Abstract
Purpose
Whether blood volume (BV) primarily determines the synchronous nature of the myocardium remains unknown. This study determined the impact of standard blood withdrawal on left ventricular mechanical dyssynchrony (LVMD) in women.
Methods
Transthoracic speckle-tracking echocardiography and central hemodynamic measurements were performed at rest and during moderate- to high-intensity exercise in healthy women (n = 24, age = 53.6 ± 16.3 year). LVMD was determined via the time to peak standard deviation (TPSD) of longitudinal and transverse strain and strain rates (LSR, TSR). Measurements were repeated within a week period immediately after a 10% reduction of BV.
Results
With intact BV, all individuals presented cardiac structure and function variables within normative values of the study population. Blood withdrawal decreased BV (5.3 ± 0.7 L) by 0.5 ± 0.1 L. Resting left ventricular (LV) end-diastolic volume (− 8%, P = 0.040) and passive filling (− 16%, P = 0.001) were reduced after blood withdrawal. No effect of blood withdrawal was observed for any measure of LVMD at rest (P ≥ 0.225). During exercise at a fixed submaximal workload (100 W), LVMD of myocardial longitudinal strain (LS TPSD) was increased after blood withdrawal (36%, P = 0.047). At peak effort, blood withdrawal led to increased LVMD of myocardial transverse strain rate (TSR TPSD) (31%, P = 0.002). The effect of blood withdrawal on TSR TPSD at peak effort was associated with LV concentric remodeling (r = 0.59, P = 0.003).
Conclusion
Marked impairments in the mechanical synchrony of the myocardium are elicited by moderate blood withdrawal in healthy women during moderate and high intensity exercise.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The dependence of heart function on the filling of the circulatory system, i.e., blood volume (BV), has been experimentally established for over a century (Diaz-Canestro et al. 2021a; Patterson and Starling 1914; Diaz-Canestro and Montero 2022a; Covell et al. 1966). At the organ level, the reduction in cardiac filling and preload induced even by moderate blood withdrawal (− 10%) results in lower myocardial contractile capacity according to the Frank-Starling mechanism, entailing a reduction in peak stroke volume (SV) and myocardial blood flow (Covell et al. 1966; Diaz-Canestro et al. 2021a). The volumetric change of internal cardiac dimensions thus affects the output, without seemingly modulating the inner structure and basal function of the heart (Diaz-Canestro and Montero 2022a; Diaz-Canestro et al. 2021a). In this regard, the cardiac wall phenotype is presumed to be unaltered, at least in the short term, by changes in BV, albeit overall measures of left ventricular (LV) mechanics are known to be modulated by LV filling (Williams et al. 2016). Likewise, subtle measurements have provided indirect evidence of large effects of LV filling on LV mechanical dyssynchrony (LVMD) (Kim et al. 2011). Importantly, the mechanical synchrony among myocardial fibers modulates the efficiency of cardiac contraction/relaxation and strongly predicts cardiac maladaptations such as LV concentric hypertrophy along with cardiovascular mortality (Shah and Solomon 2016; Biering-Sorensen et al. 2017; Modin et al. 2018; Santos et al. 2014; Morris et al. 2012; Phan et al. 2010). Yet, to date, whether BV primarily determines LVMD remains to be elucidated. The answer to this question is particularly relevant in the female population, since women are prone LV concentric remodeling and body-size adjusted hypovolemia relative to men (Diaz-Canestro and Montero 2020; Diaz-Canestro et al. 2021b; Regitz-Zagrosek and Kararigas 2017).
This study aimed to experimentally determine the impact of blood withdrawal on the mechanical synchronous nature of the myocardium in healthy women throughout the adult lifespan. Measurements were performed at rest as well as during moderate to high intensity exercise, in order to magnify potential effects of BV on LVMD in the progression towards the limits of myocardial work capacity. An exercise, rather than pharmacological, stress model was chosen for its greater physiological relevance and established major effects on myocardial mechanics (Tan et al. 2013; Williams et al. 2016). We hypothesized that moderate blood withdrawal would increase LVMD in women, an effect that would be augmented with increasing myocardial work. Central hemodynamic variables were concomitantly assessed to obtain an integrative view of the circulatory consequences of BV-dependent changes in LVMD.
Methods
Study participants
Twenty-four healthy adult female individuals (23–77 year) were recruited via electronic and printed advertisements on community notice boards in the city of Calgary. Moderate-to-vigorous physical activity (MVPA) levels were assessed as previously detailed (Montero et al. 2016). Inclusion criteria comprised healthy status according to clinical questionnaires and resting cardiac and hemodynamic screening, absence of current medical symptoms or medication limiting exercise testing, and no history of cardiac, pulmonary or neuromuscular diseases. The majority of women were postmenopausal (n = 17), the remaining ones reported to present normal menstruation (n = 7). Only individuals with complete cardiac strain analyses at all assessment points, as detailed in the “Measurements” section, were included. The study was approved by the Conjoint Health Research Ethics Board (REB18-1654) of the University of Calgary and conducted in accordance with the declaration of Helsinki. All participants provided informed oral and written consents before starting the measurements.
Study design
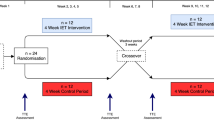
Participants were required to report to our laboratory on two occasions. Time of day of testing sessions was kept consistent for each participant with a minimum of 48 h and a maximum of 7 days between the first (baseline) and second (phlebotomy) sessions. All individuals were instructed to avoid strenuous exercise, alcohol and caffeine 24 h prior to testing as well as to maintain their usual baseline activity and daily dietary habits throughout the study period. In the phlebotomy session, measurements were performed immediately after the withdrawal of 10% of BV on an individual basis via a 20 G venflon (BD, USA) placed in the median cubital vein. All measurements were performed after a 5-h fasting period in a quiet room with controlled temperature between 22 and 23 °C.
Measurements
Blood volume (BV), red blood cell volume (RBCV), plasma volume (PV) Intravascular volumes were determined using the classic carbon monoxide (CO) rebreathing technique integrated in a semi-automated system with a very low typical error of measurement (TE ≤ 1.2%) as previously described in detail (Montero et al. 2015, 2017; Siebenmann et al. 2017). In brief, following 20 min of supine rest, 2 ml of blood (baseline) was sampled from the participant’s median cubital vein via a 20-G venflon (BD, USA) and analysed immediately in duplicate for percent carboxyhemoglobin (%HbCO), hemoglobin (Hb) concentration and hematocrit (Hct) (ABL80, Radiometer, Denmark). Then, they breathed 100% O2 for 4 min to flush the nitrogen from the airways. After closing the O2 input, a bolus of 1.5 ml/kg of 99.997% chemically pure CO (CO N47, Air Liquide, France) was administrated into the breathing circuit. The participants rebreathed this gas mixture for 10 min. An additional 2 ml blood sample was obtained and analysed in duplicate as aforementioned. The change in %HbCO is used to calculate circulating hemoglobin mass (Hbmass), taking into account the amount of CO that remains in the rebreathing circuit at the end of the procedure. BV, total red blood cell volume (RBCV) and plasma volume (PV) were determined from Hbmass, Hb concentration and Hct (Montero et al. 2015, 2017; Siebenmann et al. 2017).
Stress echocardiography and central hemodynamics Two-dimensional (2D) apical cine-loops were recorded via high-resolution ultrasound (Mindray Medical M9, USA) at supine rest, during predetermined levels of incremental exercise relative to peak heart rate (HRpeak) (80 and 100% HRpeak) and at a fixed submaximal absolute workload (100 W). Exercise measurements were performed in a supine cycle ergometer designed to facilitate the precision of echocardiography and hemodynamic measurements as well as the attainment of peak aerobic capacity in the recommended duration (7–10 min) via 10–30 W increments (Astorino et al. 2004), as previously detailed (Diaz-Canestro et al. 2021b, c). Following the American Society of Echocardiography and the European Association of Cardiovascular Imaging recommendations, cardiac chamber quantification was completed offline using the modified Simpson method (biplane method of disks) by tracing the endocardial border of the LV at end-diastole and end-systole at a target frame rate of 30 Hz (range 25–40 Hz) (Lang et al. 2015; Pellikka et al. 2007). Diastolic function was assessed via transmitral inflow velocities determined by pulsed-wave Doppler, with the sample volume placed between the mitral leaflet tips. The peak inflow velocities during early (E) and late (A) diastole were assessed, and the E/A velocity ratio was calculated. Myocardial tissue e’ and a’ velocities were measured via tissue Doppler imaging, and the E/e’ ratio was calculated. The recommended Cube algorithm was used to estimate left ventricular mass (LVmass = 0.8 (1.04 [(interventricular septum thickness at diastole (IVSd) + LV internal diameter at diastole (LVIDd) + LV posterior wall thickness at diastole (LVPWd))3 − LVIDd3]) + 0.6) (Lang et al. 2015). Left ventricular relative wall thickness (LVRWT), an index of LV concentric hypertrophy, was determined according to the formula: LVRWT = 2 × LVPWd/LVIDd. In addition, systolic arterial blood pressure (SBP), diastolic arterial blood pressure (DBP) and mean arterial blood pressure (MAP) were continuously assessed non-invasively via the volume-clamp method and finger plethysmography adjusted to the heart level (Finometer PRO, Finapres Medical Systems, Netherlands), with data exported into a pre-established acquisition software (Labchart 7, AD Instruments, UK). SV was calculated as left ventricular end-diastolic volume (LVEDV) minus left ventricular end-systolic volume (LVESV), while the product of SV and HR provided cardiac output (Q). Total peripheral resistance (TPR) was determined as the ratio of MBP and Q. Finally, the rate pressure product (RPP), an index of myocardial work and oxygen (O2) consumption, was calculated via the product of SBP and HR. Myocardial O2 consumption was not directly measured due to its invasive nature. The reproducibility of key echocardiographic and hemodynamic measurements (intraobserver coefficient of variation (CV)) during exercise in our laboratory is ≤ 6% for LV volumes and ≤ 3% for blood pressures.
Speckle-tracking strain analysis and left ventricular mechanical dyssynchrony (LVMD) Myocardial speed, longitudinal (LS) and transverse strains (TS) were analyzed offline via speckle tracking echocardiography in the standard apical 4-chamber (A4C) view with dedicated software (UltraView, Mindray Medical, USA). The longitudinal mechanical plane (henceforth referred to ‘axis’ according to the customary use in the field) spans from the base to the apex of the myocardium, while the transverse mechanical axis entails the shortest distance from the inner to the outer layer of the myocardium. Automated ECG-guided imaging was performed in all LV myocardial A4C segments (basal anterolateral, basal inferoseptal, mid anterolateral, mid inferoseptal, apical lateral, apical septal, apex). LVMD was assessed via the dispersion (standard deviation) of the time to peak (TPSD) of LS (%) and TS (%), and their respective strain rates (s−1) (LSR, TSR) (Haugaa et al. 2012). Among available methods to quantify LVMD, TPSD of LV longitudinal and transverse axes has demonstrated superior prognostic power in prior clinical studies (Haugaa et al. 2010; Lim et al. 2008; Miyazaki et al. 2008; Modin et al. 2018). Raw strain data were not analyzed due to the known frame rate-dependency (Rosner et al. 2015). The nil influence of frame rate on TPSD variables has been confirmed in our laboratory (Supplemental Fig. 1). All analyses were performed by a researcher blinded to participant characteristics and group assignment. The quality of speckle tracking analyses was verified at all assessment points (rest, 100 W, 80 and 100% HRpeak) (1–5 scale) and participants presenting with low quality or missing cine-loops were excluded from the study. Reproducibility of TPSD variables was determined using the Bland–Altman method and expressed as mean bias and SD (Biering-Sorensen et al. 2017; Bland and Altman 1986). From duplicate analyses (n = 20), the mean bias for intraobserver reproducibility was 4.0 ± 25.4, 7.1 ± 38.1, 3.6 ± 31.2 and 4.9 ± 30.7 ms for LS TPSD, LSR TPSD, TS TPSD and TSR TPSD at rest, respectively. Corresponding mean bias values for intraobserver reproducibility at the peak exercise intensity were 9.0 ± 18.1, 4.3 ± 22.4, 6.6 ± 22.1 and 1.1 ± 20.8 ms.
Statistical analysis
Statistical analysis was performed using SPSS 26.0 (SPSS, USA). Data were tested for normal distribution with the Kolmogorov–Smirnov test and for homogeneity of variances with the Levene’s test. The effect of blood withdrawal on cardiac and hemodynamic variables was assessed via paired sample t tests. The relationship of phlebotomy-induced changes (2nd visit-phlebotomy minus 1st visit-baseline values) in LVMD (∆LVMD) with cardiac function, structure and hemodynamics at rest and during exercise was evaluated using linear regression analyses and the determination of the Pearson’s correlation coefficient (r). Likewise, the potential influence of age on ∆LVMD was assessed via linear regression analyses and r. All data are reported as mean (± SD) unless otherwise stated. A two-tailed P-value less than 0.05 was considered significant.
Results
Baseline characteristics
Main characteristics of the study participants are shown in Table 1. All individuals were non-smokers, non-obese (body mass index = 22.4 ± 2.5 kg m−2) and moderately active (MVPA = 5.9 ± 2.8 h wk−1). Hormone replacement therapy (HRT) was used by a minority of participants (n = 3) and did not affect the results of the study. Resting cardiac structure and function closely concurred with normative echocardiography data according to age and sex (Kou et al. 2014; Patel et al. 2021). The established 10% blood withdrawal reduced baseline BV (5.3 ± 0.7 L) by 0.5 ± 0.1 L, including decreases of 0.3 and 0.2 l in PV and RBCV, respectively. Baseline Hb concentration (13.1 ± 0.8 vs. 13.1 ± 0.9 g dL−1, P = 0.533) and Hct (40.1 ± 2.3 vs. 39.9 ± 2.6%, P = 0.416) were not affected by blood withdrawal. In contrast, blood withdrawal induced decrements in resting LVEDV (− 8%, P = 0.040) and LV passive filling (− 16% in mitral E wave and − 13% in myocardial e’, P ≤ 0.001).
Rate pressure product (RPP), cardiac volumes and output (Q)
Figure 1 presents HR, SBP and RPP before and after blood withdrawal. Heart rate at a fixed submaximal absolute workload (100 W) was increased by blood withdrawal (121 ± 17 vs. 130 ± 21 bpm, P = 0.009). At high relative exercise intensities (80 and 100% HRpeak), blood withdrawal led to decrements in SBP and RPP (− 12 to − 15%, P ≤ 0.021). With respect to cardiac volumes, blood withdrawal did not alter LV volumes and Q at the fixed moderate submaximal workload (P ≥ 0.067), but did decrease LVEDV, SV and Q (− 11 to − 17%, P ≤ 0.009) at high relative exercise intensities (80 and 100% HRpeak).
Rate pressure product (RPP) at rest and during exercise before and after blood withdrawal. Data are reported as mean ± SEM. *P < 0.05 compared with before blood withdrawal. The magnitude of blood withdrawal was fixed (10%) for all individuals. Measurements during exercise were performed at a given absolute workload (100 W) and at relative intensities (80 and 100% of HRpeak). HR, heart rate; HRpeak, peak heart rate; RPP, rate pressure product; SBP, systolic blood pressure
Left ventricular mechanical dyssynchrony (LVMD)
LVMD in the longitudinal and transverse axes before and after blood withdrawal is displayed in Figs. 2 and 3, respectively. At rest, blood withdrawal did not alter any measure of LVMD (P ≥ 0.225). During exercise at the fixed moderate submaximal workload (100 W), LVMD of myocardial strain in the longitudinal axis was increased (+ 36%) after blood withdrawal (LS TPSD: 36.2 ± 24.9 vs. 49.1 ± 23.2 ms, P = 0.047). At peak effort (100% HRpeak) in the transverse axis, blood withdrawal augmented (+ 31%) LVMD of myocardial strain rate (TSR TPSD: 45.9 ± 15.0 vs. 60.3 ± 24.3 ms, P = 0.002).
Longitudinal left ventricular mechanical dyssynchrony (LVMD) at rest and during exercise before and after blood withdrawal. Data are reported as mean ± SEM. *P < 0.05 compared with before blood withdrawal. The magnitude of blood withdrawal was fixed (10%) for all individuals. Measurements during exercise were performed at a given absolute workload (100 W) and at relative intensities (80 and 100% of HRpeak). The longitudinal mechanical axis spans from the base to the apex of the myocardium. HR, heart rate; HRpeak, peak heart rate; LS, longitudinal strain; LSR, longitudinal strain rate; TPSD, time to peak standard deviation
Transverse left ventricular mechanical dyssynchrony (LVMD) at rest and during exercise before and after blood withdrawal. Data are reported as mean ± SEM. *P < 0.05 compared with before blood withdrawal. The magnitude of blood withdrawal was fixed (10%) for all individuals. Measurements during exercise were performed at a given absolute workload (100 W) and at relative intensities (80 and 100% of HRpeak). The transverse mechanical axis entails the shortest distance from the inner to the outer layer of the myocardium. HR, heart rate; HRpeak, peak heart rate; TPSD, time to peak standard deviation; TS, transverse strain, TSR, transverse strain rate
Regression analyses
The relationship between the effects (∆, delta change) induced by blood withdrawal on LVMD, cardiac and hemodynamic variables was determined via linear regression analyses. At rest, no relationship was found (P ≥ 0.070). At the fixed moderate submaximal workload (100 W), ∆LS TPSD was negatively associated with hemodynamic variables such as ∆SBP (r = − 0.64, P = 0.006), ∆MAP (r = − 0.66, P = 0.004) and ∆RPP (r = − 0.53, P = 0.031), while ∆TS TPSD was negatively associated with ∆SV (r = − 0.44, P = 0.046). At a relative exercise intensity (80% HRpeak), ∆LSR TPSD was positively associated with ∆LVEF (r = 0.54, P = 0.010) and negatively associated with ∆LVESV (r = − 0.63, P = 0.002). Moreover, ∆TSR TPSD was negatively associated with LVmass (r = − 0.48, P = 0.023). At peak effort, ∆TS TPSD was positively associated with ∆LVEDV (r = 0.43, P = 0.034) and ∆SV (r = 0.47, P = 0.021), while ∆TSR TPSD was positively associated with LVPWd (r = 0.62, P = 0.001) and LVRWT (r = 0.59, P = 0.003). Finally, ∆LVMD variables were not associated with age at rest (P ≥ 0.157) nor during exercise (P ≥ 0.121).
Discussion
This study determined the influence of blood withdrawal (10%) on the synchronous properties of the myocardium, represented by LV mechanical dyssynchrony (LVMD), at rest and during exercise-induced stress in healthy women. The main findings are: (i) blood withdrawal reduces cardiac filling but do not alter LVMD in resting conditions; (ii) during moderate and high intensity exercise, large impairments of LVMD are elicited by blood withdrawal; (iii) the effects of blood withdrawal on LVMD at peak effort are directly associated with a LV concentric remodeling pattern. These findings denote the intrinsic relationship between BV, cardiac filling and structure, and the mechanical synchrony of myocardial fibers in women with a cardiovascular system not altered by disease. Potential sex-specific implications will be discussed in relation to pathophysiological alterations and treatment of cardiac mechanical synchrony.
The human heart at rest is endowed with large functional reserves underlain by redundant mechanisms, which serve to cope with eventual stressors. With increasing cardiac work demands and/or preload alterations, these reserves become progressively exhausted (Diaz-Canestro and Montero 2022a; Diaz-Canestro et al. 2021a; Williams et al. 2016). It is then, at the limits of cardiac capacity, where existing differences between individuals are readily manifest (Diaz-Canestro et al. 2021b; Williams et al. 2016). In this regard, sex differences in broad rotational mechanics of the LV emerge at the highest tolerable levels of lower body negative pressure, when cardiac filling, as represented by LVEDV, is critically reduced (≤ − 25%) (Williams et al. 2016). With a lower magnitude of LVEDV reduction, minimal divergences are present in overall LV mechanics (torsion, twist) between sexes (Williams et al. 2016). At the myocardial wall level, the present study substantiates no alteration in resting LVMD in the presence of moderate decrements in LV passive filling and LVEDV (≥ − 16%) induced by standard blood withdrawal (10%, ~ 530 ml). Such functional and volumetric changes fall within the normal range of resting LV and BV drifts, which may be acutely elicited by blood donation or common surgical procedures, throughout the healthy adult lifespan (Asch et al. 2019; Miyoshi et al. 2020; Patel et al. 2021; Diaz-Canestro and Montero 2022b). Of note, age did not influence the study outcomes according to regression analyses, although larger studies are needed to confirm this result. Until further evidence is available, it can be surmised that the reserve to maintain the mechanical synchrony of the myocardium is not compromised in adult women in resting conditions, at least in the acute time frame. The deleterious impact of blood withdrawal acutely emerges with increasing myocardial work demands and will be developed hereunder.
Myocardial work can be augmented ~ 5-fold from rest to peak effort in healthy young men (Brink-Elfegoun et al. 2007). A 4-fold increase in RPP, an established index of myocardial work, was observed in our female population with intact BV at peak effort (Fig. 1). At a moderate absolute workload (100 W), HR was further increased by blood withdrawal (compared with intact BV), as expected from increased relative intensity at a given absolute workload due to decreased exercise capacity, while RPP was increased (approximately by threefold) and not affected by blood withdrawal. Notwithstanding, such a moderate workload increased the mechanical dyssynchrony of myocardial strain after blood withdrawal in the LV longitudinal axis (+ 36%) (Fig. 2). Therefore, blood withdrawal markedly impaired LVMD during cycling at 100 W, which to place it in a more familiar context is equivalent to brisk walking (~ 6 km/hr) in our study population (Bransford and Howley 1977; Pentz et al. 2021; Sims et al. 2018). At high exercise intensities, a small decrease in HRpeak was induced by blood withdrawal, possibly explained by the Bezold-Jarisch reflex, i.e., reduced preload leading to excessive ‘distortion’ of the myocardium at peak effort being sensed by ventricular mechanoreceptors in a negative feedback loop resulting in bradycardia (Crystal and Salem 2012). The fourfold increase in RPP at peak effort with intact BV was partly attenuated after blood withdrawal (− 13 to − 15%), yet LVMD was augmented. Specifically, the mechanical dyssynchrony of myocardial strain rate at 100% HRpeak in the transverse axis was augmented following blood withdrawal (+ 31%) (Fig. 3). The transverse axis refers to the horizontal dimension of the LV wall in the A4C view, which is equivalent to the radial axis in the parasternal short-axis view. As the ventricle contracts, the myocardium thickens in the transverse and radial axes. According to the present results, with reduced cardiac filling, secondary to blood withdrawal, the synchrony and thereby the efficiency of peak myocardial thickening is largely affected in women. Importantly, the strain derived from myocardial thickening is superior to that linked with myocardial shortening (reflected by longitudinal strain) in predicting ejection fraction and hard clinical outcomes after cardiac (ventricular) resynchronization therapy (CRT) (Delgado et al. 2008; Suffoletto et al. 2006; Tanaka et al. 2010). In this regard, accumulating evidence indicates that CRT induces larger mortality reductions in women than men (Cheng et al. 2014; Xu et al. 2012; Zusterzeel et al. 2015). Furthermore, women on CRT reach greater improvements than men in functional exercise capacity (Xu et al. 2012). Collectively considered, the female’s heart may be prone to develop ventricular dyssynchrony, such that even moderate changes in ventricular filling elicited by standard blood withdrawal lead to notable increments in LVMD with increasing myocardial work above resting conditions.
The frailness of myocardial synchrony in women may be partly related to sex-specific structural dimorphisms. The female heart is susceptible to a certain maladaptation involving thicker (hypertrophied) LV walls with unchanged internal dimensions, known as LV concentric remodeling (Regitz-Zagrosek and Kararigas 2017). Despite LV structural variables fell within normal values in the present healthy cohort, the effect of blood withdrawal on the transverse myocardial strain rate at peak effort was directly associated with relative LV wall thickness (LVRWT), a variable reflecting the degree of LV concentric remodeling (Biton et al. 2016). Such type of remodeling frequently results from chronically elevated total peripheral vascular resistance (TPR) and blood pressure, which obliges the heart to generate higher intraventricular pressures to eject blood, i.e., in order to overcome the afterload. Healthy normotensive women may still be exposed, throughout the lifespan, to increased afterload since the cross-sectional area of large and medium-size arteries is consistently smaller (− 10 to − 20%) compared with men, even when adjusted by body size and LVmass (Mao et al. 2008; Sandgren et al. 1999; van der Heijden-Spek et al. 2000; Hiteshi et al. 2014). Hence, the smaller heart of women must generate a great amount of contractile force (blood pressure) per unit of blood flow than men as the reduced arterial diameter of the former exponentially increases the resistance to flow, thereby overloading the female heart. Indeed, recent evidence indicates that TPR, from moderate to high intensity exercise, is largely augmented (+ 50%) in women relative to men, which approximately corresponds to the predicted effect of sex differences in arterial diameter (Diaz-Canestro et al. 2021b). Ultimately, LV concentric elicits alterations in myocardial architecture as well as histology, including increased fibrous tissue and myocardial fiber disarray (Maillet et al. 2013). The altered alignment of myocardial fibers derived from LV concentric remodeling may reasonably lead to impaired mechanical synchrony, as it has been previously suggested (Santos et al. 2014). This may be linked with the observation that CRT reverses LV concentric remodeling, and plausibly LVMD, to a higher extent in women than men (Cheng et al. 2014). Ultimately, large impairments in the mechanical synchrony of myocardial fibers in women may entail an intrinsic structural basis.
Limitations We selected healthy individuals (women) in order to limit the influence of disease-related confounding factors. Further studies, including both women and men, will elucidate the extrapolation of the present findings to particular clinical conditions, particularly those associated with acute and chronic hypovolemia (Raj and Robertson 2007), in a sex-specific manner. Alternative, less invasive methods to decrease cardiac preload include lower body negative pressure and nitrate or diuretic administration, although they all involve certain unspecific cardiovascular effects that should be controlled. Second, the investigators that performed the analyses, but not the study participants, were blinded to the experimental condition. Provided that a blinded intervention for phlebotomy could be successfully implemented, the intensity of exercise stimuli is not considered to be altered by an hypothetical nocebo effect when measurements are performed at established physiological benchmarks (American Thoracic and American College of Chest 2003). Finally, a low number of individuals (n = 3) reported the use of HRT. We did not observe different results in these individuals (as well as in the whole group of postmenopausal women), concurring with previous evidence indicating no effect of HRT on cardiovascular function during exercise in our study population (Kirwan et al. 2004).
Practical implications
This present study demonstrates the relationship of BV with the mechanical synchrony of the myocardium in women in the absence of underlying pathophysiological alterations. Specifically, a standard (10%) blood withdrawal induces marked (> 30%) impairments of LVMD in the longitudinal and transverse axes during moderate and high hemodynamic stress. Moreover, the effect of blood withdrawal on LVMD is partly a function of the geometric pattern of the LV, being magnified with higher degree of concentric remodeling. The propensity towards such type of cardiac remodeling in the female population underlines the potential major role of altered BV in deeply ingrained ventricular abnormalities with strong prognostic impact.
Data availability
All data relevant to this study are presented in the manuscript.
References
American Thoracic S, American College of Chest P (2003) ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 167 (2):211–277. https://doi.org/10.1164/rccm.167.2.211
Asch FM, Miyoshi T, Addetia K, Citro R, Daimon M, Desale S, Fajardo PG, Kasliwal RR, Kirkpatrick JN, Monaghan MJ, Muraru D, Ogunyankin KO, Park SW, Ronderos RE, Sadeghpour A, Scalia GM, Takeuchi M, Tsang W, Tucay ES, Tude Rodrigues AC, Vivekanandan A, Zhang Y, Blitz A, Lang RM, Investigators W (2019) Similarities and differences in left ventricular size and function among races and nationalities: results of the world alliance societies of echocardiography normal values study. J Am Soc Echocardiogr 32(11):1396–1406. https://doi.org/10.1016/j.echo.2019.08.012
Astorino TA, Rietschel JC, Tam PA, Taylor K, Johnson SM, Freedman TP, Sakarya CE (2004) Reinvestigation of optimal duration of VO2max testing. J Exerc Physiol 7(6):1–8
Biering-Sorensen T, Shah SJ, Anand I, Sweitzer N, Claggett B, Liu L, Pitt B, Pfeffer MA, Solomon SD, Shah AM (2017) Prognostic importance of left ventricular mechanical dyssynchrony in heart failure with preserved ejection fraction. Eur J Heart Fail 19(8):1043–1052. https://doi.org/10.1002/ejhf.789
Biton Y, Goldenberg I, Kutyifa V, Baman JR, Solomon S, Moss AJ, Szepietowska B, McNitt S, Polonsky B, Zareba W, Barsheshet A (2016) Relative wall thickness and the risk for ventricular tachyarrhythmias in patients with left ventricular dysfunction. J Am Coll Cardiol 67(3):303–312. https://doi.org/10.1016/j.jacc.2015.10.076
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1(8476):307–310
Bransford DR, Howley ET (1977) Oxygen cost of running in trained and untrained men and women. Med Sci Sports 9(1):41–44
Brink-Elfegoun T, Kaijser L, Gustafsson T, Ekblom B (2007) Maximal oxygen uptake is not limited by a central nervous system governor. J Appl Physiol (1985) 102(2):781–786. https://doi.org/10.1152/japplphysiol.00566.2006
Cheng YJ, Zhang J, Li WJ, Lin XX, Zeng WT, Tang K, Tang AL, He JG, Xu Q, Mei MY, Zheng DD, Dong YG, Ma H, Wu SH (2014) More favorable response to cardiac resynchronization therapy in women than in men. Circ Arrhythm Electrophysiol 7(5):807–815. https://doi.org/10.1161/CIRCEP.113.001786
Covell JW, Ross J Jr, Sonnenblick EH, Braunwald E (1966) Comparison of the force-velocity relation and the ventricular function curve as measures of the contractile state of the intact heart. Circ Res 19(2):364–372. https://doi.org/10.1161/01.res.19.2.364
Crystal GJ, Salem MR (2012) The Bainbridge and the “reverse” Bainbridge reflexes: history, physiology, and clinical relevance. Anesth Analg 114(3):520–532. https://doi.org/10.1213/ANE.0b013e3182312e21
Delgado V, Ypenburg C, van Bommel RJ, Tops LF, Mollema SA, Marsan NA, Bleeker GB, Schalij MJ, Bax JJ (2008) Assessment of left ventricular dyssynchrony by speckle tracking strain imaging comparison between longitudinal, circumferential, and radial strain in cardiac resynchronization therapy. J Am Coll Cardiol 51(20):1944–1952. https://doi.org/10.1016/j.jacc.2008.02.040
Diaz-Canestro C, Montero D (2020) Female sex-specific curtailment of left ventricular volume and mass in HFpEF patients with high end-diastolic filling pressure. J Hum Hypertens. https://doi.org/10.1038/s41371-020-00394-3
Diaz-Canestro C, Montero D (2022a) Blood volume primarily determines orthostatic tolerance in women. J Intern Med 291(3):371–373. https://doi.org/10.1111/joim.13397
Diaz-Canestro C, Montero D (2022b) Sex and age interaction in fundamental circulatory volumetric variables at peak working capacity. Biol Sex Differ 13(1):1. https://doi.org/10.1186/s13293-021-00409-9
Diaz-Canestro C, Pentz B, Sehgal A, Montero D (2021a) Blood withdrawal acutely impairs cardiac filling, output and aerobic capacity in proportion to induced hypovolemia in middle-aged and older women. Appl Physiol Nutr Metab 47:1–8. https://doi.org/10.1139/apnm-2021-0196
Diaz-Canestro C, Pentz B, Sehgal A, Montero D (2021b) Sex differences in cardiorespiratory fitness are explained by blood volume and oxygen carrying capacity. Cardiovasc Res. https://doi.org/10.1093/cvr/cvab028
Diaz-Canestro C, Pentz B, Sehgal A, Montero D (2021c) Sex dimorphism in cardiac and aerobic capacities: the influence of body composition. Obesity (silver Spring) 29(11):1749–1759. https://doi.org/10.1002/oby.23280
Haugaa KH, Smedsrud MK, Steen T, Kongsgaard E, Loennechen JP, Skjaerpe T, Voigt JU, Willems R, Smith G, Smiseth OA, Amlie JP, Edvardsen T (2010) Mechanical dispersion assessed by myocardial strain in patients after myocardial infarction for risk prediction of ventricular arrhythmia. JACC Cardiovasc Imaging 3(3):247–256. https://doi.org/10.1016/j.jcmg.2009.11.012
Haugaa KH, Goebel B, Dahlslett T, Meyer K, Jung C, Lauten A, Figulla HR, Poerner TC, Edvardsen T (2012) Risk assessment of ventricular arrhythmias in patients with nonischemic dilated cardiomyopathy by strain echocardiography. J Am Soc Echocardiogr 25(6):667–673. https://doi.org/10.1016/j.echo.2012.02.004
Hiteshi AK, Li D, Gao Y, Chen A, Flores F, Mao SS, Budoff MJ (2014) Gender differences in coronary artery diameter are not related to body habitus or left ventricular mass. Clin Cardiol 37(10):605–609. https://doi.org/10.1002/clc.22310
Kim MS, Kim HK, Chang SA, Kim SY, Cho GY, Kim YJ, Sohn DW, Oh BH, Park YB (2011) Impact of preload alteration on left ventricular mechanical dyssynchrony using tissue velocity imaging echocardiography. Echocardiography 28(2):196–202. https://doi.org/10.1111/j.1540-8175.2010.01288.x
Kirwan LD, MacLusky NJ, Shapiro HM, Abramson BL, Thomas SG, Goodman JM (2004) Acute and chronic effects of hormone replacement therapy on the cardiovascular system in healthy postmenopausal women. J Clin Endocrinol Metab 89(4):1618–1629. https://doi.org/10.1210/jc.2003-030324
Kou S, Caballero L, Dulgheru R, Voilliot D, De Sousa C, Kacharava G, Athanassopoulos GD, Barone D, Baroni M, Cardim N, Gomez De Diego JJ, Hagendorff A, Henri C, Hristova K, Lopez T, Magne J, De La Morena G, Popescu BA, Penicka M, Ozyigit T, Rodrigo Carbonero JD, Salustri A, Van De Veire N, Von Bardeleben RS, Vinereanu D, Voigt JU, Zamorano JL, Donal E, Lang RM, Badano LP, Lancellotti P (2014) Echocardiographic reference ranges for normal cardiac chamber size: results from the NORRE study. Eur Heart J Cardiovasc Imaging 15(6):680–690. https://doi.org/10.1093/ehjci/jet284
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging 16(3):233–270. https://doi.org/10.1093/ehjci/jev014
Lim P, Buakhamsri A, Popovic ZB, Greenberg NL, Patel D, Thomas JD, Grimm RA (2008) Longitudinal strain delay index by speckle tracking imaging: a new marker of response to cardiac resynchronization therapy. Circulation 118(11):1130–1137. https://doi.org/10.1161/CIRCULATIONAHA.107.750190
Maillet M, van Berlo JH, Molkentin JD (2013) Molecular basis of physiological heart growth: fundamental concepts and new players. Nat Rev Mol Cell Biol 14(1):38–48. https://doi.org/10.1038/nrm3495
Mao SS, Ahmadi N, Shah B, Beckmann D, Chen A, Ngo L, Flores FR, Gao YL, Budoff MJ (2008) Normal thoracic aorta diameter on cardiac computed tomography in healthy asymptomatic adults: impact of age and gender. Acad Radiol 15(7):827–834. https://doi.org/10.1016/j.acra.2008.02.001
Miyazaki C, Powell BD, Bruce CJ, Espinosa RE, Redfield MM, Miller FA, Hayes DL, Cha YM, Oh JK (2008) Comparison of echocardiographic dyssynchrony assessment by tissue velocity and strain imaging in subjects with or without systolic dysfunction and with or without left bundle-branch block. Circulation 117(20):2617–2625. https://doi.org/10.1161/CIRCULATIONAHA.107.733675
Miyoshi T, Addetia K, Citro R, Daimon M, Desale S, Fajardo PG, Kasliwal RR, Kirkpatrick JN, Monaghan MJ, Muraru D, Ogunyankin KO, Park SW, Ronderos RE, Sadeghpour A, Scalia GM, Takeuchi M, Tsang W, Tucay ES, Tude Rodrigues AC, Vivekanandan A, Zhang Y, Blitz A, Lang RM, Asch FM, Investigators W (2020) Left ventricular diastolic function in healthy adult individuals: results of the world alliance societies of echocardiography normal values study. J Am Soc Echocardiogr 33(10):1223–1233. https://doi.org/10.1016/j.echo.2020.06.008
Modin D, Biering-Sorensen SR, Mogelvang R, Jensen JS, Biering-Sorensen T (2018) Prognostic importance of left ventricular mechanical dyssynchrony in predicting cardiovascular death in the general population. Circ Cardiovasc Imaging 11(10):e007528. https://doi.org/10.1161/CIRCIMAGING.117.007528
Montero D, Cathomen A, Jacobs RA, Fluck D, de Leur J, Keiser S, Bonne T, Kirk N, Lundby AK, Lundby C (2015) Haematological rather than skeletal muscle adaptations contribute to the increase in peak oxygen uptake induced by moderate endurance training. J Physiol 593(20):4677–4688. https://doi.org/10.1113/JP270250
Montero D, Houben AJ, Koster A, Muris DM, Schram MT, Gronenschild EH, Sep SJ, Henry RM, van der Kallen CJ, Schaper NC, Dagnelie PC, van Geel TA, Kremers SP, Savelberg HH, Stehouwer CD (2016) Physical activity is associated with glucose tolerance independent of microvascular function: the Maastricht study. J Clin Endocrinol Metab 101(9):3324–3332. https://doi.org/10.1210/jc.2016-1526
Montero D, Breenfeldt-Andersen A, Oberholzer L, Haider T, Goetze JP, Meinild-Lundby AK, Lundby C (2017) Erythropoiesis with endurance training: dynamics and mechanisms. Am J Physiol Regul Integr Comp Physiol 312(6):R894–R902. https://doi.org/10.1152/ajpregu.00012.2017
Morris DA, Vaz Perez A, Blaschke F, Eichstadt H, Ozcelik C, Haverkamp W (2012) Myocardial systolic and diastolic consequences of left ventricular mechanical dyssynchrony in heart failure with normal left ventricular ejection fraction. Eur Heart J Cardiovasc Imaging 13(7):556–567. https://doi.org/10.1093/ehjci/jes042
Patel HN, Miyoshi T, Addetia K, Henry MP, Citro R, Daimon M, Gutierrez Fajardo P, Kasliwal RR, Kirkpatrick JN, Monaghan MJ, Muraru D, Ogunyankin KO, Park SW, Ronderos RE, Sadeghpour A, Scalia GM, Takeuchi M, Tsang W, Tucay ES, Tude Rodrigues AC, Vivekanandan A, Zhang Y, Schreckenberg M, Blankenhagen M, Degel M, Rossmanith A, Mor-Avi V, Asch FM, Lang RM, Investigators W (2021) Normal values of cardiac output and stroke volume according to measurement technique, age, sex, and ethnicity: results of the world alliance of societies of echocardiography study. J Am Soc Echocardiogr 34(10):1077–1085. https://doi.org/10.1016/j.echo.2021.05.012
Patterson SW, Starling EH (1914) On the mechanical factors which determine the output of the ventricles. J Physiol 48(5):357–379. https://doi.org/10.1113/jphysiol.1914.sp001669
Pellikka PA, Nagueh SF, Elhendy AA, Kuehl CA, Sawada SG, American Society of E (2007) American society of echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr 20(9):1021–1041. https://doi.org/10.1016/j.echo.2007.07.003
Pentz B, Diaz-Canestro C, Sehgal A, Montero D (2021) Effects of blood withdrawal on cardiac, hemodynamic, and pulmonary responses to a moderate acute workload in healthy middle-aged and older females. J Sci Med Sport. https://doi.org/10.1016/j.jsams.2021.10.012
Phan TT, Abozguia K, Shivu GN, Ahmed I, Patel K, Leyva F, Frenneaux M (2010) Myocardial contractile inefficiency and dyssynchrony in heart failure with preserved ejection fraction and narrow QRS complex. J Am Soc Echocardiogr 23(2):201–206. https://doi.org/10.1016/j.echo.2009.11.004
Raj SR, Robertson D (2007) Blood volume perturbations in the postural tachycardia syndrome. Am J Med Sci 334(1):57–60. https://doi.org/10.1097/MAJ.0b013e318063c6c0
Regitz-Zagrosek V, Kararigas G (2017) Mechanistic pathways of sex differences in cardiovascular disease. Physiol Rev 97(1):1–37. https://doi.org/10.1152/physrev.00021.2015
Rosner A, Barbosa D, Aarsaether E, Kjonas D, Schirmer H, D’Hooge J (2015) The influence of frame rate on two-dimensional speckle-tracking strain measurements: a study on silico-simulated models and images recorded in patients. Eur Heart J Cardiovasc Imaging 16(10):1137–1147. https://doi.org/10.1093/ehjci/jev058
Sandgren T, Sonesson B, Ahlgren R, Lanne T (1999) The diameter of the common femoral artery in healthy human: influence of sex, age, and body size. J Vasc Surg 29(3):503–510. https://doi.org/10.1016/s0741-5214(99)70279-x
Santos AB, Kraigher-Krainer E, Bello N, Claggett B, Zile MR, Pieske B, Voors AA, McMurray JJ, Packer M, Bransford T, Lefkowitz M, Shah AM, Solomon SD (2014) Left ventricular dyssynchrony in patients with heart failure and preserved ejection fraction. Eur Heart J 35(1):42–47. https://doi.org/10.1093/eurheartj/eht427
Shah AM, Solomon SD (2016) Mechanical dyssynchrony: a risk factor but not a target. Eur Heart J 37(1):60–62. https://doi.org/10.1093/eurheartj/ehv458
Siebenmann C, Keiser S, Robach P, Lundby C (2017) CORP: the assessment of total hemoglobin mass by carbon monoxide rebreathing. J Appl Physiol 123(3):645–654. https://doi.org/10.1152/japplphysiol.00185.2017
Sims DT, Onambele-Pearson GL, Burden A, Payton C, Morse CI (2018) The oxygen consumption and metabolic cost of walking and running in adults with achondroplasia. Front Physiol 9:410. https://doi.org/10.3389/fphys.2018.00410
Suffoletto MS, Dohi K, Cannesson M, Saba S, Gorcsan J 3rd (2006) Novel speckle-tracking radial strain from routine black-and-white echocardiographic images to quantify dyssynchrony and predict response to cardiac resynchronization therapy. Circulation 113(7):960–968. https://doi.org/10.1161/CIRCULATIONAHA.105.571455
Tan YT, Wenzelburger FW, Sanderson JE, Leyva F (2013) Exercise-induced torsional dyssynchrony relates to impaired functional capacity in patients with heart failure and normal ejection fraction. Heart 99(4):259–266. https://doi.org/10.1136/heartjnl-2012-302489
Tanaka H, Nesser HJ, Buck T, Oyenuga O, Janosi RA, Winter S, Saba S, Gorcsan J 3rd (2010) Dyssynchrony by speckle-tracking echocardiography and response to cardiac resynchronization therapy: results of the speckle tracking and resynchronization (STAR) study. Eur Heart J 31(14):1690–1700. https://doi.org/10.1093/eurheartj/ehq213
van der Heijden-Spek JJ, Staessen JA, Fagard RH, Hoeks AP, Boudier HA, van Bortel LM (2000) Effect of age on brachial artery wall properties differs from the aorta and is gender dependent: a population study. Hypertension 35(2):637–642. https://doi.org/10.1161/01.hyp.35.2.637
Williams AM, Shave RE, Stembridge M, Eves ND (2016) Females have greater left ventricular twist mechanics than males during acute reductions to preload. Am J Physiol Heart Circ Physiol 311(1):H76-84. https://doi.org/10.1152/ajpheart.00057.2016
Xu YZ, Friedman PA, Webster T, Brooke K, Hodge DO, Wiste HJ, Hua W, Zhang S, Hayes DL, Cha YM (2012) Cardiac resynchronization therapy: do women benefit more than men? J Cardiovasc Electrophysiol 23(2):172–178. https://doi.org/10.1111/j.1540-8167.2011.02168.x
Zusterzeel R, Spatz ES, Curtis JP, Sanders WE, Selzman KA, Pina IL, Bao H, Ponirakis A, Varosy PD, Masoudi FA, Canos DA, Strauss DG (2015) Cardiac resynchronization therapy in women versus men: observational comparative effectiveness study from the national cardiovascular data registry. Circ Cardiovasc Qual Outcomes 8(2 Suppl 1):S4-11. https://doi.org/10.1161/CIRCOUTCOMES.114.001548
Acknowledgements
The authors thank the study participants for their willingness, time and effort devoted to this study.
Funding
This work was funded by the Swiss National Science Foundation (P2ZHP3-184211, to C.D.) and the Natural Sciences and Engineering Research Council of Canada (Discovery Grant, RGPIN-2019-04833, to D.M.).
Author information
Authors and Affiliations
Contributions
Conception and Design of experiments: CD, DM. Collection, analysis and interpretation: JK, CD, KYC, MG, DM. Drafting the article or revising it critically for important intellectual content: JK, CD, KYC, MG, DM.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The study was approved by the Conjoint Health Research Ethics Board (REB18-1654) of the University of Calgary and conducted in accordance with the declaration of Helsinki. All participants provided informed oral and written consents before starting the measurements.
Additional information
Communicated by Westerterp/Westerblad .
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khor, J., Diaz-Canestro, C., Chan, K.Y. et al. Blood volume contributes to the mechanical synchrony of the myocardium during moderate and high intensity exercise in women. Eur J Appl Physiol 124, 1227–1237 (2024). https://doi.org/10.1007/s00421-023-05355-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-023-05355-5