Abstract
This study investigated the contribution of muscle architecture to the differences in the torque–velocity and power–velocity relationships between older (OM n = 9, aged 69–82 years) and younger men (YM n = 15, aged 19–35 years). Plantarflexors’ (PF) maximal isometric and concentric torques were recorded at 0.87, 1.75, 2.62, 3.49 and 4.36 rad s−1. Physiological cross-sectional area (PCSA) was calculated as the ratio of muscle volume (determined by magnetic resonance imaging) to muscle fascicle length (L f, measured by ultrasonography). GM PCSA and L f of the OM were, respectively, 14.3% (P < 0.05) and 19.3% (P < 0.05) smaller than of the YM. In the OM, GM maximum isometric torque and maximum contraction velocity (V max), estimated from Hill’s equation were, respectively, 48.5 and 38.2% lower (P < 0.001) than in the YM. At all contraction velocities, the OM produced less torque than the YM (46.3% of YM at 0.87 rad s−1 to 14.7% at 4.36 rad s−1, P < 0.001). Peak power (PP) of the OM was 80% lower than that of the YM and normalisation of PP to muscle volume only reduced this difference by 10%. Normalisation of torque to PCSA reduced, but did not eliminate, differences in torque between YM and OM (9.6%) and differences in torque/PCSA increased with contraction velocity (P < 0.05). After normalisation of velocity to L f, the difference in V max between the OM and the YM was reduced to 15.9%. Thus, although muscle architecture contributes significantly to the differences in the torque– and power–velocity properties of OM and YM, other contractile factors, intrinsic to the muscle, seem to play a role. It is noteworthy that the deficit in PP between OM and YM is far greater than that of muscle torque, even after normalisation of PP to muscle volume. This finding likely plays an important role in the loss of mobility in old age.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The torque–velocity relationship is known to be markedly affected by ageing (Vandervoort et al. 1990; Poulin et al. 1992; Hortobagyi et al. 1995; Porter et al. 1995, 1997; Lindle et al. 1997; Gajdosik et al. 1999; Lynch et al. 1999; Hughes et al. 2001; Pousson et al. 2001; Lanza et al. 2003; Bazzucchi et al. 2005). The loss of torque has been shown to be contraction mode-specific. Since eccentric torque seems to be preserved in older individuals (Vandervoort et al. 1990; Poulin et al. 1992; Hortobagyi et al. 1995; Porter et al. 1995, 1997; Lindle et al. 1997; Lynch et al. 1999), while isometric (Jubrias et al. 1997; Frontera et al. 2000a; Akima et al. 2001; Klein et al. 2001) and concentric torques have been found to be markedly depressed (Vandervoort et al. 1990; Poulin et al. 1992; Hortobagyi et al. 1995; Porter et al. 1995; Lindle et al. 1997; Lynch et al. 1999; Hughes et al. 2001; Pousson et al. 2001). The deficit in concentric peak torque between young and elderly individuals has also been found to increase with contraction velocity (Gajdosik et al. 1999; Frontera et al. 2000a; Lanza et al. 2003; Bazzucchi et al. 2005).
The main cause of the lower isometric torques produced by older individuals is the reduction in muscle cross-sectional area (CSA), reflecting a loss of sarcomeres in parallel (Doherty 2003; Morse et al. 2005). However, in the case of dynamic contractions, the loss of torque should be due to both a decrease in the number of sarcomeres in parallel and also in series (fascicle length) since both CSA and fascicle length are determinants of the force–velocity relationship (Lieber and Friden 2000). We have previously shown that sarcopenia is not only associated with a decrease in muscle physiological CSA (PCSA) but also with a decrease in the intrinsic force of skeletal muscle (force/PCSA). In two previous studies we also showed that fascicle length of the plantarflexor muscles is significantly shorter in older individuals compared to younger adults (Narici et al. 2003; Morse et al. 2005). These two findings predict that muscle architecture plays a substantial role in the loss of dynamic torque in old age. However, no study so far has investigated the role of muscle architecture on the changes of the torque–velocity characteristics with old age. Hence the purpose of the present study was to compare the torque-velocity (T–V) properties of young and older individuals and assess to what extent differences in T–V properties could be explained by changes in muscle architecture.
Methods
Participants
Twenty-four healthy, recreationally active male volunteers participated in this study; nine of whom were older men (OM age 74.7 ± 4.0 years (range 69–82 years), height 1.723 ± 0.046 m, mass 76.6 ± 8.0 kg, mean ± SD) and 15 of whom were young men (YM age 25.3 ± 4.5 years (range 19–35 years), height 1.790 ± 0.074 m, mass 79.8 ± 10.9 kg). All OM participants were medically screened by their General Practitioner prior to inclusion in the study; the YM were also screened, but via a health status questionnaire. Exclusion criteria included known cardiovascular, myopathic, neurological and inflammatory conditions. All participants gave written informed consent prior to commencement of the study and were familiarised with the proceedings on a separate test session prior to data collection. The study was approved by the local University Ethics Committee.
Muscle isometric and concentric torque
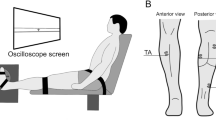
Plantarflexor torque measurements were assessed using a Cybex® dynamometer (Cybex Norm, Cybex International Inc., NY, USA) with the participants lying prone, the ankle at 0° (neutral) and the knee in extension. The left foot was tightly secured to the footplate with straps to minimise heel displacement during contractions and was positioned on the footplate so that the lateral malleoli was aligned to the dynamometers’ axis of rotation.
Following a familiarisation session, three warm up isometric maximal voluntary contractions (MVC) were attempted prior to two MVCs performed at 0°, with at least 1 min rest between contractions. Maximal concentric isokinetic torque of the plantarflexors was then measured at 0.87, 1.75, 2.62, 3.49 and 4.36 rad s−1 (50, 100, 150, 200 and 250° s-1) to assess the torque–velocity relationship. The angular velocities of the isokinetic contractions were distributed in a randomised order. At each velocity, four concentric contractions were performed in succession through the range of motion from −20° to 30°, with the foot placed at −20° prior to each movement. Verbal encouragement was given for all contractions, with at least 1 min rest between contractions and 5 min rest between the different types of contractions. Data was sampled with a Macintosh G4 computer using a data acquisition system (Biopac Systems Inc., USA). Maximal isokinetic torque was determined from the peak torque at 0° for each velocity. The torque–velocity relationship of the gastrocnemius medialis muscle (GM) was determined from the observed peak torque values corrected for the relative portion of the GM volume in the plantarflexors (assumed to be 21.8% in YM and 20.9% in OM; Morse et al. 2005).
The GM torque–velocity experimental values were then fitted with Hill’s equation: (P + a)(V + b) = (Po + a) b (Hill 1938), where a and b are constants with the dimension of force and velocity, respectively. In order to obtain these consants, Hill’s hyperbolic curve was linearised by plotting (Po−V)/V versus P, where Po is the maximal isometric torque (MVC), P is the torque developed at different velocities of shortening, and V is the velocity of shortening. This process yields a linear curve of intercept a/b and slope 1/b. To estimate the role of muscle architecture on the torque–velocity relationship of YM and OM, torque was normalised to PCSA (thereby accounting for differences in the number of sarcomeres in-parallel) and velocity was normalised to fibre fascicle length (thereby accounting for differences in the number of sarcomeres in-series).
Muscle volume
Magnetic resonance imaging (MRI) was used to assess muscle volume of the GM muscle. Serial axial plane scans were performed along the length of the GM using a fixed 0.2-T MRI scanner (E-Scan; ESAOTE Biomedica, Genova, Italy). Scans were performed using a T1 weighted 3D isotropic profile with the following scanning parameters time to echo 16 ms; repetition time 38 ms; field of view 180 × 180 mm; matrix 256 × 192 pixels. Participants were supine throughout the scanning process and for 15 min prior to adjust for fluid shifts. The initial scan was centred over the knee to include the origin of both heads of the gastrocnemius muscle, with subsequent scans performed distally. A total of three to four scans were obtained, with each scan containing seven axial images 1 cm apart (slice thickness 8 mm and gap 2 mm). Cod liver oil markers on the skin were used to align each scan with the previous one. In total, the distance covered by the axial packages was 280 mm.
From each slice the anatomical cross sectional area (ACSA) of the GM was measured using imaging software (NIH Image, version 1.61/ppc; National Institutes of Health, Bethesda, USA). The sum of the ACSAs by the number of scans (1 cm thickness) was used to estimate the muscle volume of the GM (VOL).
Muscle architecture
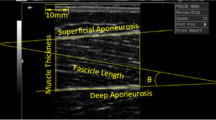
Muscle architecture measurements were assessed using B-Mode, real time ultrasonography (HDI-3000, ATL, Bothell, USA) as described previously (Morse et al. 2005). The participants laid prone, with the foot secured at 0° using the Cybex dynamometer as described above. Images were obtained along the mid-sagittal line of the GM at the mid distance between the proximal and distal tendon insertions of each muscle identified by ultrasound (7.5 MHz linear array probe), ensuring that the orientation of the ultrasound probe coincided with that of the muscle fascicle orientation. The head of the probe, covered with water-soluble transmission gel, was held perpendicular to the dermal surface to provide an image including both superficial and deep aponeurosis, and a number of clearly visible fasciculi that could be followed between the aponeuroses. Ultrasound scans were recorded on VHS and subsequently digitised on a Macintosh G4 computer and analyzed off-line with image analysis software (NIH image version 1.61/ppc, National Institute of Health, Bethesda, USA). Resting GM fibre fascicle length (L f) was defined as the length of the fascicle between the deep and superficial aponeuroses. Where a small portion of a fascicle extended beyond which was acquired in the ultrasound image, the length of the missing portion was estimated using linear extrapolation of the fascicle and aponeurosis.
Muscle PCSA
Physiological cross sectional area [PCSA (cm2)] of the GM was calculated as the ratio between VOL (cm3) and Lf (cm).
Statistical analyses
Torque–velocity data were analysed using repeated measures ANOVA (group × velocity) (SPSS software package, version 11.5). Statistical significance between groups was assessed using independent Student’s t tests. Data were expressed as mean ± SE unless otherwise stated. Differences were considered significant at P < 0.05.
Results
Compared to the YM, GM muscle volume of the OM was 31.3% smaller (303.3 ± 16.8 and 208.2 ± 7.3 cm3, respectively, P < 0.001), L f of the GM was 19.3% shorter (62.7 ± 3.2 and 50.6 ± 1.6 mm, respectively, P < 0.05) and GM PCSA was 14.3% smaller (48.5 ± 1.7 and 41.6 ± 2.2 cm2, respectively, P < 0.05). The torque–velocity (T–V) and power–velocity curves for the OM and YM fitted with Hill’s equation are presented in Fig. 1. The isometric GM torque of the OM was 51.5% that of the YM (OM 13.6 ± 1.1 Nm and YM 26.4 ± 1.8 Nm, P < 0.001) and the estimated maximum velocity of shortening (V max) was 61.9% that of the YM (6.89 and 11.14 rad s−1, respectively, Fig. 1). At all contraction velocities, the OM produced significantly less torque than the YM (46.3% of YM at 0.87 rad s−1 to 14.7% of YM at 4.36 rad s−1, P < 0.001). When Hill’s constants were compared, the mean value of a/Po (concavity of Hill’s curve), were higher in the OM than in the YM (0.35 for the OM and 0.28 for the YM, P < 0.05). Also, the mean value of b/Vo was higher in the OM than in the YM (0.38 in the OM and 0.28 in the YM, P < 0.01).
Both absolute peak power (PP) and the optimum velocity at peak power (V opt) were lower in the OM (Fig. 1), for PP was 10.7 ± 1.4 W in the OM and 53.4 ± 6.1 W in the YM (81% difference, P < 0.001) and V opt was 2.6 ± 0.3 rad s−1 in the OM and 6.1 ± 0.8 rad s−1 in the YM (62% difference, P < 0.001).
PP normalised to muscle volume was 83.8 ± 13.4 W l−1 in the OM and 303.1 ± 25.0 W l−1 in the YM (P < 0.001), that is to say, 72% lower in the OM.
Figure 2 shows the Hill’s plots of the normalised torque/PCSA-velocity curves for YM and OM. As it may be observed from this figure, normalisation of each individuals torque to PCSA reduced but did not eliminate the difference in absolute torque between the YM and OM. In isometric conditions, the torque normalised to PCSA of the OM was 61.0% of that of the YM (0.34 ± 0.03 N m cm−2 and 0.55 ± 0.04 N m cm−2, respectively), compared to a ratio of 51.4% of absolute OM and YM torques. That is to say, the difference in PCSA accounted for 9.6% of the difference in absolute torque between the OM and the YM, leaving 39% of the discrepancy in torque/PCSA accounted for by other factors. The difference in normalised torque became greater with increasing velocity (44.6% at 0.87 rad s−1, 56.3% at 1.75 rad s−1, 67.9% at 2.62 rad s−1, 74.1% at 3.49 rad s−1 and 81.8% at 4.36 rad s−1, P < 0.05). To account for differences in fibre length (thus in the number of sarcomere in series) the torque–velocity curves of the OM and YM fitted with Hill’s equation were plotted by normalising velocity to the respective participants GM fascicle lengths (Fig. 3). In order to operate this normalisation, the velocity in radians s−1 was converted to velocity in mm s−1 by multiplying the value in rad s−1 by 16.0, a conversion factor enabling to express velocity from radial to a linear units published by Wickiewicz et al. (1984, c.f. Table 4). As shown by Fig. 3, normalisation of the torque–velocity curves to fascicle length reduced the difference in the estimated maximum shortening velocity of the OM and YM curves. Before normalisation, the estimated V max of the OM was 61.8% that of the YM (Fig. 1). After normalisation to fascicle length, V max of the OM was 77.7% that of the YM; that is to say, the difference in Lf accounted for 15.9% of the difference V max between the OM and the YM.
Discussion
The present study has shown that muscle architecture significantly contributes to the loss of dynamic torque in old age. The GM muscle of older men was weaker at all contraction velocities in comparison to the younger males. In order to appreciate the influence of muscle architecture to the loss of static and dynamic torque, the effect of changes in muscle PCSA ought to be considered.
Influence PCSA on the torque–velocity relationship
The loss of torque was not fully accounted for by the smaller PCSA of the OM. The isometric torque of the OM was 49% lower than that of the YM, while PCSA of the OM was only 14.3% smaller than that of the YM. Thus, when the torque–velocity relation was normalised to PCSA, that is to say for the total number of sarcomere in parallel, at all velocities, the specific torque (torque/PCSA) of the OM was still significantly lower (by 44–82%) than that of the YM. Therefore, factors other than muscle size must account for this phenomenon; these may include: (1) a deterioration of muscle quality (single fibre specific force), (2) a reduced agonist muscle activation in the OM, (3) an increased antagonist muscle coactivation, and (4) a shift in the length-tension relation of muscle fibres with old age, due to the shorter fascicle length. Several studies have shown a lower specific force of muscle fibres of older individuals (Larsson et al. 1997; D’Antona et al. 2003; Frontera et al. 2000b). This phenomenon has been attributed both to a decrease in the number of cross-bridges, due to decreased myofibrilar density (D’Antona et al. 2003), as well as to a reduction in excitation–contraction coupling (Delbono et al. 1997; Payne and Delbono 2004). For instance, Larsson et al. (1997) found specific tension of freeze dried as well as of skinned human fibres to be lower by 16–25% for fibres expressing type I myosin heavy chain (MHC) isoform and by 20–28% for fibres expressing type IIa MHC. Similarly, Frontera et al. (2000b) found the specific force of type I and type II of older men (mean age 74 years) to be lower on average by 30% than that of younger males (mean age 36.5 years).
Reduced agonist activation and an increased antagonist muscles coactivation may also have been a potential cause of the observed loss of specific force in static and dynamic contractions. Although, in static contractions, we previously reported in these older males a reduced voluntary activation of the plantarflexor muscles and a similar antagonist coactivation compared to younger males (Morse et al. 2004), other authors found no deficit in plantarflexors’ activation with ageing (Vandervoort and McComas 1986) and an increased antagonists’ coactivation (Klein et al. 2001; Macaluso et al. 2002). However, in dynamic contractions, the voluntary drive to the agonist muscles and the degree of coactivation have both been found to be unaffected by age and load when young (26 year old) and older (65 and 75 years old) males were compared (McNeil et al. 2007). Hence it seems unlikely that differences in voluntary activation or antagonist co-activation accounted for the different torque–velocity properties observed in the present study. Furthermore, had differences in neural activation played a role, a poor fit of the experimental points would have been found when applying Hill’s model since, by definition, each point of the torque–velocity curve should belong to the same value of activation (Hill 1938; Wilkie 1949). Instead, the linearization of Hill’s curve (obtained by plotting by (Po-P)/V against P) used to calculate Hill’s constants (see “Methods”) always yielded a very good linear fit of the experimental points (with R 2 values of 0.95–0.99).
In this study, consistent with previous observations by our group (Narici et al. 2003) and by others (Kubo et al. 2003), gastrocnemius fibre fascicle length was found to be significantly shorter in the older males. This reduction in fibre fascicle length, implying a decrease in the number of sarcomeres in series, would be expected to result in lesser shortening of muscle fibres upon contraction. Since the fascicles of the gastrocnemius are known to operate in the ascending limb of the length–force relation (Herzog et al. 1991), less shortening would cause the fascicles to operate away from the optimum fascicle length. However, because tendon stiffness tends to decline with ageing, this may compensate for the reduced shortening of fibre fascicles and still enable fascicles to operate close to the optimum region of the length-force relation (Narici and Maganaris 2006).
One important functional implication of the decrease in specific force observed in the present study is that for any given contraction velocity the plantarflexor muscles of older individuals are markedly weaker than those of younger men. Furthermore, the results showed that the greater the contraction velocity, the greater the torque deficit between the older and younger men. For instance, whereas for isometric contractions the intrinsic torque deficit was 37%, at the highest recorded contraction velocity (4.36 rad s−1) the intrinsic torque deficit was 78%. The most likely explanation for this finding is that whereas isometric torque only strictly depends on the number of sarcomere in parallel, dynamic torque depends both on the force generated by the sarcomere in parallel and on the rate of change in length of the sarcomere in series, as dictated by the force–velocity relation. Hence as the velocity of shortening increases, the influence of fascicle length over PCSA prevails, and thus normalisation to PCSA has a maximum effect when considering the isometric torque and no effect when considering V max.
In order to appreciate the role of contraction speed, it is necessary to consider the effect of fibre fascicle length.
Influence of fascicle length on the torque–velocity relationship
The present study showed that, irrespective of the torque level, the velocity of shortening of the OM plantarflexor muscles was significantly lower than of the YM. Since fibre length is a major determinant of both maximal and submaximal shortening velocity (Lieber and Friden 2000), it was expected that the differences in the T–V between the OM and YM would be substantially reduced, after normalisation of shortening velocity to fascicle length, and that the importance of the contribution of fascicle length increased with shortening velocity. Our results show that fascicle length accounted for about 16% of the total difference (38%) in estimated V max between the OM and YM; that is to say, the shorter fascicle length (hence the lower number of sarcomeres in series) accounted for almost half of the difference in estimated V max between the OM and YM. Other factors that may have contributed for the lower V max of the OM are: (1) changes in MHC composition, (2) a decrease in the intrinsic speed of shortening of the myosin molecule, (3) a lower agonist muscle activation and (4) a greater antagonist muscle co-activation. Although selective atrophy of type 2 muscle fibres has long been thought to occur with ageing, recently a thorough analysis of cross-sectional and longitudinal data on MHC composition, evaluated on muscle homogenates, revealed no selective fibre atrophy in very old compared to younger adults (Andersen 2003). However, D’Antona et al. (2003, 2007) suggest that physical activity level plays an important role in determining MHC composition of older individuals: whereas a decrease in physical activity (common in ageing, c.f. Morse et al. 2004) tends to favour the expression of a slower myosin phenotype, an increase in physical activity increases the expression of type 2A fibres. Although, as pointed out by D’Antona et al. (2007), no ‘typical’ MHC profile exists in ageing, one important finding is that the maximum shortening velocity (Vo) of the type 2A fibres, the most abundant fast fibres in humans (since the proportion of type 2X is extremely low: 0–8%, Andersen et al. 2003; D’Antona et al. 2003) is lower than in young individuals, irrespective of physical activity level. This finding, together with the observation of a decrease in the intrinsic speed of the myosin molecule (Höök et al. 2001), is likely to account for a substantial part of the reduction in shortening velocity measured in vivo.
Theoretically, other factors that may have potentially accounted for the lower shortening velocity of older muscle are differences in voluntary activation and in co-activation. However, as discussed in the preceding section, it seems unlikely that these factors played a significant role in the differences in torque–velocity properties observed between the OM and YM of this study.
Absolute and normalised muscle power
The present study has shown that GM muscle power is 80% lower in the OM. Only about 10% of this difference was accounted by the smaller muscle volume of the OM, since peak power normalised to muscle volume was 72% lower in the OM (OM 83.4 W l−1, YM 303.1 W l−1). Clearly, other factors besides muscle volume must account for this remarkable specific power deficit. It seems likely that major key-players in this phenomenon are, (1) reduced single fibre specific force, (2) decreased intrinsic speed of the myosin molecule and (3) loss of sarcomeres in-series. The influence of factors 2 and 3 seems reflected by the lower optimum velocity at peak power (2.6 rad s−1 in the OM and 6.1 rad s−1 in the YM). However, without the availability of muscle biopsies in these individuals, it seems difficult at present to establish the exact contribution of these factors to the observed differences in specific power.
It seems noteworthy that the above values of normalised isokinetic power of the YM closely match those that can be estimated from Margaria’s stair climbing test (Margaria et al. 1966). These authors reported that the average power developed during stair climbing at maximum speed, approximates 1.4 kg m s−1 per kg of body mass, i.e., 13.7 W kg−1, which for a typical 75 kg man would yield an average power of 1027 W. Given that, for an average sedentary young man, thigh muscle volume is 6.5 l (Grassi et al. 1991) and that the knee extensors occupy about 48% of this volume (Narici 1992), it can be estimated that the power per litre of muscle mass would be 1027 W/3.12 l, that is, 329 W l−1, a good match indeed!
Conclusions
Changes in muscle architecture were found to contribute significantly to the differences in torque–velocity properties between older and younger males. Our results suggest that the reduction in PCSA, representing the loss of sarcomere in parallel, accounts for about one-sixth of the total decrease in isometric torque measured in vivo. Additional factors, such as a decrease in single fibre specific tension and a possible shift in muscle fascicles’ length–tension relation are proposed to account for the remaining discrepancy. It was estimated that the decrease in fibre fascicle length, representing a loss of sarcomere in series, accounted for about 1/2 of the total loss in maximum shortening velocity estimated from Hill’s equation. Other factors such as, changes in MHC composition (influenced both by the ageing process and by physical activity level) and in the intrinsic speed of the myosin molecule likely account for the remaining speed discrepancy. Although, at any given submaximal velocity value, the contribution of PCSA to muscle torque decreased with increasing velocity, and vice versa for fascicle length, the differences in muscle architecture only partly account for the differences in torque–velocity characteristics between young and older males.
A general, but most important conclusion is that when considering the loss of muscle function in old age, it is power more than strength that is drastically reduced. Our findings on the OM show this very clearly, whereas the loss of muscle strength was 48.5%, the decrease in muscle power was 80%. Considering that most daily actions require the development of power (e.g., stair negotiation, walking to the grocery or lifting one’s body from a bed or chair), such a huge loss of muscle power is bound to be a main cause of the loss of mobility and self-sufficiency in old age and warrant interventions aimed at preserving muscle power in older individuals.
References
Akima H, Kano Y, Enomoto Y, Ishizu M, Okada M, Oishi Y, Katsuta S, Kuno S (2001) Muscle function in 164 men and women aged 20–84 year. Med Sci Sports Exerc 33:220–226
Andersen JL (2003) Muscle fibre type adaptation in the elderly human muscle. Scand J Med Sci Sports 13:40–47
Bazzucchi I, Marchetti M, Rosponi A, Fattorini L, Castellano V, Sbriccoli P, Felici F (2005) Differences in the force/endurance relationship between young and older men. Eur J Appl Physiol 93:390–397
D’Antona G, Pellegrino MA, Adami R, Rossi R, Carlizzi CN, Canepari M, Saltin B, Bottinelli R (2003) The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J Physiol 552:499–511
D’Antona G, Pellegrino MA, Carlizzi CN, Bottinelli R (2007) Deterioration of contractile properties of muscle fibres in elderly subjects is modulated by the level of physical activity. Eur J Appl Physiol (Epub ahead of print)
Delbono O, Renganathan M, Messi ML (1997) Excitation-Ca2+ release–contraction coupling in single aged human skeletal muscle fiber. Muscle Nerve Suppl 5:S88–92
Doherty TJ (2003) Invited review: aging and sarcopenia. J Appl Physiol 95:1717–1727
Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R (2000a) Aging of skeletal muscle: a 12-year longitudinal study. J Appl Physiol 88:1321–1326
Frontera WR, Suh D, Krivickas LS, Hughes VA, Goldstein R, Roubenoff R (2000b) Skeletal muscle fiber quality in older men and women. Am J Physiol––Cell Physiol 279:C611–618
Gajdosik RL, Vander Linden DW, Williams AK (1999) Concentric isokinetic torque characteristics of the calf muscles of active women aged 20–84 years. J Orthop Sports Phys Ther 29:181–190
Grassi B, Cerretelli P, Narici MV, Marconi C (1991) Peak anaerobic power in master athletes. Eur J Appl Physiol 62:394–399
Herzog W, Hasler E, Abrahamse SK (1991) A comparison of knee extensor strength curves obtained theoretically and experimentally. Med Sci Sports Exerc 23(1):108–114
Hill AV (1938) The heat of shortening and the dynamic constants of muscle. In: Proc R Soc B, 126, 136–195
Höök P, Sriramoju V, Larsson L (2001) Effects of aging on actin sliding speed on myosin from single skeletal muscle cells of mice, rats, and humans. Am J Physiol––Cell Physiol 280:C782–788
Hortobagyi T, Zheng D, Weidner M, Lambert NJ, Westbrook S, Houmard JA (1995) The influence of aging on muscle strength and muscle fiber characteristics with special reference to eccentric strength. J Gerontol Ser A—Biol Sci Med Sci 50:B399–406
Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, Fiatarone Singh MA (2001) Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol Ser A—Biol Sci Med Sci 56:B209–217
Jubrias SA, Odderson IR, Esselman PC, Conley KE (1997) Decline in isokinetic force with age: muscle cross-sectional area and specific force. Pflugers Arch––Eur J Physiol 434:246–253
Klein CS, Rice CL, Marsh GD (2001) Normalized force, activation, and coactivation in the arm muscles of young and old men. J Appl Physiol 91:1341–1349
Kubo K, Kanehisa H, Azuma K, Ishizu M, Kuno SY, Okada M, Fukunaga T (2003) Muscle architectural characteristics in young and elderly men and women. Int J Sports Med 24(2):125–130
Lanza IR, Towse TF, Caldwell GE, Wigmore DM, Kent-Braun JA (2003) Effects of age on human muscle torque, velocity, and power in two muscle groups. J Appl Physiol 95:2361–2369
Larsson L, Li X, Frontera WR (1997) Effects of aging on shortening velocity and myosin isoform composition in single human skeletal muscle cells. Am J Physiol 272:C638–649
Lieber RL, Friden J (2000) Functional and clinical significance of skeletal muscle architecture. Muscle and Nerve 23:1647–1666
Lindle RS, Metter EJ, Lynch NA, Fleg JL, Fozard JL, Tobin J, Roy TA, Hurley BF (1997) Age and gender comparisons of muscle strength in 654 women and men aged 20–93 year. J Appl Physiol 83:1581–1587
Lynch NA, Metter EJ, Lindle RS, Fozard JL, Tobin JD, Roy TA, Fleg JL, Hurley BF (1999) Muscle quality. I. Age-associated differences between arm and leg muscle groups. J Appl Physiol 86:188–194
Macaluso A, Nimmo MA, Foster JE, Cockburn M, McMillan NC, De Vito G (2002) Contractile muscle volume and agonist–antagonist coactivation account for differences in torque between young and older women. Muscle Nerve 25(6):858–863
Margaria R, Agemo P, Rovelli E (1966) Measurement of muscular power (anaerobic) in man. J Appl Physiol 21:1662–1664
McNeil CJ, Vandervoort AA and Rice CL (2007) Peripheral impairments cause a progressive age-related loss of strength and velocity-dependent power in the dorsiflexors. J Appl Physiol (In press)
Morse C, Thom J, Birch K, Narici M (2005) Changes in triceps surae muscle architecture with sarcopenia. Acta Physiol Scand 183:291–298
Morse CI, Thom JM, Davis MG, Fox KR, Birch KM, Narici MV (2004) Reduced plantarflexor specific torque in the elderly is associated with a lower activation capacity. Eur J Appl Physiol 92:219–226
Narici MV (1992) Antropometria volumetrica. In: Bozzetti F and Guarnieri R (eds) Manuale di nutrizione artificiale, Masson (Publ), Milan, vol 59, pp 793–804
Narici MV, Maganaris CN, Reeves ND, Capodaglio P (2003) Effect of aging on human muscle architecture. J Appl Physiol 95:2229–2234
Narici MV, Maganaris CN (2006) Adaptability of elderly human muscles and tendons to increased loading. J Anat 208(4):433–443
Payne AM, Delbono O (2004) Neurogenesis of excitation–contraction uncoupling in aging skeletal muscle. Exerc Sport Sci Rev 32:36–40
Porter MM, Myint A, Kramer JF, Vandervoort AA (1995) Concentric and eccentric knee extension strength in older and younger men and women. Can J Appl Physiol 20:429–439
Porter MM, Vandervoort AA, Kramer JF (1997) Eccentric peak torque of the plantar and dorsiflexors is maintained in older women. J Gerontol Ser A—Biol Sci Med Sci 52:B125–131
Poulin MJ, Vandervoort AA, Paterson DH, Kramer JF, Cunningham DA (1992) Eccentric and concentric torques of knee and elbow extension in young and older men. Can J Sport Sci 17:3–7
Pousson M, Lepers R, Van Hoecke J (2001) Changes in isokinetic torque and muscular activity of elbow flexors muscles with age. Exp Gerontol 36:1687–1698
Vandervoort AA, Kramer JF, Wharram ER (1990) Eccentric knee strength of elderly females. J Gerontol 45:B125–128
Vandervoort AA, McComas AJ (1986) Contractile changes in opposing muscles of the human ankle joint with aging. J Appl Physiol 61(1):361–367
Wickiewicz TL, Roy RR, Powell PL, Perrine JJ, Edgerton VR (1984) Muscle architecture and force–velocity relationships in humans. J Appl Physiol 57(2):435–443
Wilkie DR (1949) The relation between force and velocity in human muscle. J Physiol 110(3–4):249–280
Acknowledgments
The authors would like to thank Ian Rothwell for his technical assistance. This study was supported by the European Commission Framework V funding (‘Better-Ageing’ Project, No. QLRT-2001-00323).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thom, J.M., Morse, C.I., Birch, K.M. et al. Influence of muscle architecture on the torque and power–velocity characteristics of young and elderly men. Eur J Appl Physiol 100, 613–619 (2007). https://doi.org/10.1007/s00421-007-0481-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-007-0481-0