Abstract
Purpose
To examine differences in the electromyographic vs torque (EMG-T) relationship, as well as muscle strength and indicators of muscle mass and quality between young versus postmenopausal females, and explore whether the potential differences in the EMG-T relationships could be explained by differences in muscle mass.
Methods
Thirty young (age = 20.7 ± 2.8 y) and 30 postmenopausal (age = 56.3 ± 4.7 y) females completed maximal isometric strength testing (MVIT) and isometric ramp contractions at 40% and 70% MVIT, during which electromyographic signals were collected to quantify the slopes (Slope40; Slope70) and intercepts (Intercept40; Intercept70) of the EMG-T relationships. Muscle mass and quality measurements were also completed.
Results
Postmenopausal females exhibited lower skeletal muscle mass (− 2.3 ± 1.5 kg), fat-free mass index (− 1.1 ± 0.7 kg·m−2), MVIT (− 17.1 ± 16.3 Nm), phase angle (− 0.5 ± 0.0°), muscle cross-sectional area (− 5.5 ± 1.1 cm2), muscle quality (− 0.1 ± 0.0 a.u), Slope40 (− 0.0003 ± 0.0002 mV·%MVIT−1), Slope70 (− 0.0003 ± 0.0002 mV·%MVIT−1), and had a higher echo intensity (+ 9.8 ± 2.8 a.u), Intercept40 (+ 0.001 ± 0.001 mV), and Intercept70 (+ 0.004 ± 0.003 mV) (p ≤ 0.001–0.04) than the young females. The EMG-T relationship variables were correlated with both muscle mass and quality after controlling for bodyweight. When controlling for muscle mass and bodyweight, group differences in the slopes of the EMG-T relationship and muscle strength were eliminated.
Conclusion
Muscle mass and quality are primary contributors to the decrements in neuromuscular function observed in postmenopausal versus young females, and the preservation of muscle mass should be prioritized in the years leading up to, during, and immediately after menopause.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aging is associated with a general loss of functional ability and independence, and it is of primary importance to understand the contributing factors to be able to better slow the rate of decline in an increasingly growing population of older adults (Milanovic et al. 2013). Skeletal muscle health and function appear to be primary contributing factors (Tieland et al. 2018), and age-related decreases in skeletal muscle mass (SMM; i.e., sarcopenia) and skeletal muscle quality have been implicated as major contributors to decreased functional ability (Cramer et al. 2017; Jenkins et al. 2015; Keller and Engelhardt 2013; Phillips et al. 1993). While these impairments have been shown to decline with age independent of sex (Lang et al. 2010), the rate of decline appears to increase rather dramatically at or near the time of menopause in females. For example, Aloia et al. (1991) reported an accelerated loss of total body potassium, a marker of lean body mass, at menopause. Further, Phillips et al. (1993) reported a dramatic reduction in specific muscle force at the time of menopause. Consequently, a higher incidence of sarcopenia has been reported among females than males between the ages of 60 and 70 y (Baumgartner et al. 1998; Kirchengast and Huber 2009; Shafiee et al. 2017). Despite these observations, however, there is a relative lack of research regarding the influence of menopause on neuromuscular function in females.
Central nervous system limitations are another major factor contributing to age-related decreases in force production capabilities. Specifically, deficits in voluntary activation, or the ability to fully activate the motor unit (MU) pool and drive active MUs to maximal discharge rates (Tieland et al. 2018; Jenkins et al. 2017), are thought to contribute to the observation of lower electromyographic signal (EMG) amplitudes at a variety of torque requirements in older versus younger adults (Clark et al. 2010; Rozand et al. 2020). The relationship between muscle activity measured through surface electromyography (sEMG) and isometric torque, or the EMG versus torque (EMG-T) relationship, provides complex information regarding neural and muscular contributions to force production. Consequently, it has been suggested that the EMG-T relationship may give insight into the MU activation strategies or muscle activation strategies used to produce force during progressive, non-fatiguing contractions (Herda et al. 2011; Paquin and Power 2018). Indeed, Herda et al. (2011) reported that the EMG-T relationship distinguished between young adults with high- (> 90%) versus moderate- (< 90%) voluntary activation capabilities. Thus, the EMG-T relationship may be a useful tool to examine neuromuscular impairments associated with age and/or menopause.
One issue with inferring differences in neuromuscular control between subjects utilizing the EMG-T relationship is the known influence of peripheral factors such as muscle geometry on the EMG signal. In fact, Martinez-Valdes et al. (2018) recently reported that differences in EMG amplitude between the vastus lateralis (VL) and vastus medialis muscles are primarily explained by differences in motor unit action potential (MUAP) sizes (r = 0.73–0.85), which likely reflect muscle fiber geometries. Interestingly, Sterczala et al. (2018) reported smaller MUAP amplitudes of high-threshold MUs in older than younger individuals and suggested that this was caused by atrophy of the fibers that comprised the higher-threshold MUs. Therefore, due to the influence of peripheral factors such as muscle fiber sizes on the amplitude of the EMG signal, it is plausible that any age- or menopause-related differences in the EMG-T relationship may be influenced by muscle atrophy, and that older adults would have less steep slopes compared to their younger counterparts due to preferential atrophy of fibers comprising higher threshold MUs.
The primary purposes of this study were (a) to examine differences in the EMG-T relationship, as well as muscle strength and indicators of muscle mass and quality between young versus postmenopausal females, and (b) to explore whether the potential differences in the EMG-T relationships could be explained by differences in muscle mass in our sample.
Participants
Thirty healthy young (mean ± SD; age = 20.7 ± 2.8 y; weight = 70.3 ± 13.9 kg; height = 163.8 ± 7.4 cm) and thirty healthy postmenopausal (56.3 ± 4.7 y; 70.1 ± 10.3 kg; 162.3 ± 6.5 cm; years post-menopause = 6.6 ± 3.9 y) females volunteered for and completed this study. To be eligible, each participant must have been untrained and reported completing < 150 min·week−1 of moderate or < 75 min·week−1 of vigorous-intensity exercise (per the American Heart Association guidelines (Piercy and Troiano 2018)), and have been a female between the ages of 18–29 or 45–65 y, free of any cardiovascular, metabolic, or neuromuscular disease, and could not have been currently taking any medications known to alter energy metabolism as determined by a health history questionnaire. To be eligible for the postmenopausal group, participants must have gone without a menstrual cycle for more than one year. No participants in the postmenopausal group had, or were undergoing hormone replacement therapy prior to or upon enrollment in the study. Participants were a random subset from two larger clinical trials (i.e., 30 participants from each) that were approved by and conducted in accordance with the guidelines and regulations of the Oklahoma State University’s Institutional Review Board (IRB Approval #s: ED-17–157 and ED-18-101).
Experimental design
Each participant visited the laboratory on three separate occasions. During visit 1, participants completed the informed consent, health and exercise history questionnaire, and had their height and weight measured. Following an overnight fast, participants returned for visit two, and their body composition was assessed using a medical body composition analyzer. During visit 3, ultrasound-based assessments of VL muscle size and echo intensity (EI) were performed. After the ultrasound measurements, the participants completed maximal voluntary isometric leg-extension tests before completing submaximal isometric leg-extension tests, during which they traced a ramped torque template. During the submaximal contractions, EMG signals were collected from the VL to quantify the EMG-T relationship for each participant. To avoid any influence of the menstrual cycle in the young females, all testing was conducted during the last half of the follicular phase for these participants.
Isometric strength testing
Subjects were seated on an isokinetic dynamometer (Biodex System 4; Biodex Medical Systems, Inc. Shirley, NY, USA) with the trunk and pelvis secured with straps and the lateral epicondyle aligned with the dynamometer head. All isometric testing was performed with a 90° knee joint angle and the lever arm pad positioned 3–4 cm proximal to the medial malleolus. Once secured in the dynamometer, participants completed three, 3-s warm-up leg extension muscle actions at intensities of 25, 50, and 75% of their perceived effort with 30-s of rest between each set of muscle actions. Following a 60-s rest period, the participants performed two, 5-s maximal voluntary contractions (MVIC) separated by at least 60-s of rest. If the second MVIC attempt rendered a higher peak value compared to the first attempt, the participant was given another 60-s rest period and additional attempts were completed until a true maximum was achieved. All torque signals were displayed in real-time on an external computer monitor for visual feedback and strong verbal encouragement was provided. The maximal voluntary isometric torque (MVIT) output (Nm) for each subject achieved in a 1-s window during either MVIC and was used to scale the subsequent submaximal, trapezoidal ramp contractions. Following MVIC testing, participants performed two trapezoidal ramp contractions at 40 and 70% MVIT by tracing a ramped torque trajectory displayed on an external computer monitor. The trajectories increased linearly at a rate of 10% MVIT·s−1 to 40 or 70% MVIT, were held for 10-s, and then decreased linearly at a rate of 10% MVIT·s−1 until returning to baseline. If the participant was unable to accurately trace a torque trajectory, they were provided a standard rest period and the tracing was repeated until two accurate trapezoidal ramp contractions were executed.
Electromyography
Parallel-bar, bipolar, sEMG sensors with an interelectrode distance of 10 mm (Delsys DE-2.1, Delsys, Inc. Natick, MA, USA) were placed on the VL to quantify EMG amplitude during the trapezoidal ramp contractions. Prior to sensor placement, the surface of the skin was prepared by shaving and removing dead skin via careful abrasion, adhesive taping, and cleansing with alcohol. For the VL, the center of the bipolar electrode was placed at 66% of the distance between the anterior superior iliac spine and the lateral superior border of the patella (Hermens et al. 2000) and was oriented parallel to the angle of pennation of the muscle fibers (~ 20% (Fukumoto et al. 2012)). A reference electrode (UltraStim USX2000, Axelgaard Manufacturing Co., Ltd., Fallbrook, CA, USA) was placed on the spinous process of the C7.
Signal processing
The EMG and torque signals were sampled simultaneously at 2 kHz using a Delsys Bagnoli Desktop data acquisition system (Delsys, Inc., Natick, MA, USA), recorded to a desktop computer, and processed offline using custom-written Labview software (v.12.0, National Instruments, Austin, TX, USA). The EMG signals were amplified using the built-in sensor amplifier using a gain of 10 VN ± 1%, a common-mode rejection ratio of − 92 dB, and an input impedance of > 101 5Ω//0.2 pF. The EMG signals were then zero-meaned and bandpass filtered from 10 to 499 Hz using a zero-phase shift, 4th-order Butterworth filter. The torque signals were low-pass filtered at 20 Hz using a zero-phase shift, 4th-order Butterworth filter.
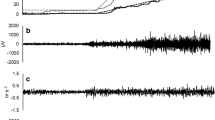
During the MVICs, MVIT and EMG amplitude values were calculated from the 1000 ms epoch corresponding to the highest average torque value that occurred during the MVIC plateau. For each 5% increment of torque during the increasing portion of the submaximal ramp contractions at 40 and 70% MVIC, the average torque achieved and the associated EMG amplitude values were calculated (Fig. 1). The EMG amplitude values were expressed as the root mean square value in mV. Subsequently, the EMG-T relationship was determined for each subject during the submaximal ramp contractions by regressing EMG amplitude against average torque, which provided a slope (mV·MVIT%−1) and y-intercept (mV) value for each subject during both 40 (Slope40; Intercept40) and 70% (Slope70; Intercept70) submaximal ramp contractions.
a The filtered and rectified electromyographic (EMG) and filtered torque signal collected during a trapezoidal tracing at 70% of maximal voluntary isometric torque (MVIT) for one participant. The vertical dashed lines illustrate the bins in which EMG root mean square amplitude (RMS) and average torque were calculated during the linearly increasing torque segment. b Shows the EMG RMS versus average torque values from the corresponding 5% MVIC bins for the same participant for whom the signals are displayed in figure A, as well as the line and equation characterizing the EMG versus torque relationship for this subject. c The mean EMG versus average torque values during the 40% trapezoidal tracing with the composite regression equation in the young and postmenopausal females. d The mean EMG versus average torque values during the ramp contraction at 70% trapezoidal tracing with the composite regression equation in the young and postmenopausal females. In C and D, the black circles and lines represent the young females, whereas the orange-filled circles and orange lines represent the postmenopausal
Body composition
Participants were asked to arrive at the laboratory euhydrated prior to body composition analysis. Height and weight were measured using an eye-level beam physician scale (Physician Scale 439; Detecto, Webb City, MO, US) and a calibrated platform scale (ADAM CPWplus 150 M, Oxford, CT, USA), respectively. Following a 10-h overnight fast, SMM (kg), fat-free mass index (FFMI; FM (kg) / Height (m)2), and phase angle (PA; degree) were assessed using 8-point bioelectrical impedance analysis (BIA; SECA mBCA 514, Hamburg, DE) as described in detail previously (Bosy-Westphal et al. 2013). During BIA, resistance (R) and reactance (Xc) were measured in ohms (Ω), and PA (˚) was calculated using the following formula:
(Barbosa-Silva et al. 2005) .
Skeletal muscle ultrasound
During all ultrasound testing, the participant’s right leg was examined while in the supine position with a pillow placed behind the knees and the leg braced in a custom-made foot mold to prevent rotation of the femur. Ultrasound images were obtained using a brightness mode (B-mode) ultrasound device (General Electric LOGIQ S8, Wauwatosa, WI) and a multi-frequency linear-array probe (Model ML6-15-D 4–15 MHz, 50-mm field of view). A generous amount of transmission gel was applied to the skin and the ultrasound probe to maximize acoustic coupling and to reduce near-field artifacts, and minimal pressure was applied to the probe during all measurements to limit compression of the muscle. The frequency was set at 12 MHz, the image gain at 50 dB, the dynamic range at 72 dB, and the image depth was set between 5–9 cm. Three panoramic ultrasound images of the VL were taken at 50% of the distance from the anterior superior iliac spine to the proximal border of the patella. The muscle cross-sectional area (mCSA) of the VL was then analyzed using image analysis software (ImageJ, version 1.50i) by selecting a region of interest including as much muscle as possible without including surrounding fascia using the built-in polygon function. VL EI was quantified as arbitrary unit (a.u.) values between 0 (black) and 255 (white) by gray-scale analysis of each image using the histogram function of ImageJ from the same region of interest as mCSA. Muscle quality (MQ) was then quantified by normalizing VL mCSA to VL EI (cm2/a.u.).
Nutritional status
Participants were asked to record and turn in dietary logs containing all food and calorie-containing beverages consumed during two week days, and one weekend day. Participants recorded food immediately after consumption and provided information regarding the method of preparation and serving sizes. Calories (kcals) and protein (g) intake were then calculated by entering all foods and drinks consumed into ESHA’s Food Processor® Nutrition Analysis Software (https://www.esha.com, ESHA Research, Oak Brook, Il, USA) (Bazzano et al. 2002; Liu et al. 2013).
Statistical analyses
Independent-sample t-tests were used to examine age-related differences in the dependent variables, which included age, height, weight, caloric and protein intakes, SMM, FFMI, MVIT, PA, mCSA, EI, MQ, Slope40, Intercept40, Slope70, and Intercept70. The assumption of homogeneity was assessed for all t-tests using Levene’s Test of Equality of Variances. If violated, the degrees of freedom were adjusted using the Welch-Satterthwaite method. Normality was also assessed using the Shapiro-Wilks test. Any non-normally distributed variables were analyzed using Mann–Whitney tests. Pearson’s partial correlation coefficients were calculated to examine the relationship among all of the dependent variables after adjusting for bodyweight because bodyweight has been shown to exert a substantial influence on neuromuscular function outcomes (Folland et al. 2008; Oba et al. 2014; Weir et al. 1999) and it displayed collinearity with the other neuromuscular function variables in this study (i.e. MVIT, SMM, mCSA, MQ, PA, and EMG-T slopes and intercepts). Age was not included in the correlation analysis, because it was non-continuous due to the recruitment of two distinct age groups. Strengths of association were deemed small, moderate, or strong when the coeffeicient values were between 0.1–0.3, 0.3–0.5, and > 0.5, respectively.
Based on our initial correlation analyses, we then conducted six separate analyses of covariance (ANCOVAs) to explore whether potential between-group differences in the EMG-T relationship and MVIT could be explained by differences in indicators of muscle mass or muscle quality and bodyweight. For these analyses, the dependent variables of interest were MVIT, Slope40, and Slope70, the independent variable was a group (i.e., young vs. postmenopausal), and the covariates were bodyweight and SMM or bodyweight and MQ. All statistical analyses were performed using GraphPad Prism 8 (GraphPad Software Inc, San Diego, CA) and IBM SPSS Statistics (v. 26, IBM Corp, Armonk, NY). Unless otherwise reported, all mean differences are reported as the mean or median ± 95% confidence interval difference. The a priori type I error rate was set at 0.05.
Results
All data were normally distributed except for age (p < 0.001), height (p = 0.032), weight (p = 0.002), caloric intake (p = 0.025), protein intake (p = 0.029), MQ (p = 0.008), Slope40 (p = 0.046), Slope70 (p < 0.001), and Intercept70 (p < 0.001), and the homogeneity of variances assumption was met for all variables except for age (p = 0.01) and Intercept40 (p = 0.046). Table 1 includes age-related differences among the dependent variables. In summary, the postmenopausal females had lower SMM (− 2.3 ± 1.5 kg; p = 0.004), FFMI (− 1.1 ± 0.7 kg/m2; p = 0.002), MVIT (− 17.1 ± 16.3 Nm; p = 0.04), PA (− 0.5 ± 0.0°; p < 0.001), mCSA (− 5.5 ± 1.1 cm2; p < 0.001), MQ (− 0.1 ± 0.0 a.u.; p < 0.001), Slope40 (− 0.0003 ± 0.0002 mV·%MVIT−1; p = 0.008), and Slope70 (− 0.0003 ± 0.0002 mV·%MVIT−1; p = 0.005) and had greater EI (+ 9.8 ± 2.8 a.u.; p = 0.001), Intercept40 (+ 0.0013 ± 0.0011 mV; p = 0.02), and Intercept70 (+ 0.0033 ± 0.0024 mV; p < 0.001) compared to the young females (Table 1). The relationships among the dependent variables after partialling out bodyweight are included in Table 2.
The ANCOVA analyses indicated that the age-related differences for Slope40 and Slope70 were both eliminated (both p ≥ 0.70) when co-varying for bodyweight and SMM, and both bodyweight (p ≤ 0.008; n2p ≥ 0.12) and SMM (p ≤ 0.001; n2p ≥ 0.22) were significant adjusters of the means for both Slope40 and Slope70. Similarly, when co-varying for bodyweight and MQ, the age-related differences for Slope40 and Slope70 were eliminated (both p ≥ 0.27), although while MQ was not a significant adjuster of the means (p ≥ 0.13; n2p ≤ 0.04), bodyweight was (p ≤ 0.002; n2p ≥ 0.16).
For MVIT, the ANCOVA analyses indicated that the age-related difference was eliminated (p = 0.89) when co-varying for bodyweight and SMM, but while SMM was a significant adjuster of the means (p < 0.001; n2p = 0.21), bodyweight was not (p = 0.36; n2p = 0.02). Finally, when controlling for bodyweight and MQ, the age-related difference in MVIT was also eliminated (p = 0.84), and both MQ (p = 0.002; n2p = 0.16) and bodyweight (p = 0.01; n2p = 0.11) were significant adjusters of the mean.
Discussion
The primary purposes of this study were (a) to examine differences in the EMG-T relationship, as well as muscle strength and indicators of muscle mass and quality between young versus postmenopausal females, and (b) to explore whether the potential differences in the EMG-T relationships could be explained by differences in muscle mass in our sample. The results of this study indicated that the young females exhibited steeper slopes and lower y-intercepts characterizing the EMG-T relationship during ramp contractions at both 40 and 70% of MVIT when compared to their postmenopausal female counterparts. We also observed differences in MVIT, SMM, FFMI, PA, mCSA, EI, and MQ among the young versus postmenopausal females. After controlling for bodyweight, measurements of SMM, MVIT, PA, mCSA, and EI were consistently related to the EMT-T slopes and intercepts. Additionally, the differences among the neuromuscular function indices (i.e., slopes of EMG-T relationship and MVIT) were eliminated after accounting for SMM or MQ and bodyweight, although SMM seems to be a more consistent, significant adjuster than MQ. Therefore, it appears that the observed differences in neuromuscular function among the young versus postmenopausal females in this study may primarily be driven by age-related differences in skeletal muscle mass.
The postmenopausal females in the present study exhibited lower Slope40 and Slope70, but greater Intercept40 and Intercept70 (Fig. 1c, d). Thus, while the postmenopausal females displayed greater EMG amplitudes at very low torques, the young females displayed a more progressive increase in EMG amplitude across the torque spectrum. Moreover, young females were 17% stronger compared to postmenopausal females. Though the loss of SMM is a major consequence of aging, there are other factors needed to fully explain age-associated impairments in muscle strength, such as decreases in skeletal muscle quality and intrinsic force generation capacity, as well as neural decrements that influence muscle activation (Cardozo et al. 2013; Clark and Fielding 2012). Interestingly, the EMG-T relationship has been shown to be sensitive to differences in voluntary activation capacity in young adults (Herda et al. 2011). Herda et al. (2011) observed greater increases in EMG amplitude from 30 to 90% MVIT in participants able to achieve > 90% voluntary activation than in those who achieved < 90% voluntary activation. In a recent systematic review, Rozand et al. (2020) reported a modest reduction of voluntary activation in the knee extensors of older versus younger adults. Therefore, it is possible that an age-associated reduction in voluntary activation contributed to the age-related differences observed for the slopes and intercepts of the EMG-T relationship in the present study. However, this is highly speculative and, as we discuss further below, it is extremely likely that the age-related differences in the EMG-T relationship were heavily influenced by differences in muscle fiber size.
While aging is generally characterized by a progressive decrease in SMM, a few studies indicate that menopause may cause an acceleration in this decline. For example, Greendale et al. (2019) reported a 0.2% decline of SMM per year during the menopause transition period and Aloia et al. (1991) reported an accelerated loss of total body potassium (a marker of lean body mass) after menopause and that the rate of loss was greatest for those ≤ 3 y (− 1.3%) when compared to those > 6 y (− 0.5%) post-menopause. In the present study, the postmenopausal females, who were ~ 56 y of age and ~ 6.6 y post-menopause, displayed 12% lower total SMM and 32% lower VL mCSA than the young females. Thus, our cross-sectional data, in combination with the data presented by Aloia et al. (1991), suggest that postmenopausal females experience a significant increase in the rate of SMM loss in the years immediately following menopause, causing large decreases in SMM by the middle of their sixth decade. Indeed, higher sarcopenia prevalence has generally been reported among females than in males between the ages of 60 and 70 y (Baumgartner et al. 1998; Kirchengast and Huber 2009; Shafiee et al. 2017).
The age-related loss of skeletal muscle mass, or sarcopenia, is characterized by progressive atrophy and loss of both type I and type II muscle fibers, although several studies have suggested that these changes appear to be accelerated for the latter (Larsson et al. 1978; Brooke and Kaiser 1970; Verdijk et al. 2007). This decrease in fiber size and function has also been suggested as a primary driver of age-related decreases in muscle strength (Cramer et al. 2017; Jenkins et al. 2015). Phillips et al. (1993) reported a drastic drop off in specific strength (strength/CSA) in females at the time of menopause that was prevented in individuals on hormone replacement therapy, and also reported strong correlations between strength and CSA. It has also been proposed that age-related decreases in muscle quality also contribute to age-related decreases in neuromuscular function. EI and PA have been suggested as surrogate measurements of muscle quality and cellular health, respectively (Garlini et al. 2019; Reimers et al. 1993). Indeed, EI has been shown to be strongly correlated with MRI-based assessments of intramuscular fat (Young et al. 2015), is inversely associated and is a significant independent predictor of maximal strength in older adult males (Cadore et al. 2012; Watanabe et al. 2013) and females (Fukumoto et al. 2012), and has been shown to increase with age (Magrini et al. 2018). Similarly, PA is thought to be associated with cellular health, has been used as a prognostic indicator in various health conditions (Garlini et al. 2019) and, accordingly, has been shown to decline with age (Barbosa-Silva et al. 2005; Dittmar 2003; Norman et al. 2015). In addition to EI and PA, we also normalized VL mCSA to EI as a more wholistic indicator of MQ. In the present study, EI was 13% greater, while PA and MQ were 10 and 45% lower in the postmenopausal versus young females, respectively. Thus, our data suggest that in addition to significant decreases in SMM, there are likely decreases in cellular health and/or muscle quality that may be contributing to the age-related decreases in neuromuscular function observed in the postmenopausal versus young females in this study.
In addition to the age-comparisons, we explored the relationships among muscle mass and quality and indices of neuromuscular function. After removing the influence of bodyweight, which has been shown to exert a significant influence on neuromuscular measurements (Folland et al. 2008; Oba et al. 2014; Weir et al. 1999) and indeed displayed collinearity with MVIT, SMM, mCSA, MQ, PA, and the EMG-T variables in the present investigation, both our indicators of muscle mass (i.e., SMM and mCSA) and quality (i.e., EI, PA, MQ) were consistently, moderately related to the neuromuscular function indices (i.e., MVIT, Slope40/Slope70, Intercept40/Intercept70). In addition to the aforementioned possibility that the age-related differences in MVIT and the EMG-T slopes and intercepts were caused by age-associated reductions in voluntary activation, it is also highly plausible and, in our opinion, more likely that differences in muscle mass and quality are responsible. Indeed, EMG amplitude is influenced by muscle architecture (Lowery et al. 2004). It has also been reported (Martinez-Valdes et al. 2018) that the difference in the absolute EMG amplitudes obtained at 70% MVIC between the vastus medialis versus VL are largely explained (57% of variability) by MUAP amplitudes, which have been proposed as a non-invasive indicator of muscle fiber size (Pope et al. 2016). Furthermore, in females, both Miller et al. (2013) and Trappe et al. (2003) have reported significant age-related decreases in myosin heavy chain IIa fiber size, specifically. Therefore, the observed relationships among muscle size, quality, and the EMG-T slopes in the present study, together with these previous studies, may indicate that the observed differences in the EMG-T relationship and MVIT are heavily influenced by age-related differences in the peripheral properties of skeletal muscle. To further explore this possibility, we also examined whether controlling for bodyweight and SMM or for bodyweight and MQ would eliminate the age-related differences in MVIT and slopes of the EMG-T relationships. Indeed, when accounting for these variables, the differences were eliminated, which provides additional evidence that the differences in neuromuscular function, as assessed with MVIT and the EMG-T relationship, are largely driven by differences in muscle mass and quality in young versus postmenopausal females. Additional studies are needed to more closely examine age-related differences in the EMG-T relationship, MUAP versus recruitment threshold relationship, and muscle mass to better understand the utility and limitations of these measurements.
Overall our data suggest that postmenopausal females exhibit significant reductions in muscle mass, muscle quality, and neuromuscular function compared to their younger female counterparts. Furthermore, our data suggest that although muscle quality is influential, muscle mass is likely a critical driver of the declines in neuromuscular function observed in these females, suggesting that the preservation of muscle mass should be a primary goal for females in the years leading up to, during, and immediately after menopause.
Data availability
Data will be made available upon reasonable request.
Abbreviations
- ANCOVA:
-
Analysis of Covariance
- BIA:
-
Bioelectrical impedance analysis
- EI:
-
Echo intensity
- EMG:
-
Electromyography
- EMG-T:
-
Electromyographic vs. Torque
- FFMI:
-
Fat-free mass index
- Intercept40, Intercept70 :
-
Intercept at 40%/70% maximal voluntary isometric torque
- MVIC:
-
Maximal voluntary isometric contraction
- MVIT:
-
Maximal voluntary isometric torque
- MU:
-
Motor Unit
- MUAP:
-
Motor Unit Action Potential Amplitude
- Mcsa:
-
Muscle cross-sectional area
- MQ:
-
Muscle quality
- PA:
-
Phase angle
- SMM:
-
Skeletal muscle mass
- Slope40, Slope70 :
-
Slope at 40%/70% maximal voluntary isometric torque
- sEMG:
-
Surface electromyography
- US:
-
Ultrasound
- VL:
-
Vastus lateralis
References
Aloia JF, McGowan DM, Vaswani AN, Ross P, Cohn SH (1991) Relationship of menopause to skeletal and muscle mass. Am J Clin Nutr 53(6):1378–1383. https://doi.org/10.1093/ajcn/53.6.1378
Barbosa-Silva MC, Barros AJ, Wang J, Heymsfield SB, Pierson RN Jr (2005) Bioelectrical impedance analysis: population reference values for phase angle by age and sex. Am J Clin Nutr 82(1):49–52. https://doi.org/10.1093/ajcn.82.1.49
Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD (1998) Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147(8):755–763. https://doi.org/10.1093/oxfordjournals.aje.a009520
Bazzano LA, He J, Ogden LG, Loria CM, Vupputuri S, Myers L, Whelton PK (2002) Agreement on nutrient intake between the databases of the First National Health and Nutrition Examination Survey and the ESHA Food Processor. Am J Epidemiol 156(1):78–85. https://doi.org/10.1093/aje/kwf003
Bosy-Westphal A, Schautz B, Later W, Kehayias JJ, Gallagher D, Muller MJ (2013) What makes a BIA equation unique? Validity of eight-electrode multifrequency BIA to estimate body composition in a healthy adult population. Eur J Clin Nutr 67(Suppl 1):S14-21. https://doi.org/10.1038/ejcn.2012.160
Brooke MH, Kaiser KK (1970) Muscle fiber types: how many and what kind? Arch Neurol 23(4):369–379. https://doi.org/10.1001/archneur.1970.00480280083010
Cadore EL, Izquierdo M, Conceicao M, Radaelli R, Pinto RS, Baroni BM, Vaz MA, Alberton CL, Pinto SS, Cunha G, Bottaro M, Kruel LF (2012) Echo intensity is associated with skeletal muscle power and cardiovascular performance in elderly men. Exp Gerontol 47(6):473–478. https://doi.org/10.1016/j.exger.2012.04.002
Cardozo C, Goncalves M, Hallal C, Marques N (2013) Age-Related Neuromuscular Adjustments Assessed by EMG. In: Hande Turker (ed) Electrodiagnosis in New Frontiers of Clinical Research, IntechOpen. https://doi.org/10.5772/55053
Clark DJ, Fielding RA (2012) Neuromuscular contributions to age-related weakness. J Gerontol A Biol Sci Med Sci 67(1):41–47. https://doi.org/10.1093/gerona/glr041
Clark DJ, Patten C, Reid KF, Carabello RJ, Phillips EM, Fielding RA (2010) Impaired voluntary neuromuscular activation limits muscle power in mobility-limited older adults. J Gerontol A Biol Sci Med Sci 65(5):495–502. https://doi.org/10.1093/gerona/glq012
Cramer JT, Jenkins NDM, Mustad VA, Weir JP (2017) Isokinetic dynamometry in healthy versus sarcopenic and malnourished elderly: beyond simple measurements of muscle strength. J Appl Gerontol 36(6):709–732. https://doi.org/10.1177/0733464815584669
Dittmar M (2003) Reliability and variability of bioimpedance measures in normal adults: effects of age, gender, and body mass. Am J Phys Anthropol 122(4):361–370. https://doi.org/10.1002/ajpa.10301
Folland JP, Mc Cauley TM, Williams AG (2008) Allometric scaling of strength measurements to body size. Eur J Appl Physiol 102(6):739–745. https://doi.org/10.1007/s00421-007-0654-x
Fukumoto Y, Ikezoe T, Yamada Y, Tsukagoshi R, Nakamura M, Mori N, Kimura M, Ichihashi N (2012) Skeletal muscle quality assessed from echo intensity is associated with muscle strength of middle-aged and elderly persons. Eur J Appl Physiol 112(4):1519–1525. https://doi.org/10.1007/s00421-011-2099-5
Garlini LM, Alves FD, Ceretta LB, Perry IS, Souza GC, Clausell NO (2019) Phase angle and mortality: a systematic review. Eur J Clin Nutr 73(4):495–508. https://doi.org/10.1038/s41430-018-0159-1
Greendale GA, Sternfeld B, Huang M, Ha Karvonen-Gutierrez C, Ruppert K, Cauley JA, Finkelstein JS, Jiang SF, Karlamangla AS (2019) Changes in body composition and weight during the menopause transition. JCI Insight. https://doi.org/10.1172/jci.insight.124865
Herda TJ, Walter AA, Costa PB, Ryan ED, Stout JR, Cramer JT (2011) Differences in the log-transformed electromyographic-force relationships of the plantar flexors between high- and moderate-activated subjects. J Electromyogr Kinesiol 21(5):841–846. https://doi.org/10.1016/j.jelekin.2011.05.008
Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G (2000) Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol 10(5):361–374. https://doi.org/10.1016/s1050-6411(00)00027-4
Jenkins ND, Housh TJ, Palmer TB, Cochrane KC, Bergstrom HC, Johnson GO, Schmidt RJ, Cramer JT (2015) Relative differences in strength and power from slow to fast isokinetic velocities may reflect dynapenia. Muscle Nerve 52(1):120–130. https://doi.org/10.1002/mus.24505
Jenkins NDM, Miramonti AA, Hill EC, Smith CM, Cochrane-Snyman KC, Housh TJ, Cramer JT (2017) Greater neural adaptations following high- vs low-load resistance training. Front Physiol 8:331. https://doi.org/10.3389/fphys.2017.00331
Keller K, Engelhardt M (2013) Strength and muscle mass loss with aging process. Age and strength loss. Muscles Ligaments Tendons J 3(4):346–350
Kirchengast S, Huber J (2009) Gender and age differences in lean soft tissue mass and sarcopenia among healthy elderly. Anthropol Anz 67(2):139–151. https://doi.org/10.1127/0003-5548/2009/0018
Lang T, Streeper T, Cawthon P, Baldwin K, Taaffe DR, Harris TB (2010) Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporos Int 21(4):543–559. https://doi.org/10.1007/s00198-009-1059-y
Larsson L, Sjodin B, Karlsson J (1978) Histochemical and biochemical changes in human skeletal muscle with age in sedentary males, age 22–65 years. Acta Physiol Scand 103(1):31–39. https://doi.org/10.1111/j.1748-1716.1978.tb06187.x
Liu L, Wang PP, Roebothan B, Ryan A, Tucker CS, Colbourne J, Baker N, Cotterchio M, Yi Y, Sun G (2013) Assessing the validity of a self-administered food-frequency questionnaire (FFQ) in the adult population of Newfoundland and Labrador. Canada Nutr J 12:49. https://doi.org/10.1186/1475-2891-12-49
Lowery MM, Stoykov NS, Dewald JP, Kuiken TA (2004) Volume conduction in an anatomically based surface EMG model. IEEE Trans Biomed Eng 51(12):2138–2147. https://doi.org/10.1109/TBME.2004.836494
Magrini MA, Colquhoun RJ, Barrera-Curiel A, Thiele RM, DeFreitas JM, Smith DB, Jenkins NDM (2018) Muscle Size, Strength, Power, and Echo Intensity, but Not Specific Tension, Are Affected by Age in Physically Active Adults. Isokinet Exerc Sci 26(2):95–103. https://doi.org/10.3233/IES-172194
Martinez-Valdes E, Negro F, Falla D, De Nunzio AM, Farina D (2018) Surface electromyographic amplitude does not identify differences in neural drive to synergistic muscles. J Appl Physiol 124(4):1071–1079. https://doi.org/10.1152/japplphysiol.01115.2017
Milanovic Z, Pantelic S, Trajkovic N, Sporis G, Kostic R, James N (2013) Age-related decrease in physical activity and functional fitness among elderly men and women. Clin Interv Aging 8:549–556. https://doi.org/10.2147/CIA.S44112
Miller MS, Bedrin NG, Callahan DM, Previs MJ, Jennings ME 2nd, Ades PA, Maughan DW, Palmer BM, Toth MJ (2013) Age-related slowing of myosin actin cross-bridge kinetics is sex specific and predicts decrements in whole skeletal muscle performance in humans. J Appl Physiol 115(7):1004–1014. https://doi.org/10.1152/japplphysiol.00563.2013
Norman K, Wirth R, Neubauer M, Eckardt R, Stobaus N (2015) The bioimpedance phase angle predicts low muscle strength, impaired quality of life, and increased mortality in old patients with cancer. J Am Med Dir Assoc 16(2):173e.117-173.e122. https://doi.org/10.1016/j.jamda.2014.10.024
Oba Y, Hetzler RK, Stickley CD, Tamura K, Kimura IF, Heffernan TP Jr (2014) Allometric scaling of strength scores in NCAA division I-A football athletes. J Strength Cond Res 28(12):3330–3337. https://doi.org/10.1519/JSC.0000000000000548
Paquin J, Power GA (2018) History dependence of the EMG-torque relationship. J Electromyogr Kinesiol 41:109–115. https://doi.org/10.1016/j.jelekin.2018.05.005
Phillips SK, Rook KM, Siddle NC, Bruce SA, Woledge RC (1993) Muscle weakness in women occurs at an earlier age than in men, but strength is preserved by hormone replacement therapy. Clin Sci (Lond) 84(1):95–98. https://doi.org/10.1042/cs0840095
Piercy KL, Troiano RP (2018) Physical activity guidelines for Americans from the US department of health and human services. Circ Cardiovasc Qual Outcomes 11(11):e005263. https://doi.org/10.1161/CIRCOUTCOMES.118.005263
Pope ZK, Hester GM, Benik FM, DeFreitas JM (2016) Action potential amplitude as a noninvasive indicator of motor unit-specific hypertrophy. J Neurophysiol 115(5):2608–2614. https://doi.org/10.1152/jn.00039.2016
Reimers K, Reimers CD, Wagner S, Paetzke I, Pongratz DE (1993) Skeletal muscle sonography: a correlative study of echogenicity and morphology. J Ultrasound Med 12(2):73–77. https://doi.org/10.7863/jum.1993.12.2.73
Rozand V, Sundberg CW, Hunter SK, Smith AE (2020) Age-related deficits in voluntary activation: a systematic review and meta-analysis. Med Sci Sports Exerc 52(3):549–560. https://doi.org/10.1249/MSS.0000000000002179
Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R (2017) Prevalence of sarcopenia in the world: a systematic review and meta- analysis of general population studies. J Diabetes Metab Disord 16:21. https://doi.org/10.1186/s40200-017-0302-x
Sterczala AJ, Herda TJ, Miller JD, Ciccone AB, Trevino MA (2018) Age-related differences in the motor unit action potential size in relation to recruitment threshold. Clin Physiol Funct Imaging 38(4):610–616. https://doi.org/10.1111/cpf.12453
Tieland M, Trouwborst I, Clark BC (2018) Skeletal muscle performance and ageing. J Cachexia Sarcopenia Muscle 9(1):3–19. https://doi.org/10.1002/jcsm.12238
Trappe S, Gallagher P, Harber M, Carrithers J, Fluckey J, Trappe T (2003) Single muscle fibre contractile properties in young and old men and women. J Physiol 552(Pt 1):47–58. https://doi.org/10.1113/jphysiol.2003.044966
Verdijk LB, Koopman R, Schaart G, Meijer K, Savelberg HH, van Loon LJ (2007) Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Metab 292(1):E151-157. https://doi.org/10.1152/ajpendo.00278.2006
Watanabe Y, Yamada Y, Fukumoto Y, Ishihara T, Yokoyama K, Yoshida T, Miyake M, Yamagata E, Kimura M (2013) Echo intensity obtained from ultrasonography images reflecting muscle strength in elderly men. Clin Interv Aging 8:993–998. https://doi.org/10.2147/CIA.S47263
Weir JP, Housh TJ, Johnson GO, Housh DJ, Ebersole KT (1999) Allometric scaling of isokinetic peak torque: the Nebraska Wrestling Study. Eur J Appl Physiol Occup Physiol 80(3):240–248. https://doi.org/10.1007/s004210050588
Young HJ, Jenkins NT, Zhao Q, McCully KK (2015) Measurement of intramuscular fat by muscle echo intensity. Muscle Nerve 52(6):963–971. https://doi.org/10.1002/mus.24656
Acknowledgments
Thank you to all of the participants of this study for volunteering their time and effort. Thank you to Tyler W.D. Muddle, Ryan J. Colquhoun, and Patrick M. Tomko for your roles in data collection. The data included in this manuscript include those collected during projects supported by the Center for Integrative Research on Childhood Adversity (Award Number P20GM109097) and the American Heart Association (Award Number: 18AIREA33960528).
Funding
The data included in this manuscript include those collected during projects supported by the Center for Integrative Research on Childhood Adversity under Award Number P20GM109097 and the American Heart Association (Award Number: 18AIREA33960528).
Author information
Authors and Affiliations
Contributions
N.F.B. carried out data analysis and interpretation, and was the primary author. E.M.R. helped carry out data acquisition and interpretation. N.F.B. and N.D.M.J. created figures and tables. N.D.M.J. was the primary manuscript reviser and a substantial contributor to study concept, study design, and interpretation. All authors read, revised, and approved the final version of this manuscript. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no competing interests.
Ethics approval
This study was approved by and conducted in accordance with the guidelines and regulations of the Oklahoma State University’s Institutional Review Board (IRB Approval #s: ED-17-157 and ED-18-101) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
All participants were fully informed of the parameters of the study, and what would be expected of them if they were to participate. Participants were allotted adequate time to ask questions about the study prior to volunteering to participate, and if at any point during the duration of the study they wanted to terminate participation, they were knowingly allowed to do so. All participants signed an informed consent form prior to any data collection.
Consent for publication
We agree that if this paper is accepted the journal may publish its work.
Additional information
Communicated by William J. Kraemer.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Banks, N.F., Rogers, E.M. & Jenkins, N.D.M. Electromyographic amplitude versus torque relationships are different in young versus postmenopausal females and are related to muscle mass after controlling for bodyweight. Eur J Appl Physiol 121, 479–488 (2021). https://doi.org/10.1007/s00421-020-04532-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-020-04532-0