Abstract
Hypoxia decreases baroreflex sensitivity (BRS) and can be a sufficient cause for syncope in healthy individuals. Carbohydrate loading enhances efferent sympathetic activity, which affects cardiac contractility, heart rate and vascular resistance, the main determinants of blood pressure. Thus, in both normoxia and hypoxia, carbohydrate loading may be more than simply metabolically beneficial, as it may affect blood pressure regulation. We hypothesised that carbohydrate loading will, in both normoxia and hypoxia, alter the regulation of blood pressure, as reflected in a change in baroreflex sensitivity. Fourteen subjects participated in two experiments, composed of a 15-min normoxic period, after which the subjects ingested water or an equal amount of water with carbohydrates. A 30-min rest period was then followed by a 10-min second normoxic and a 30-min hypoxic period. Blood pressure and heart rate were monitored continuously during the experiment to determine BRS. Despite an increased sympathetic activation, reflected in increased heart rate (P < 0.001) BRS was lower (P < 0.01) after carbohydrate loading, as compared to the water experiment, in both normoxic [23.7 (12.4) versus 28.8 (13.8) ms/mmHg] and hypoxic [16.8 (11.0) versus 24.3 (12.3) ms/mmHg] phases of the present study. As BRS was decreased in acute hypoxic exposure, the results confirm that hypoxia interferes with blood pressure regulation. However, although oral carbohydrate loading induced sympathoexcitation, it did not improve blood pressure regulation in hypoxia, as evident from the BRS data. Baroreflex effects of other forms of carbohydrate loading, not causing postprandial blood shifts to digestive system, should therefore be investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The maintenance of normal blood pressure depends on the activity of a baroreflex system. In response to a sudden increase in blood pressure, for example, baroreflex activation establishes a certain level of parasympathetic activation and sympathetic inhibition (La Rovere et al. 1995). The capability of baroreflex system to reflexly correct blood pressure to its optimal values is referred to as baroreflex sensitivity (BRS).
It is generally recognised that the reduction in nervous system responsiveness indicates the inability to adapt the body to challenging conditions (Saito et al. 2005). In the view of BRS, a lower ability of autonomic control mechanisms to compensate for blood pressure fluctuations is a sign of baroreflex malfunction (Lanfranchi and Somers 2002).
It has been proposed that hypoxia decreases BRS (Sagawa et al. 1997; Roche et al. 2002), that susceptibility to syncope may be increased at high altitude (Nicholas et al. 1992), and that exposure to acute severe hypoxia can be a sufficient cause for syncope in healthy individuals (Westendorp et al. 1997). To a certain extent, however, these negative effects of hypoxia may be attenuated by carbohydrate loading. Namely, ingestion of carbohydrates improves oxygen delivery during acute hypoxia (Golja et al. 2008) and intensifies cardiac autonomic modulation (Klemenc et al. 2008). Furthermore, as ingestion of carbohydrates increases sympathetic activity in both animals and humans (Welle et al. 1981; Berne et al. 1989; Paolisso et al. 1997), it seems reasonable to expect that carbohydrate loading would not only affect cardiac modulation (Klemenc et al. 2008; Paolisso et al. 1997) or metabolism (Opie 1995; Golja et al. 2008) in hypoxia, but that its symathoexcitatory effects (acting upon cardiac contractility, heart rate, and vascular resistance—the main determinants of blood pressure) will also alter the baroreflex blood pressure regulation.
In summary, we hypothesised that the sympatho-excitatory effects of carbohydrate ingestion will, in both normoxia and hypoxia, reduce the vagal activity at the level of sinoatrial node and affect the level of sympathetic vasoconstriction, which will, in turn, alter the baroreflex blood pressure regulation. Should this change be such to improve the baroreflex sensitivity, carbohydrate loading might be used as a factor opposing hypoxia-induced syncope.
Materials and methods
The exact protocol of the study was presented in detail elsewhere (Golja et al. 2008; Klemenc et al. 2008) the main methods are presented below.
Ethical clearance
The protocol of the study was approved by the Ethics Committee of the Republic of Slovenia.
Subjects
Fourteen young, healthy volunteers, eight females and six males, participated in the study. Mean mass of the subjects was 73 (18) kg, mean height 173 (10) cm and mean age 24 (2) years. The subjects were screened to exclude those with histories of diabetes and cardiovascular disorders. None of the subjects had lung disease and none of them reported breathing disorders including obstructive sleep apnea. The subjects did not take any medications. They were asked to refrain from alcohol and caffeine and to avoid any physically demanding activities on the day prior to the experiment, or on the day of the experiment. Also, they were instructed to consume nothing but water ad libitum on the day of the experiment. To eliminate the effects of circadian rhythm, both experiments were performed at the same time of the day. To eliminate the effects of menstrual cycle, all eight female subjects were tested in follicular phase of the menstrual cycle.
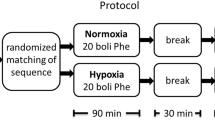
Protocol
The study was composed of two similar experiments, which were both performed in the morning and were separated by a minimum of 24 h.
At the beginning of the experiment, a continuous blood pressure monitor and electrocardiogram (Colin BP-508, Komaki City, Japan), as well as a finger pulse oximeter (Nellcor Oximax N-550, Pleasanton, CA, USA) were mounted, and glucose concentration in capillary blood was determined with a glucose monitor (Accutrend GCT, Roche Diagnostics, Germany). The subjects were provided with a mouthpiece and a sample of air from the mixing box, connected to the expiratory side of the mouthpiece, was continuously analysed with O2/CO2 gas analyser (Servomex 1440, Crowborough, UK). Subjects rested supine on an examination table throughout the experiment.
The order of the two experiments was balanced between the subjects. Both experiments were composed of a 15 min control normoxic period (first normoxia, FN), after which the subjects ingested either water (Water) or water with carbohydrates (CHO). In the CHO experiment, each subject ingested a 10% water solution of sucrose in the amount of 4 kcal per kg body mass (4 kcal ≈ 1 g sucrose). In the Water experiment, the subjects ingested an equal volume of water.
As soon as the subjects ingested the drink, a rest period of 30 min was initiated to allow enough time for carbohydrate absorption; no data were recorded during this time. Following the 30-min rest period, the second normoxic interval of 10 min was recorded (second normoxia, SN). Then, gas mixture was switched between normoxic and hypoxic (12.86% O2 in N2) gas mixture without the subjects’ knowledge. The subjects inspired the hypoxic gas mixture for 30 min. The following hypoxic intervals were then recorded: first hypoxia (FH) between 1st and 10th minute, second hypoxia (SH) between 11th and 20th minute, and third hypoxia (TH) between 21st and 30th minute. In all subjects, glucose concentration in capillary blood was again determined in the middle of hypoxic exposure. We have intentionally controlled blood glucose at two time points only, first, because the dynamics of glucose uptake into blood after glucose ingestion has been well described previously (Daly et al. 2000), and second, more importantly, because blood sampling is an invasive sympatho-excitatory procedure, which can interfere with autonomic responses associated with BRS. Furthermore, ECG, oxygen saturation (SaO2, %) and blood pressure (SAP, DAP, mmHg) were continuously monitored during both the experiments.
BRS analysis
In the present study, an indirect technique for measuring the baroreflex sensitivity was used. Namely, changes in cardiac R–R interval (in ms) were measured against a given change in blood pressure (in mmHg) (Lanfranchi and Somers 2002). A continuous blood pressure measurement was performed by applanation tonometry method with a Colin monitor (Colin BP-508, Komaki City, Japan), which was shown to provide reliable beat-to-beat measurement of blood pressure in a variety of autonomic testing conditions, as compared to the standard intra-arterial monitoring (Kemmotsu et al. 1991). Recordings of blood pressure and ECG signal were continuously digitalised and stored to a computer.
Sequences of three or more consecutive heart beats in which progressive increases/decreases in systolic blood pressure were followed by progressive decreases/increases in heart rate (measured as progressive lengthening/shortening of cardiac R–R intervals) were first identified (Bertinieri et al. 1985). The criteria to detect a sequence was an increase/decrease in systolic blood pressure of more than 1 mmHg and a concordant change in R–R interval of more than 4 ms. Nevrokard BRS Analysis software (Nevrokard, Izola, Slovenia) was used for this purpose. On each sequence, linear regression was performed. Finally, from all the regression lines obtained from all sequences and using a high r 2 value (≥0.85), a single mean linear relation was calculated. Its slope was recognised as baroreflex sensitivity (Lanfranchi and Somers 2002; Bertinieri et al. 1985). Namely, the higher the slope, the greater the change in heart rate to a given change in blood pressure, thus the higher BRS and the higher the efficiency of autonomic reflex mechanisms to correct for the fluctuations in blood pressure.
Statistical analysis
Differences between and within the two experiments were assessed with a two-factor analysis of variance (ANOVA) with repeated measures on both factors. Whenever ANOVA yielded significant differences, data were further analysed with a Tukey HSD post hoc test. The level of <0.05 was adopted as statistically significant. All data are presented as mean (standard deviation).
Results
Blood glucose
In the water experiment, glucose concentration in capillary blood was 4.9 (0.8) mmol/L at the beginning, and 4.6 (1.1) mmol/L in the middle of hypoxic period, thus 55 min following the ingestion; the difference was not statistically significant (P > 0.05). In the CHO experiment, glucose concentration was 4.5 (0.8) mmol/L at the beginning of the experiment, and was significantly (P < 0.001) increased to 6.8 (1.6) mmol/L in the middle of hypoxic period. The difference in glucose concentration between the two trials was not statistically significant (P > 0.05) at the beginning of the experiment, but was highly significant (P < 0.001) in the hypoxic period.
Haemoglobin saturation
During normoxia, hemoglobin saturation did not differ (P > 0.05) between the two experiments. It decreased in both experiments during the hypoxic exposure. Haemoglobin saturation decreased from 99 (1)% during normoxia to 86 (6)% in the last third of hypoxia in the water experiment and from 99 (1)% in normoxia to 90 (5)% in the last third of hypoxia in the CHO experiment. Haemoglobin saturation was significantly (P < 0.001) higher in the CHO as compared to the water experiment throughout the hypoxic exposure. See Golja et al. (2008) for a detailed explanation of ventilatory responses following carbohydrate loading.
Heart rate
Heart rate was similar between the two experiments in the FN (62 (8) min−1 in the CHO; 63 (7) min−1 in the water experiment), but was significantly (P < 0.001) higher in the CHO (66 (10) min−1), as compared to the water experiment (60 (7) min−1) in the SN. In both experiments, heart rate was further increased by hypoxia (77 (12) min−1 and 75 (12) min−1 in the SH and TH of the CHO; 71 (9) min−1and 73 (10) min−1 in the SH and TH of the water experiment). Heart rate was significantly higher (P < 0.001) in the CHO as compared to the water experiment in the SH phase, but the difference decreased in the TH phase, where it did not reach statistical significance.
Systolic blood pressure
Compared to the first normoxia period (111 (11) mmHg), systolic arterial pressure remained statistically unchanged in the second normoxic period (117 (11) mmHg), and increased (P < 0.01) during the second (125 (12) mmHg) and last third (124 (15) mmHg) of hypoxic exposure in the CHO experiment. Systolic arterial pressure remained statistically unchanged throughout the control experiment, with the values of 118 (15), 116 (14), 125 (16), and 123 (21) mmHg for FN, SN, SH and TH, respectively. Systolic arterial pressure was not significantly different (P > 0.05) between the two experiments at any phase of the experiment (Fig. 1).
Diastolic blood pressure
Compared to the first normoxia period [61 (10) mmHg], diastolic arterial pressure remained statistically unchanged in the second normoxic period [63 (9) mmHg], and increased (P < 0.01) in the last third [70 (12) mmHg] of the hypoxic exposure in the CHO experiment. In the control experiment, DAP remained statistically unchanged throughout the experiment, with the values of 68 (11), 63 (10), 65 (11) and 66 (12) mmHg for FN, SN, SH and TH, respectively. Apart from first normoxia (P < 0.05), diastolic arterial pressure was not significantly different (P > 0.05) between the two experiments at any other phase of the experiment (Fig. 1).
Baroreflex sensitivity
Ingestion of water or carbohydrates induced differences in BRS between the two experiments already in the second normoxic period, with BRS being lower (P < 0.01) in the CHO experiment. Baroreflex sensitivity, expressed as an average change in R–R interval (ms) to a change in systolic blood pressure (mmHg) in different phases of the two experiments is presented in Table 1.
Baroreflex sensitivity then decreased significantly with hypoxic exposure in both experiments, with BRS remaining significantly lower in CHO, as compared to the control experiment. The difference between the two experiments diminished towards the end of the hypoxic exposure, when, according to the existing data (Daly et al. 2000), the effects of carbohydrate loading fade. Relative changes in baroreflex sensitivity, as compared to the first normoxia (baseline) condition are presented on Fig. 2.
Discussion
The results of the present study confirm that baroreflex sensitivity is decreased in acute hypoxia, which is in agreement with previous reports (Sagawa et al. 1997; Roche et al. 2002). The observed decrease in baroreflex sensitivity in hypoxia sheds some light on why acute exposure to hypoxia can result in syncope (Nicholas et al. 1992; Westendorp et al. 1997). Furthermore, in contrast to the potentially beneficial higher autonomic cardiac modulation, which was observed in hypoxia after carbohydrate loading (Klemenc et al. 2008), the results of the present study suggest that oral carbohydrate loading, although it evidently causes sympathoexcitation, exerts no beneficial effect on the baroreflex blood pressure regulation, as evident from a significantly lower baroreflex sensitivity in the CHO, as compared to the control experiment, in both normoxic and hypoxic phases of the present study.
It is expected that the results of the present study are a consequence of two main interfering reflexes, a chemoreflex and a baroreflex. First, chemoreflex was activated by a decrease in partial pressure of oxygen in the arterial blood. As chemoreflex is sympato-excitatory reflex in nature (Du and Chen 2007), exposure to hypoxia increases sympathetic nerve discharge directed to vascular beds (Saito et al. 1988), ventilation, heart rate and blood pressure (Marshall 1994). Apart from sympathetic nerve discharge, which was not measured in the present study, all the other responses were observed [for discussion of ventilation results see Golja et al. (2008), for sympathetic cardiac modulation see Klemenc et al. (2008)].
In addition to chemoreflex-induced sympathetic excitation due to hypoxia, ingestion of carbohydrates exerted an additional sympatho-excitatory effect, evidently both in normoxic and hypoxic conditions of the study. However, although heart rate was increased after CHO ingestion due to sympathetic activation, no differences in arterial blood pressure were observed between the two conditions. We believe this was due to the second reflex, active in the present study, i.e. the baroreflex.
In contrast to chemoreflex, baroreflex is a sympatho-inhibitory reflex in nature (Du and Chen 2007). Baroreflex blood pressure regulation is achieved by the regulation of sympathetic outflow to peripheral vasculature, which determines the vascular resistance to blood flow (van de Vooren et al. 2007; Du and Chen 2007; Liu et al. 2002) and venous return (Pang 2001), as well as by both parasympathetic and sympathetic (Lanfranchi and Somers 2002) regulation of cardiac contractility and heart rate. There is no reason to believe that cardiac contractility was decreased in the present study. Therefore, as heart rate was higher in the CHO experiment, and as blood pressure did not vary between the two conditions, the compensatory baroreflex reduction of vascular resistance must have taken place.
In hypoxic periods of the present study, baroreflex sensitivity was diminished in both, CHO and water experiment, with BRS being even lower in CHO than in water experiment. This suggests that the effects of CHO ingestion were superimposed to those of hypoxia. If one examines the mathematical formula (ΔBP/ΔRR) that denotes each single sequence, from which baroreflex sensitivity is calculated, it becomes clear that decreasing the denominator at a similar blood pressure change would result in higher BRS. Both, hypoxia and carbohydrate loading decrease the denominator, but BRS was nevertheless lower (not higher) in the present experiment. This suggests that, mathematically speaking, the numerator must have also been diminished accordingly to result in lower BRS. The numerator (ΔBP) is affected by altering either cardiac output or total peripheral resistance. Any changes in cardiac output induced by hypoxia or carbohydrate loading would result in the opposite effect than actually observed, so it is the total peripheral resistance that remains to be discussed.
We know that acute hypoxia will not only induce higher muscle sympathetic nerve activity (Saito et al. 1988), but also induce vasodilatation locally. This will diminish total peripheral resistance and transiently decrease venous return. As diminished venous return will provide a smaller window for spontaneous fluctuations of blood pressure, ΔBP will be lower, which will then result in a smaller BRS. Furthermore, a baroreflex sympatho-inhibitory reduction of peripheral resistance [being a compensatory action to hypoxic (central and cardiac) sympathoexcitation] would also exert the same effect on BRS. Although peripheral resistance was not measured in the present experiment, the observations of Somers et al. (1991), who investigated chemo- and baroreflex control of muscle sympathetic nerve activity and concluded that in hypoxia baroreflex activation can abolish muscle sympathetic nerve activity, speak in favour of such explanation. Finally, as supported by the results of the present study, a hypertonic solution in digestive system, although sympathoexcitatory in nature, would cause a shift of blood towards the digestive system, which would in turn affect venous return and thus decrease BRS—a phenomenon that has recently been reported (Brown et al. 2008) and could explain a lower BRS in CHO as compared to water experiment in the present study. Putting it all together, a combination of local hypoxic vasodilatation, combined with a higher baroreflex inhibition of sympathetic outflow (being a compensatory reaction to hypoxic sympathoexcitation), would cause a well documented diminishment of BRS at altitude and may be an underlying factor for higher incidence of syncope at high altitude.
It has to be noted that some authors (Halliwill and Minson 2002, 2005) did not observe any changes in BRS during acute normobaric hypoxia (oxygen fraction in inspired air (FiO2) = 0.12; partial pressure of oxygen (PiO2) = 91 mmHg), but the absence of effect might be at least partially explained by the level of hypoxia used. Namely, authors who assessed BRS in a more severe hypoxia, reported a decrease in BRS in both hypobaric (Sagawa et al. 1997) and normobaric (Roche et al. 2002) hypoxic conditions. Sagawa et al. (1997), for example, observed a decrease in BRS with hypoxia level of PiO2 = 82 mmHg (= 4,300 m), but not 90 mmHg (=3,800 m). Similarly, Roche et al. (2002) reported tachycardia and a decrease in BRS without any significant alteration of the systolic and diastolic pressure with hypoxia level of PiO2 = 84 mmHg (FiO2 = 0.11). However, as the level of hypoxia used in the present study was PiO2 = 98 mmHg (FiO2 = 0.1286) and we still observed a significant diminishment in BRS, the level of hypoxia used cannot explain the lack of hypoxic effect on BRS in the studies of Halliwill and Minson (2002, 2005). Instead, a shorter measuring interval [3 min (Halliwill and Minson 2002) compared to 10 min in the present study (which was likely more informative due to higher number of sequences detected)] or a different methodological approach [observations of minute heart rate (Halliwill and Minson 2002, 2005) compared to R–R intervals in the present study] may explain the differences between their results and the results of Sagawa et al. (1997), Roche et al. (2002), and the present study.
Finally, although blood pressure values, which were similar between the two experiments in all phases of the experiment, do not support the phenomenon of postprandial hypotension, a significantly reduced BRS in the CHO experiment, when compared to the water experiment, suggests that BRS was likely affected by a postprandial shift of blood to the digestive system, which altered blood pressure regulation in a way to counteract the observed sympathoexcitatory effects of carbohydrate loading. Thus, although carbohydrate loading does have the potential to alter blood pressure regulation due to its sympathoexcitatory nature, the ingestion of carbohydrates may not be the suitable application of carbohydrates to the body due to the postprandial blood shifts to the digestive system.
Before drawing the final conclusions, we believe some of the study limitations have to be noted. First, we have to keep in mind that BRS is not a direct reflection of blood pressure buffering (van de Vooren et al. 2007). However, assessing BRS will nevertheless provide indirect information about the efficiency of baroreflex system to maintain optimal blood pressure in a variety of conditions (Jordan et al. 2002). Second, syncope at high altitude is most often induced within the first 24 h of arrival at high altitude (Nicholas et al. 1992), thus over a time period which is usually longer than the majority of experimental acute hypoxic exposures. Thus, in real life, a longer interplay between local, respiratory, cardiovascular, and cerebrovascular control systems takes place than in experimental conditions, but mid-term measurements of parameters, such as, for example, muscle sympathetic nerve activity that may significantly add to the conclusions of the present study, may well not be feasible over such time period. Nevertheless, a few results on the interactive effects of chemoreflex and baroreflex control on muscle sympathetic nerve activity that have been reported by now (Somers et al. 1991), speak in favour of our conclusions. And third, syncope is a multifactor event (Blaber et al. 2003), thus investigating factors that affect BRS at high altitude will provide only a limited, yet indispensable insight into the associated physiological mechanisms.
Conclusions
The results of the present study suggest that hypoxia interferes with blood pressure regulation, as baroreflex sensitivity is decreased in acute hypoxic conditions. A compensatory interplay between chemo- and baroreflex responses may explain why acute hypoxic exposure can result in syncope. Furthermore, although the sympathoexcitatory effects of carbohydrate loading are evident, the results of the present study suggest that oral ingestion of carbohydrates has no beneficial effect on baroreflex blood pressure regulation in hypoxia, possibly because a postprandial shift of blood to the digestive system after carbohydrate ingestion may counteract the sympathoexcitatory effects of carbohydrate loading.
References
Berne C, Fagius J, Niklasson F (1989) Sympathetic response to oral carbohydrate administration. Evidence from microelectrode nerve recordings. J Clin Invest 84:1403–1409
Bertinieri G, Di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A, Mancia G (1985) A new approach to analysis of the arterial baroreflex. J Hypertens Suppl 3:S79–S81
Blaber AP, Hartley T, Pretorius PJ (2003) Effect of acute exposure to 3660 m altitude on orthostatic responses and tolerance. J Appl Physiol 95:591–601
Brown CM, Dulloo AG, Yepuri G, Montani JP (2008) Fructose ingestion acutely elevates blood pressure in healthy young humans. Am J Physiol Regul Integr Comp Physiol 294:R730–R737
Daly ME, Vale C, Walker M, Littlefield A, George K, Alberti M, Mathers J (2000) Acute fuel selection in response to high-sucrose and high-starch meals in healthy men. Am J Clin Nutr 71:1516–1524
Du YH, Chen AF (2007) A “love triangle” elicited by electrochemistry—complex interactions amongst cardiac sympathetic afferent, chemo- and baro-reflexes. J Appl Physiol 102:9–10
Golja P, Flander P, Klemenc M, Maver J, Princi T (2008) Carbohydrate ingestion improves oxygen delivery in acute hypoxia. High Alt Med Biol 9:53–62
Halliwill JR, Minson CT (2002) Effect of hypoxia on arterial baroreflex control of heart rate and muscle sympathetic nerve activity in humans. J Appl Physiol 93:857–864
Halliwill JR, Minson CT (2005) Cardiovagal regulation during combined hypoxic and orthostatic stress: fainters vs. nonfainters. J Appl Physiol 98:1050–1056
Jordan J, Tank J, Shannon JR, Diedrich A, Lipp A, Schroder C, Arnold G, Sharma AM, Biaggioni I, Robertson D, Luft FC (2002) Baroreflex buffering and susceptibility to vasoactive drugs. Circulation 105:1459–1464
Kemmotsu O, Ueda M, Otsuka H, Yamamura T, Winter DC, Eckerle JS (1991) Arterial tonometry for noninvasive, continuous blood pressure monitoring during anesthesia. Anesthesiology 75:333–340
Klemenc M, Maver J, Princi T, Flander P, Golja P (2008) The effect of sucrose ingestion on autonomic nervous system function in young subjects during acute moderate hypoxia. Eur J Appl Physiol 104:803–812
La Rovere MT, Mortara A, Schwartz PJ (1995) Baroreflex sensitivity. J Cardiovasc Electrophysiol 6:761–774
Lanfranchi PA, Somers VK (2002) Arterial baroreflex function and cardiovascular variability: interactions and implications. Am J Physiol Regul Integr Comp Physiol 283:R815–R826
Liu HK, Guild SJ, Ringwood JV, Barrett CJ, Leonard BL, Nguang SK, Navakatikyan MA, Malpas SC (2002) Dynamic baroreflex control of blood pressure: influence of the heart vs. peripheral resistance. Am J Physiol Regul Integr Comp Physiol 283:R533–R542
Marshall JM (1994) Peripheral chemoreceptors and cardiovascular regulation. Physiol Rev 74:543–594
Nicholas R, O’Meara PD, Calonge N (1992) Is syncope related to moderate altitude exposure? JAMA 268:904–906
Opie LH (1995) Glucose and the metabolism of ischaemic myocardium. Lancet 345:1520–1521
Pang CC (2001) Autonomic control of the venous system in health and disease: effects of drugs. Pharmacol Ther 90:179–230
Paolisso G, Manzella D, Ferrara N, Gambardella A, Abete P, Tagliamonte MR, De Lucia D, Furgi G, Picone C, Gentile S, Rengo F, Varricchio M (1997) Glucose ingestion affects cardiac ANS in healthy subjects with different amounts of body fat. Am J Physiol 273:E471–E478
Roche F, Reynaud C, Garet M, Pichot V, Costes F, Barthelemy JC (2002) Cardiac baroreflex control in humans during and immediately after brief exposure to simulated high altitude. Clin Physiol Funct Imaging 22:301–306
Sagawa S, Torii R, Nagaya K, Wada F, Endo Y, Shiraki K (1997) Carotid baroreflex control of heart rate during acute exposure to simulated altitudes of 3,800 m and 4,300 m. Am J Physiol 273:R1219–R1223
Saito M, Mano T, Iwase S, Koga K, Abe H, Yamazaki Y (1988) Responses in muscle sympathetic activity to acute hypoxia in humans. J Appl Physiol 65:1548–1552
Saito S, Tanobe K, Yamada M, Nishihara F (2005) Relationship between arterial oxygen saturation and heart rate variability at high altitudes. Am J Emerg Med 23:8–12
Somers VK, Mark AL, Abboud FM (1991) Interaction of baroreceptor and chemoreceptor reflex control of sympathetic nerve activity in normal humans. J Clin Invest 87:1953–1957
van de Vooren H, Gademan MG, Swenne CA, TenVoorde BJ, Schalij MJ, Van der Wall EE (2007) Baroreflex sensitivity, blood pressure buffering, and resonance: what are the links? Computer simulation of healthy subjects and heart failure patients. J Appl Physiol 102:1348–1356
Welle S, Lilavivat U, Campbell RG (1981) Thermic effect of feeding in man: increased plasma norepinephrine levels following glucose but not protein or fat consumption. Metabolism 30:953–958
Westendorp RG, Blauw GJ, Frolich M, Simons R (1997) Hypoxic syncope. Aviat Space Environ Med 68:410–414
Acknowledgments
P. Golja was a recipient of a post-doc scholarship of the Slovenian Ministry of Science and Higher Education.
Conflict of interest
The authors declare no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Narihiko Kondo.
Rights and permissions
About this article
Cite this article
Klemenc, M., Golja, P. Baroreflex sensitivity in acute hypoxia and carbohydrate loading. Eur J Appl Physiol 111, 2509–2515 (2011). https://doi.org/10.1007/s00421-011-1875-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-011-1875-6