CHO oxidation (P < 0.01). RER and CHO oxidation were higher and fat oxidation was lower in the high-CHO than in the high-PRO conditions during NX2 (P < 0.05 for all), HX (P < 0.01 for all), HX-EX1 (P < 0.01 for all) and HX-EX2 (P < 0.01 for RER, P = 0.005 for CHO oxidation and P < 0.01 for fat oxidation). Over the whole session, the CHO oxidation was 19.4 ± 4.0 % higher in the high-CHO than in the high-PRO condition (P < 0.001).
Respiratory exchange ratio (RER), energy expenditure, fat and CHO oxidations (mean ± SD) during the 15 min of the first and second phases of normoxia (NX1 and NX2, respectively), of hypoxia at rest (HX) and of the first and second phases of exercise in hypoxia (HX-EX1 and HX-EX2, respectively). *Different from the high-CHO breakfast condition;  different from the previous phase for both conditions (all P < 0.05)
different from the previous phase for both conditions (all P < 0.05)
Lactate levels measured during exercise were higher in the high-CHO than in the high-PRO conditions (4.2 ± 0.9 and 3.5 ± 1.1 mmol L−1, respectively, P < 0.05). However, no difference was observed 5 min after the exercise in hypoxia was completed (2.9 ± 1.7 and 3.4 ± 1.7 mmol L−1, respectively, ns).
Subjective parameters of tolerance to hypoxia and exercise
Lake Louise scores were low and not different between the high-CHO and the high-PRO conditions (1.4 ± 1.5 and 2.1 ± 1.2, respectively, ns). The scores on the Borg scales showed that the exercise was considered fairly light and were not different in the high-CHO and high-PRO conditions (11.8 ± 2.1 and 11.8 ± 1.7, respectively, ns).
Discussion
This study showed that a high-CHO meal induced an improvement in SpO2 during exercise performed in hypoxia as compared to a high-protein meal. This was associated with higher CHO oxidation leading probably to an increase in \( \dot{V}{\text{CO}}_{2} \) and \( \dot{V}_{E} \), but autonomous nervous system activity seemed not to be implied in these changes. During hypoxia at rest we observed no effect of the meal composition on SpO2.
Indeed, no significant differences in most of the ventilatory parameters were observed at rest between the macronutrient versions of the breakfast. In particular, neither \( \dot{V}{\text{CO}}_{2} \), \( \dot{V}_{E} \) nor SpO2 differed between breakfast conditions. Thus, using a high-CHO compared to a high-protein meal, our results do not replicate the effects that Golja et al. (2008) reported with a CHO beverage (sugar in water) compared to water. The delay between consumption of the preload and the onset of hypoxia being similar in both studies, it is likely that the differences between these results are in relation with the choice of the preload. In the Golja et al. (2008) study, glucose was ingested in a proportion of 1 g kg−1 (73 g ranging from ~53 to ~113 g) and compared to water whereas in the present study we chose to provide a fixed amount of ~100 g in the high-CHO condition for all subjects, representing a CHO load ranging from 1.0 to 1.4 g kg−1 and compared it to a protein load. Since the lowest CHO content that we were able to achieve while using the same food items across conditions was 47.3 g for the high-protein version, the CHO difference between our conditions was lower than in the Golja et al. (2008) study. This smaller difference in the CHO load may contribute to explaining why the expected effects on SpO2 and ventilatory parameters were not observed. However, interstitial glucose levels and glucose oxidation were higher after the high-CHO than after the high-protein breakfast, showing that the preload differences were effective during hypoxia exposure at rest. Moreover, \( \dot{V}{\text{O}}_{2} \) was lower after the high-CHO than after the high-protein breakfast, a likely consequence of the lower O2 cost of CHO than protein for providing energy, showing that the macronutrients of the breakfasts were contributing to metabolism during this phase of hypoxia at rest. Our results are in agreement with the ones by Swenson et al. (1997) who used similar levels of CHO content (~68 and ~45 % in the high and low-CHO versions, respectively) in the diet preceding exposure to hypoxia than we used in our meals (~69 and ~35 % in the high and low-CHO versions, respectively). Their conclusion was that the difference in RER between their meal conditions was not different enough (0.94 vs. 0.87 in the high and low-CHO conditions, respectively), to modify the values of SpO2. In our study, the difference in RER between conditions had a similar magnitude (0.90 vs. 0.84 in the high and low-CHO conditions, respectively) and may not have been large enough to modify ventilation, at least in a resting state. Thus, our and Swenson et al. (1997) results suggest that a meal containing a high but not extreme CHO level is not potent enough for large alteration of the ventilatory response to hypoxia at rest and for reducing oxygen desaturation compared to a moderate-CHO and high-protein one.
The expected CHO-induced modifications of ventilatory responses to hypoxia were observed during exercise. The high-CHO version of the breakfast increased \( \dot{V}_{E} \), \( \dot{V}{\text{CO}}_{2} \), and SpO2 compared to its high-protein version. However, it is noteworthy that only \( \dot{V}{\text{CO}}_{2} \) was still significantly different between conditions during the second phase of exercise in hypoxia, with the difference in SpO2 and \( \dot{V}_{E} \) just failing to reach significance. Thus, the high-CHO meal was efficient in increasing CO2 production, \( \dot{V}_{E} \) and for attenuating oxygen desaturation during an exercise performed in hypoxia ~90 min later. This improvement in SpO2 of about 3 % was maintained during the whole exercise session, despite reaching significance only during the first 15 min (HX-EX1), and could have positive consequences on exercise performance in hypoxia (Chapman et al. 2011). Indeed, it is well established that even a slight decrease in saturation (−3 %) contributes to the decline of \( \dot{V}{\text{O}}_{{ 2 {\text{max}}}} \) and performance at altitude (Chapman et al. 2011; Wehrlin and Hallen 2006). Moreover, oxygen availability has been reported to alter performance through changes in the central motor output to the working muscle (Amann et al. 2006). Hypoxia will, however, affect various performance types differently, for example, impairing a 5-km time trial (Amann et al. 2006), but not a sprint-like exercise to exhaustion such as a Wingate test (Calbet et al. 2003). Recently, a CHO supplement consumed via energy drinks was found to successfully reduce perceived exertion and to improve performance on a specific time-trial test (Oliver et al. 2012), but this was assessed after 22 days of chronic exposure to altitude and CHO consumption. Our study was not aimed at assessing such performance and further research is needed to show whether this small improvement in oxygen delivery to the tissues may improve performance in a time-trial test. Moreover, neither the Lake Louise score or the Borg scale were different between conditions, suggesting that this difference in SpO2 was not associated with an improved tolerance to hypoxia or a reduced perceived exertion. Since this hypoxia was very well tolerated (Lake Louise score ~1.8) and perceived exertion moderate (Borg scale ~11), the conditions were probably not difficult enough to reveal any effect of this difference in SpO2.
Similar to hypoxia at rest, CHO oxidation was higher in the high-CHO than in the high-PRO conditions during exercise, but the interstitial glucose level was no more different, whatever the chosen lag. This may illustrate the increased capture of glucose by active muscles. A greater reliance on glucose oxidation during exercise at high altitude than at sea level for the same absolute intensity has been reported (Brooks et al. 1992; Friedmann et al. 2004; Lundby and Van Hall 2002; Peronnet et al. 2006). It should be noted that this was observed at the same relative intensity in some (Friedmann et al. 2004; Peronnet et al. 2006), but not all studies (Bouissou et al. 1987; Lundby and Van Hall 2002). This increased reliance was proposed to be endogenous rather than exogenous since CHO consumed during exercise was not oxidized to a greater degree in hypoxia simulating an altitude of 4.300 m than in normoxia (Peronnet et al. 2006). Our study was not designed to determine the utilization of the CHO content of the breakfast in hypoxia compared to normoxia, but it showed that at the same absolute workload, the high-CHO meal was followed by a 15 ± 4 % increase in glucose oxidation compared to the high-protein meal (~50 and ~43 g over the 30 min in the high-CHO and the high-PRO conditions, respectively). The higher glycolysis rate in the high-CHO than in the high-protein conditions was suggested by the ~17 % increase in blood lactate level during exercise. Although the rate of appearance of lactate in hypoxia is discussed (Hochachka et al. 2002), hypoxia and exercise conditions were the same between both breakfast conditions and the difference is likely to be attributed to the difference in glycolysis rate. It is important to note that calculation of substrate oxidation from gas exchange is very sensitive to changes in ventilation, with a possible transient dissociation between \( \dot{V}{\text{CO}}_{2} \) at the mouth and in tissues (Jeukendrup and Wallis 2005). In the present study, substrate oxidation values were analyzed when \( \dot{V}_{E} \) and \( \dot{V}{\text{CO}}_{2} \) had reached a stable state either at rest or during exercise, arguing for \( \dot{V}{\text{CO}}_{2} \) measuring gas exchange at the tissue level.
Contrary to our hypothesis, sympathovagal balance was not modified by the macronutrient content of the meal. In our study, similarly to previous results (Charlot et al. 2011; Pivik and Dykman 2004; Pivik et al. 2006) the postprandial increase in HR was not associated with a consistent increase in SNS activity or decrease in PNS activity. As expected, hypoxia and exercise modified the sympathovagal balance toward its SNS component (Favret and Richalet 2007) but the macronutrient version of the meal did not change these effects. Thus, our results do not argue in favour of a role for the autonomous activity in the reduced desaturation observed after the high-CHO breakfast.
Similarly to previous studies (Golja et al. 2008; Hansen et al. 1972; Klemenc and Golja 2011; Klemenc et al. 2008; Lawless et al. 1999; Swenson et al. 1997), there was no control normoxic condition in the present experiment. Although this did not preclude testing our primary hypothesis, i.e., the difference in response to hypoxia according to the macronutrient content of the prior meal, this does not authorize any comparison of our results with the tolerance to exercise in normoxia.
On the practical side, this study suggests that consumption of a high-CHO meal prior to an exercise in hypoxia (alpinism, high altitude competitions…) may represent a better choice that a high-protein meal if the objective is to limit arterial desaturation in oxygen. Conversely, in a training context such as “training high-living low” (Hoppeler and Vogt 2001), it could be a better option to consume a high-protein meal, leading to greater oxygen desaturation if hypoxia-induced adaptations are the main objectives. This effect of glucose may enhance well described increased performance effect of a CHO supplementation that seems to be mediated by an improved neural drive and an attenuated central fatigue more than by glycogen sparing (Karelis et al. 2010).
In conclusion, this study showed that a high-CHO meal consumed 90 min prior to a moderate exercise session performed in hypoxia simulating a 3,500 m altitude, significantly increases CO2 production, ventilation and improves oxygen saturation compared to a high-protein meal. These effects during exercise were associated with an increase in CHO oxidation without differences in systemic glucose levels or sympathovagal balance, and no impact on tolerance to hypoxia or perceived exertion. This contribution of the CHO content of the meal preceding exercise in hypoxia should be considered according to the specific objectives of these environmental conditions.
References
Amann M, Eldridge MW, Lovering AT, Stickland MK, Pegelow DF, Dempsey JA (2006) Arterial oxygenation influences central motor output and exercise performance via effects on peripheral locomotor muscle fatigue in humans. J Physiol 575:937–952
Baldari C, Bonavolonta V, Emerenziani GP, Gallotta MC, Silva AJ, Guidetti L (2009) Accuracy, reliability, linearity of Accutrend and Lactate Pro versus EBIO plus analyzer. Eur J Appl Physiol 107:105–111
Beidleman BA, Rock PB, Muza SR, Fulco CS, Gibson LL, Kamimori GH, Cymerman A (2002) Substrate oxidation is altered in women during exercise upon acute altitude exposure. Med Sci Sports Exerc 34:430–437
Bickerman HA, Bouvet W, Harrer CJ, Oyler JR, Seitz CP (1945) Aviation nutrition studies. 1. Effects of pre-flight and in-flight means of varying compositions with respect to carbohydrate, protein and fat. Aviat Med 16:69–84
Bigard AX, Douce P, Merino D, Lienhard F, Guezennec CY (1996) Changes in dietary protein intake fail to prevent decrease in muscle growth induced by severe hypoxia in rats. J Appl Physiol 80:208–215
Billat LV (1996) Use of blood lactate measurements for prediction of exercise performance and for control of training. Recommendations for long-distance running. Sports Med 22:157–175
Borg G (1970) Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med 2:92–98
Bouissou P, Guezennec CY, Defer G, Pesquies P (1987) Oxygen consumption, lactate accumulation, and sympathetic response during prolonged exercise under hypoxia. Int J Sports Med 8:266–269
Boyne MS, Silver DM, Kaplan J, Saudek CD (2003) Timing of changes in interstitial and venous blood glucose measured with a continuous subcutaneous glucose sensor. Diabetes 52:2790–2794
Brooks GA, Wolfel EE, Groves BM, Bender PR, Butterfield GE, Cymerman A, Mazzeo RS, Sutton JR, Wolfe RR, Reeves JT (1992) Muscle accounts for glucose disposal but not blood lactate appearance during exercise after acclimatization to 4,300 m. J Appl Physiol 72:2435–2445
Calbet JA, De Paz JA, Garatachea N, Cabeza de Vaca S, Chavarren J (2003) Anaerobic energy provision does not limit Wingate exercise performance in endurance-trained cyclists. J Appl Physiol 94:668–676
Chapman RF, Stager JM, Tanner DA, Stray-Gundersen J, Levine BD (2011) Impairment of 3,000 m run time at altitude is influenced by arterial oxyhemoglobin saturation. Med Sci Sports Exerc 43:1649–1656
Charlot K, Pichon A, Chapelot D (2011) Exercise prior to a freely requested meal modifies pre and postprandial glucose profile, substrate oxidation and sympathovagal balance. Nutr Metab (Lond) 8:66
Clarke WL, Anderson S, Farhy L, Breton M, Gonder-Frederick L, Cox D, Kovatchev B (2005) Evaluating the clinical accuracy of two continuous glucose sensors using continuous glucose-error grid analysis. Diabetes Care 28:2412–2417
Eckberg DL (1997) Sympathovagal balance: a critical appraisal. Circulation 96:3224–3232
Eckman M, Barach B et al (1945) Effect of diet on altitude tolerance. J Aviat Med 16:328–340
Favret F, Richalet JP (2007) Exercise and hypoxia: the role of the autonomic nervous system. Respir Physiol Neurobiol 158:280–286
Ferrannini E (1988) The theoretical bases of indirect calorimetry: a review. Metabolism 37:287–301
Friedmann B, Bauer T, Menold E, Bartsch P (2004) Exercise with the intensity of the individual anaerobic threshold in acute hypoxia. Med Sci Sports Exerc 36:1737–1742
Geoffrey M, Brazg R, Richard W (2011) FreeStyle Navigator Continuous Glucose Monitoring System with TRUstart algorithm, a 1-hour warm-up time. J Diabetes Sci Technol 5:99–106
Golja P, Flander P, Klemenc M, Maver J, Princi T (2008) Carbohydrate ingestion improves oxygen delivery in acute hypoxia. High Alt Med Biol 9:53–62
Gonzalez NC, Clancy RL, Moue Y, Richalet JP (1998) Increasing maximal heart rate increases maximal O2 uptake in rats acclimatized to simulated altitude. J Appl Physiol 84:164–168
Goodwin ML, Harris JE, Hernandez A, Gladden LB (2007) Blood lactate measurements and analysis during exercise: a guide for clinicians. J Diabetes Sci Technol 1:558–569
Hansen JE, Hartley LH, Hogan RP 3rd (1972) Arterial oxygen increase by high-carbohydrate diet at altitude. J Appl Physiol 33:441–445
Hochachka PW, Beatty CL, Burelle Y, Trump ME, McKenzie DC, Matheson GO (2002) The lactate paradox in human high-altitude physiological performance. News Physiol Sci 17:122–126
Hoppeler H, Vogt M (2001) Hypoxia training for sea-level performance. Training high-living low. Adv Exp Med Biol 502:61–73
Jeukendrup AE, Wallis GA (2005) Measurement of substrate oxidation during exercise by means of gas exchange measurements. Int J Sports Med 26(Suppl 1):S28–S37
Karelis AD, Smith JW, Passe DH, Peronnet F (2010) Carbohydrate administration and exercise performance: what are the potential mechanisms involved? Sports Med 40:747–763
Klemenc M, Golja P (2011) Baroreflex sensitivity in acute hypoxia and carbohydrate loading. Eur J Appl Physiol 111:2509–2515
Klemenc M, Maver J, Princi T, Flander P, Golja P (2008) The effect of sucrose ingestion on autonomic nervous system function in young subjects during acute moderate hypoxia. Eur J Appl Physiol 104:803–812
Kovatchev BP, Shields D, Breton M (2009) Graphical and numerical evaluation of continuous glucose sensing time lag. Diabetes Technol Ther 11:139–143
Larsen PD, Tzeng YC, Sin PY, Galletly DC (2010) Respiratory sinus arrhythmia in conscious humans during spontaneous respiration. Respir Physiol Neurobiol 174:111–118
Lawless NP, Dillard TA, Torrington KG, Davis HQ, Kamimori G (1999) Improvement in hypoxemia at 4600 meters of simulated altitude with carbohydrate ingestion. Aviat Space Environ Med 70:874–878
Lundby C, Van Hall G (2002) Substrate utilization in sea level residents during exercise in acute hypoxia and after 4 weeks of acclimatization to 4100 m. Acta Physiol Scand 176:195–201
McNarry MA, Lewis MJ (2012) Heart rate variability reproducibility during exercise. Physiol Meas 33:1123–1133
Mollard P, Bourdillon N, Letournel M, Herman H, Gibert S, Pichon A, Woorons X, Richalet JP (2010) Validity of arterialized earlobe blood gases at rest and exercise in normoxia and hypoxia. Respir Physiol Neurobiol 172:179–183
Montano N, Ruscone TG, Porta A, Lombardi F, Pagani M, Malliani A (1994) Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. Circulation 90:1826–1831
Morel OE, Aubert R, Richalet JP, Chapelot D (2005) Simulated high altitude selectively decreases protein intake and lean mass gain in rats. Physiol Behav 86:145–153
Niskanen JP, Tarvainen MP, Ranta-Aho PO, Karjalainen PA (2004) Software for advanced HRV analysis. Comput Methods Programs Biomed 76:73–81
Oliver SJ, Golja P, Macdonald JH (2012) Carbohydrate supplementation and exercise performance at high altitude: a randomized controlled trial. High Alt Med Biol 13:22–31
Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, Sandrone G, Malfatto G, Dell’Orto S, Piccaluga E et al (1986) Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res 59:178–193
Paul D, Jacobs KA, Geor RJ, Hinchcliff KW (2003) No effect of pre-exercise meal on substrate metabolism and time trial performance during intense endurance exercise. Int J Sport Nutr Exerc Metab 13:489–503
Peronnet F, Massicotte D (1991) Table of nonprotein respiratory quotient: an update. Can J Sport Sci 16:23–29
Peronnet F, Massicotte D, Folch N, Melin B, Koulmann N, Jimenez C, Bourdon L, Launay JC, Savourey G (2006) Substrate utilization during prolonged exercise with ingestion of (13)C-glucose in acute hypobaric hypoxia (4,300 m). Eur J Appl Physiol 97:527–534
Pinna GD, Maestri R, Torunski A, Danilowicz-Szymanowicz L, Szwoch M, La Rovere MT, Raczak G (2007) Heart rate variability measures: a fresh look at reliability. Clin Sci (Lond) 113:131–140
Pitzalis MV, Mastropasqua F, Massari F, Forleo C, Di Maggio M, Passantino A, Colombo R, Di Biase M, Rizzon P (1996) Short- and long-term reproducibility of time and frequency domain heart rate variability measurements in normal subjects. Cardiovasc Res 32:226–233
Pivik RT, Dykman RA (2004) Cardiovascular effects of morning nutrition in preadolescents. Physiol Behav 82:295–302
Pivik RT, Dykman RA, Tennal K, Gu Y (2006) Skipping breakfast: gender effects on resting heart rate measures in preadolescents. Physiol Behav 89:270–280
Pyne DB, Boston T, Martin DT, Logan A (2000) Evaluation of the Lactate Pro blood lactate analyser. Eur J Appl Physiol 82:112–116
Rebrin K, Steil GM (2000) Can interstitial glucose assessment replace blood glucose measurements? Diabetes Technol Ther 2:461–472
Richalet JP, Larmignat P, Poitrine E, Letournel M, Canoui-Poitrine F (2012) Physiological risk factors for severe high-altitude illness: a prospective cohort study. Am J Respir Crit Care Med 185:192–198
Rowlands DS, Hopkins WG (2002) Effects of high-fat and high-carbohydrate diets on metabolism and performance in cycling. Metabolism 51:678–690
Sinnreich R, Kark JD, Friedlander Y, Sapoznikov D, Luria MH (1998) Five minute recordings of heart rate variability for population studies: repeatability and age-sex characteristics. Heart 80:156–162
Sutton JR, Coates G, Houston CS (1991) Lake Louise consensus on definition and quantification of altitude illness. In: Sutton JR, Coates G, Houston CS (eds) Hypoxia and mountain medicine. Pergamon Press, New York, pp 327–330
Swenson ER, MacDonald A, Vatheuer M, Maks C, Treadwell A, Allen R, Schoene RB (1997) Acute mountain sickness is not altered by a high carbohydrate diet nor associated with elevated circulating cytokines. Aviat Space Environ Med 68:499–503
Tanner RK, Fuller KL, Ross ML (2010) Evaluation of three portable blood lactate analysers: lactate Pro, Lactate Scout and Lactate Plus. Eur J Appl Physiol 109:551–559
Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (1996) Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 93:1043–1065
Wehrlin JP, Hallen J (2006) Linear decrease in VO2max and performance with increasing altitude in endurance athletes. Eur J Appl Physiol 96:404–412
Weippert M, Kumar M, Kreuzfeld S, Arndt D, Rieger A, Stoll R (2010) Comparison of three mobile devices for measuring R–R intervals and heart rate variability: polar S810i, Suunto t6 and an ambulatory ECG system. Eur J Appl Physiol 109:779–786
Weir JB (1949) New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109:1–9
Welle S, Lilavivat U, Campbell RG (1981) Thermic effect of feeding in man: increased plasma norepinephrine levels following glucose but not protein or fat consumption. Metabolism 30:953–958
Whitley HA, Humphreys SM, Campbell IT, Keegan MA, Jayanetti TD, Sperry DA, MacLaren DP, Reilly T, Frayn KN (1998) Metabolic and performance responses during endurance exercise after high-fat and high-carbohydrate meals. J Appl Physiol 85:418–424
Acknowledgments
The authors would like to thank subjects for their participation.
Conflict of interest
The authors declare no conflict of interest of any kind in this research.
Ethical standards
The authors certify that this experiment complied with the current French laws. The protocol was conducted according to the standards set by the Declaration of Helsinki and was officially approved by the representatives of the regional board of the National Ethics Committee (Comité de Protection des Personnes no 10).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Guido Ferretti.
Rights and permissions
About this article
Cite this article
Charlot, K., Pichon, A., Richalet, JP. et al. Effects of a high-carbohydrate versus high-protein meal on acute responses to hypoxia at rest and exercise. Eur J Appl Physiol 113, 691–702 (2013). https://doi.org/10.1007/s00421-012-2472-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-012-2472-z