Abstract
Echocardiography can be used to estimate myocardial contractility by the assessment of the circumferential end-systolic stress-corrected left ventricular (LV) fractional shortening measured at midwall level (stress-corrected MWS). Whether stress-corrected MWS at rest predicts exercise peak oxygen uptake (peak V̇O2) is unknown. Also, it is not known whether the propagation rate of the early LV filling wave (E wave propagation rate, V̇ p), a new pre-load insensitive index of LV diastolic function, and echocardiographically assessed indices of arterial stiffness correlate to peak V̇O2. Accordingly, we performed echocardiographic studies and exercise tests with respiratory gas analysis in 15 young healthy male subjects (mean age 27 years, range 18–36). Neither stress-corrected-MWS (r=0.20, P=NS) nor ejection fraction (r=−0.05, P=NS) correlated significantly with peak V̇O2. Adjustment for age and resting heart rate had no effect on the results. In separate multiple regression models adjusting for standard covariates (age, LV size and heart rate), peak V̇O2 correlated with V̇ p (beta=0.98, P<0.01), as well as with E/A (beta=0.85, P<0.01), and with the isovolumic relaxation time (indicator of LV relaxation) (beta=−0.59, P<0.05). Arterial stiffness indices showed no significant relation to peak V̇O2. We conclude that in young healthy male subjects, resting myocardial contractility and arterial stiffness are not significant correlates of peak V̇O2, whereas LV diastolic function, and in particular V̇ p, influences the variability of peak V̇O2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peak oxygen consumption (peak V̇O2) is a measure of exercise performance. While resting left ventricular (LV) ejection fraction (EF, the percentage of LV end-diastolic volume ejected in systole) does not predict peak V̇O2, an index of LV diastolic function, the ratio of early (E) to atrial contraction-dependent (A) transmitral waveform peak velocities (E/A), is related to peak V̇O2 (Vanoverschelde et al. 1993). However, EF is influenced by preload and afterload and is not a reliable indicator of LV myocardial contractility (de Simone et al. 1999). LV myocardial contractility can be assessed echocardiographically by means of circumferential LV midwall end-systolic stress-adjusted midwall fractional shortening (MWS), the stress-corrected MWS (de Simone et al. 1994). In addition, stress-corrected MWS is more closely related than EF to E/A (Palmieri et al. 1999a) and to LV early relaxation (Bella et al. 2001), measured by means of isovolumic relaxation time (IVRT). The E/A is a loading-dependent indicator of LV diastolic function which is only in part related to LV relaxation (Oh et al. 1997), whereas the relation of E wave propagation rate (V̇ p, the propagation rate of the early transmitral filling wave, a more load-independent index of LV diastolic dynamic) (Garcia et al. 2000) to peak V̇O2 has not been reported.
In addition, arterial mechanics have a role in determining exercise capability (Vaitkevicius et al. 1993; Yin et al. 1981). Arterial stiffness contributes to generate the blood pressure profile during LV ejection phase and is, therefore, a determinant of LV afterload (Stergiopulos et al. 1999). The LV function and arterial mechanics are inter-related (Starling 1993). However, relationships of echocardiographic indices of arterial stiffness with peak V̇O2 have not been reported. Therefore, we assessed whether stress-corrected MWS, V̇ p and echocardiographic indices of arterial stiffness are correlates of peak V̇O2.
Methods
This study enrolled 15 healthy young male volunteers who trained for 60–90 min, two times per week (Table 1). All participants underwent echocardiography and, subsequently, exercise testing. Both evaluations were performed before noon on a single day while subjects were fasting. The study protocol was approved by the local ethics committee and each participant gave informed consent.
Echocardiography
The LV structure and function were evaluated according to a standard protocol (Palmieri et al. 1999b) using a General Electric VIVID 7 echocardiographic machine (Horten, Norway, software version 1.1.5/1004) equipped with a multifrequency matrix probe 1.5–4 MHz (model M3S), a digital storage system and videotape recorder. Two-dimensionally guided M-mode tracings were used to measure LV structure, if they were correctly oriented anatomically. Alternatively, linear measurements of LV structure from two-dimensional parasternal long-axis views were used (Schiller et al. 1989).
The LV measurements were averaged from two to five cardiac cycles from views in which LV internal dimension was maximized following recommendation of the American Society of Echocardiography (Sahn et al. 1978). End-diastolic LV dimensions were used to calculate LV mass by a formula that yields values which are highly correlated to LV weight determined by autopsy (Devereux et al. 1986). Also, procedures followed for assessment of LV structure and function are known to be highly reliable (Palmieri et al. 1999b). All measurements were made by a single reader (V.P.), blinded to the subject’s name and clinical information.
Haemodynamics and vascular compliance
Echocardiographic stroke volume was assessed by Doppler, as the product of the time–velocity integral of the aortic flow at the aortic anulus and the aortic anulus area (Devereux et al. 1997). Cardiac output was stroke volume × heart rate, and stroke index was stroke volume/body surface area. Total arterial compliance was assessed as the ratio of brachial pulse pressure/Doppler stroke index (Palmieri et al. 2003). The arterial compliance of the descending aorta was assessed by sampling blood flow using Doppler echocardiography at the transition from thoracic to abdominal aortic segment:
with the assumption that the higher the diastolic flow compared to that in systole, the higher the contribution of the aortic elasticity to forward blood flow (Shimamoto et al. 1996).
LV systolic chamber function and myocardial contractility
The EF, LV MWS and the circumferential end-systolic stress were computed using echocardiographic data (de Simone et al. 1994). The EF was:
LV volumes were calculated from LV linear measurements (Teichholz et al. 1976). The EF represents global LV chamber systolic function if ventricular wall motion is uniform. However, because ejection-phase indices of LV performance depend on afterload, measurement of myocardial afterload is helpful to determine whether LV function reflects myocardial contractile performance. A noninvasive measure of myocardial afterload is the circumferential end-systolic stress, measured using the end-systolic LV measurement and simultaneous cuff blood pressure (Gaasch et al. 1989):
where LVIDs is LV internal systolic diameter, and PWTs is the LV posterior wall thickness in systole. Ejection-phase indices of LV function are negatively related to circumferential end-systolic stress in both normotensive and hypertensive subjects (de Simone et al. 1994). Because the majority of the myocardial fibres at the mid LV cavity are oriented circumferentially, LV shortening was also assessed at the midwall (Shimizu et al. 1991) and compared to MWS predicted for measured circumferential end-systolic stress calculated using an equation developed in a reference population (de Simone et al. 1994). The ratio per cent of observed over predicted MWS was termed stress-corrected MWS and indicates stress-corrected myocardial contractility (de Simone et al. 1994). This approach allows assessment of myocardial contractility because myocardial shortening and stress are on the same axis.
LV diastolic dynamics
All parameters of diastolic function were measured off-line by an in-house non-commercial multi-media system (EchoReviewSystem). The LV filling was evaluated by pulsed Doppler, placing the sample volume in the centre of the tips of the mitral leaflets, with the cursor beam perpendicular to the mitral plane in the apical four-chamber view. Peak E and A waveform velocities were measured starting from the E–A waveform with highest E wave. The E deceleration time (EDT), and indicator of LV passive relaxation, was calculated as the time from E peak to the point identified by the tangent to the E waveform descending shoulder on the baseline. The V̇ p was assessed by colour M-mode, with cursor-beam placed through the left ventricle perpendicular to the mitral anulus. The aliasing velocity limit of the colour Doppler scale was set at ±55 cm s−1, shifting the colour baseline toward the LV apex to see low flow velocities. The sweep rate was at 100 cm s−1, while the subject was asked to hold his breath. The V̇ p was identified by a standard method previously described (Garcia et al. 1998). The IVRT was assessed in apical five-chamber view by continuous Doppler, using colour Doppler guide to identify the inflow–outflow interface in the LV output tract. The IVRT, indicating the early LV relaxation phase, was measured as the time from the end of aortic flow to the beginning of the E wave while the subject was invited to hold the breath.
Exercise
Exercise was a multistage treadmill protocol with 2-min step increments until exhaustion (modified Bruce protocol). Respiratory gas analysis was recorded on metabolic chart (Benchmark Exercise Test System, Morgan, Bologna, Italy). The V̇O2, carbon dioxide production and minute ventilation were measured as moving averages of eight breaths. During each stage of exercise, 12-lead ECG, heart rate and blood pressure were recorded. All subjects were encouraged to exercise until exhaustion, and had a peak respiratory exchange ratio >1.1. Maximal exercise capability was defined by the peak V̇O2, expressed as the average of the last minute of exercise.
Statistical analysis
Data in tables are means with standard deviations and ranges in parentheses. All continuous variables were log-transformed before the use of parametric tests. Bivariate Pearson correlation was used to evaluate relationships of peak V̇O2 to LV systolic and diastolic function, arterial function, exercise blood pressure and heart rate. Separate and alternative multiple regression analyses were used to assess relations of peak V̇O2 to indices of LV systolic function, arterial compliance and exercise blood pressure controlling for resting heart rate and age, potentially expressing duration and level of physical training, as well as to evaluate relations of peak V̇O2 to LV diastolic function controlling for age, resting heart rate and LV diastolic diameter. P<0.05 indicated statistical significance.
Results
The EF was not related to peak V̇O2 (Table 2), as confirmed by a subsequent partial correlation controlling for circumferential end-systolic stress (partial r=−0.08). Similarly, the stress-corrected MWS did not show a significant relation to peak V̇O2 (r=0.20, P=0.4). After accounting for age and resting heart rate, relations of EF and stress-corrected MWS to peak V̇O2 remained non-significant. The peak V̇O2 did not show significant relations to LV diastolic and systolic diameters. The LV mass index as well as stroke volume showed no statistically significant relation to peak V̇O2.
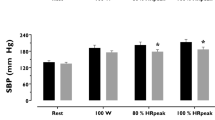
Peak V̇O2 was related to the exercise percentage increment of systolic blood pressure (r=0.53, P<0.05), but not to the exercise percentage increase in diastolic blood pressure or heart rate (r=0.06, r=0.1 respectively). Peak V̇O2 did not show a relationship to peak exercise systolic (r=0.31) and diastolic (r=-0.16) blood pressure, or peak heart rate (r=0.13). After adjustment for age and heart rate, the correlation of exercise percentage increment of systolic blood pressure with peak V̇O2 was confirmed (beta=0.78, P<0.05).
The peak V̇O2 showed no statistically significant relations to the pulse pressure/stroke index and the descending aorta compliance index (Table 2). Adjustment for mean blood pressure, a potential confounding factor, did not impact on results (data not shown).
In contrast, peak V̇O2 showed a positive relationship with V̇ p (Table 2). The peak V̇O2 also showed a relation to the E/A. The relation of peak V̇O2 to E/A was due to the relation between peak E wave velocity and peak V̇O2 (r=0.71, P=0.01), whereas peak A wave velocity bore no correlation to peak V̇O2. Peak E wave was not significantly related to LV diastolic diameter. Relations of indicators of early and passive LV relaxation, IVRT and EDT, to peak V̇O2, did not reach the statistical significance. In multivariate analyses adjusting for age, LV diastolic diameter (index of LV pre-load) and heart rate, relations of E/A and V̇ p to peak V̇O2 were confirmed. The relation of V̇ p to peak V̇O2 remained (beta=0.76, P<0.01) in a multivariate model controlling for age, heart rate, LV mass and circumferential end-systolic stress.
In a multiple regression analysis controlling for age, resting heart rate and stress-corrected MWS, peak V̇O2 was related to V̇p (beta=0.96, P=<0.01, R 2=0.77). In a subsequent model including E/A for V̇p and using the same set of covariates, E/A showed an independent relation to peak V̇O2 (beta=0.80, P<0.05, R 2=0.40). In another multiple regression model including IVRT as a parameter of diastolic function, and age, resting heart rate and stress-corrected MWS as covariates, longer IVRT did not show a significant relation to peak V̇O2 (beta=−0.46, R 2=0.24). Similarly, when EDT was considered as a LV diastolic function parameter, EDT did not show an independent relation to peak V̇O2 (beta=0.19, R 2=0.11). In a multivariate model, peak V̇O2 was related to V̇ p (beta=0.90, P<0.01, R 2=0.81) independently of age, resting heart rate, stress-corrected MWS and IVRT.
Discussion
We examined the relationships of myocardial systolic and diastolic function and arterial stiffness to peak V̇O2 in a group of 15 healthy young male subjects. The first new finding of our study was that peak V̇O2 showed no significant correlation with resting stress-corrected MWS, an index of myocardial contractility. We also confirmed that at rest LV chamber systolic function, measured as EF, showed no significant relation to peak V̇O2 (Di Bello et al. 1995; Dubach et al. 1997; Leung et al. 1997). Peak V̇O2 is related to peak cardiac output (Di Bello et al. 1996; Schulman et al. 1996). In a group of mildly hypertensive and normotensive subjects, stress-corrected MWS was lower in hypertensive than in normotensive subjects both at baseline and during exercise despite an approximately similar peak V̇O2 reached by the two groups (Palatini et al. 2001).
We found a strong correlation between V̇ p, a measure of efficiency of LV diastolic function not previously used in similar studies, and peak V̇O2. Moreover, V̇ p was more strongly related than E/A, IVRT and EDT to peak V̇O2; the relation of V̇ p to peak V̇O2 was independent of age, heart rate, LV loading-condition, myocardial contractility and IVRT. Although the relation between LV diastolic function and peak V̇O2 was expected (Di Bello et al. 1996; Vanoverschelde et al. 1993), we demonstrated that LV haemodynamic conditions and LV relaxation account only in part for the relation between the V̇ p and peak V̇O2. It appeared that the atrio–ventricular pressure gradient (determining the E peak velocity) and the intra-ventricular flow propagation rate (measured by means of V̇ p) at the beginning of the diastolic interval are important correlates of peak V̇O2. In addition, 81% of the variability of peak V̇O2 was explained by a multivariate model including V̇ p, whereas the multivariate model including E/A for V̇ p explained only 40% of the variability of peak V̇O2. Given the fact that the increase in cardiac output during exercise is in large part achieved by increasing heart rate (Wyns et al. 1982), and that, in elite athletes, greater venous return appears to contribute to maintaining LV end-diastolic volume at a constant level despite increase in heart rate and shortening of diastolic interval during exercise (Di Bello et al. 1995), the data suggest that better LV diastolic properties quantified at rest may predict a better adaptation of the pump performance during exercise with favourable direct impact on peak V̇O2 (Clausen et al. 1976; Vanoverschelde et al. 1993).
We also evaluated the relations of echocardiographic indices of arterial stiffness at rest with peak V̇O2, and found no significant relations of peak V̇O2 to the pulse pressure/stroke index or to an index of compliance of the descending aorta. However, the relations were in the direction of higher peak V̇O2 associated with lower arterial stiffness, independent of covariates. During exercise, systolic blood pressure increased only by an average of 49%, whereas heart rate increased by a mean of 192%. Increments in systolic blood pressure correlated with peak V̇O2, suggesting that in normal young subjects exercise-induced increase in systolic blood pressure, to which arterial stiffness may contribute, may represent the capability of the left ventricle to generate stroke work rather than a factor limiting LV ejection phase. However, the relations of arterial stiffness and exercise capability may change in case of increased arterial stiffness, as seen in hypertensive subjects, and in the elderly.
Conclusions
In normal young men, peak V̇O2 is not related to echocardiographically estimated parameters of LV chamber and myocardial systolic function at rest. Conversely, LV diastolic dynamic at rest correlated with peak V̇O2, independent of myocardial contractility, heart rate and preload. In addition, echocardiographic indices of arterial stiffness did not show a significant relationship with peak V̇O2.
References
Bella JN, Palmieri V, Liu JE, Kitzman DW, Oberman A, Hunt SC, Hopkins PN, Rao DC, Arnett DK, Devereux RB (2001) Relationship between left ventricular diastolic relaxation and systolic function in hypertension: The Hypertension Genetic Epidemiology Network (HyperGEN) Study. Hypertension 38:424–428
Clausen JP, Trap-Jensen J (1976) Heart rate and arterial blood pressure during exercise in patients with angina pectoris. Effects of training and of nitroglycerin. Circulation 53:436–442
De Simone G, Devereux RB, Roman MJ, Ganau A, Saba PS, Alderman MH, Laragh JH (1994) Assessment of left ventricular function by the midwall fractional shortening/end-systolic stress relation in human hypertension. J Am Coll Cardiol 23:1444–1451 [erratum in J Am Coll Cardiol (1994) 24:844]
De Simone G, Devereux RB, Celentano A, Roman MJ (1999) Left ventricular chamber and wall mechanics in the presence of concentric geometry. J Hypertens 17:1001–1006
Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N (1986) Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 57:450–458
Devereux RB, Roman MJ, Paranicas M, O’Grady MJ, Wood EA, Howard BV, Welty TK, Lee ET, Fabsitz RR (1997) Relations of Doppler stroke volume and its components to left ventricular stroke volume in normotensive and hypertensive American Indians: the Strong Heart Study. Am J Hypertens 10:619–628
Di Bello V, Talarico L, Di Muro C, Santoro G, Bertini A, Giorgi D, Caputo MT, Bianchi M, Cecchini L, Giusti C (1995) Evaluation of maximal left ventricular performance in elite bicyclists. Int J Sports Med 16:498–506
Di Bello V, Santoro G, Talarico L, Di Muro C, Caputo MT, Giorgi D, Bertini A, Bianchi M, Giusti C (1996) Left ventricular function during exercise in athletes and in sedentary men. Med Sci Sports Exerc 28:190–196
Dubach P, Myers J, Dziekan G, Goebbels U, Reinhart W, Muller P, Buser P, Stulz P, Vogt P, Ratti R (1997) Effect of high intensity exercise training on central hemodynamic responses to exercise in men with reduced left ventricular function. J Am Coll Cardiol 29:1591–1598
Gaasch WH, Zile MR, Hoshino PK, Apstein CS, Blaustein AS (1989) Stress-shortening relations and myocardial blood flow in compensated and failing canine hearts with pressure-overload hypertrophy. Circulation 79:872–883
Garcia MJ, Thomas JD, Klein AL (1998) New Doppler echocardiographic applications for the study of diastolic function. J Am Coll Cardiol 32:865–875
Garcia MJ, Smedira NG, Greenberg NL, Main M, Firstenberg MS, Odabashian J, Thomas JD (2000) Color M-mode Doppler flow propagation velocity is a preload insensitive index of left ventricular relaxation: animal and human validation. J Am Coll Cardiol 35:201–208
Leung DY, Griffin BP, Snader CE, Luthern L, Thomas JD, Marwick TH (1997) Determinants of functional capacity in chronic mitral regurgitation unassociated with coronary artery disease or left ventricular dysfunction. Am J Cardiol 79:914–920
Oh JK, Appleton CP, Hatle LK, Nishimura RA, Seward JB, Tajik AJ (1997) The noninvasive assessment of left ventricular diastolic function with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr 10:246–270
Palatini P, Frigo G, Visentin P, Mario L, Mormino P, Pessina AC (2001) Left ventricular contractile performance in the early stage of hypertension in humans. Eur J Appl Physiol 85:118–124
Palmieri V, Bella JN, DeQuattro V, Roman MJ, Hahn RT, Dahlof B, Sharpe N, Lau CP, Chen WC, Paran E, de Simone G, Devereux RB (1999a) Relations of diastolic left ventricular filling to systolic chamber and myocardial contractility in hypertensive patients with left ventricular hypertrophy (The PRESERVE Study). Am J Cardiol 84:558–562
Palmieri V, Dahlöf B, DeQuattro V, Sharpe N, Bella JN, de Simone G, Paranicas M, Fishman D, Devereux RB (1999b) Reliability of echocardiographic assessment of left ventricular structure and function: the PRESERVE study. J Am Coll Cardiol 34:1625–1632
Palmieri V, Bella JN, Roman MJ, Gerdts E, Papademetriou V, Wachtell K, Nieminen MS, Dahlof B, Devereux RB (2003) Pulse pressure/stroke index and left ventricular geometry and function: the LIFE Study. J Hypertens 21:781–787
Sahn DJ, DeMaria A, Kisslo J, Weyman A (1978) Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation 58:1072–1083
Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I (1989) Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, subcommittee on quantitation of two-dimensional echocardiograms. J Am Soc Echocardiogr 2:358–367
Schulman SP, Fleg JL, Goldberg AP, Busby-Whitehead J, Hagberg JM, O’Connor FC, Gerstenblith G, Becker LC, Katzel LI, Lakatta LE, Lakatta EG (1996) Continuum of cardiovascular performance across a broad range of fitness levels in healthy older men. Circulation 94:359–367
Shimamoto H, Shimamoto Y (1996) Lisinopril reverses left ventricular hypertrophy through improved aortic compliance. Hypertension 28:457–463
Shimizu G, Hirota Y, Kita Y, Kawamura K, Saito T, Gaasch WH (1991) Left ventricular midwall mechanics in systemic arterial hypertension. Myocardial function is depressed in pressure-overload hypertrophy. Circulation 83:1676–1684
Starling MR (1993) Left ventricular-arterial coupling relations in the normal human heart. Am Heart J 125:1659–1666
Stergiopulos N, Westerhof N (1999) Role of total arterial compliance and peripheral resistance in the determination of systolic and diastolic aortic pressure. Pathol Biol (Paris) 47:641–647
Teichholz LE, Kreulen T, Herman MV, Gorlin R (1976) Problems in echocardiographic volume determinations: echocardiographic- angiographic correlations in the presence of absence of asynergy. Am J Cardiol 37:7–11
Vaitkevicius PV, Fleg JL, Engel JH, O’Connor FC, Wright JG, Lakatta LE, Yin FC, Lakatta EG (1993) Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation 88:1456–1462
Vanoverschelde JJ, Essamri B, Vanbutsele R, d’Hondt A, Cosyns JR, Detry JR, Melin JA (1993) Contribution of left ventricular diastolic function to exercise capacity in normal subjects. J Appl Physiol 74:2225–2233
Wyns W, Melin JA, Vanbutsele RJ, De Coster PM, Steels M, Piret L, Detry JM (1982) Assessment of right and left ventricular volumes during upright exercise in normal men. Eur Heart J 3:529–536
Yin FC, Weisfeldt ML, Milnor WR (1981) Role of aortic input impedance in the decreased cardiovascular response to exercise with aging in dogs. J Clin Invest 68:28–38
Acknowledgement
This study was supported in part by the Educational Fund of PhD program “XVI ciclo”, 2002.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Palmieri, V., Palmieri, E.A., Arezzi, E. et al. Peak exercise oxygen uptake and left ventricular systolic and diastolic function and arterial mechanics in healthy young men. Eur J Appl Physiol 91, 664–668 (2004). https://doi.org/10.1007/s00421-003-0999-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-003-0999-8