Abstract
Activation of glial cells (reactive gliosis) and the purinergic pathway, together with metalloproteinase (MMP)-induced remodeling of the neural extracellular matrix (nECM), drive maladaptive changes in the spinal cord following peripheral nerve injury (PNI). We evaluated the effects on spinal maladaptive plasticity through administration of oxidized ATP (oxATP), an antagonist of P2X receptors (P2XR), and/or GM6001, an inhibitor of MMPs, in rats following spared nerve injury (SNI) of the sciatic nerve. With morpho-molecular techniques, we demonstrated a reduction in spinal reactive gliosis and changes in the neuro-glial-nECM crosstalk via expression remodeling of P2XR, nerve growth factor (NGF) receptors (TrkA and p75), and histone deacetylase 2 (HDAC2) after treatments with oxATP/GM6001. Altogether, our data suggest that MMPs and purinergic inhibition have a modulatory impact on key proteins in the neuro-glial-nECM network, acting at different levels from intracellular signaling to epigenetic modifications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glial cells are well-recognized components of the synaptic complex, together with neurons and the surrounding extracellular matrix (ECM), constituting a more exhaustive model of the “classic” neuronal-based synaptic structure, both in physiology (Dityatev and Rusakov 2011) and in pathology (De Luca and Papa 2016). The matrix-neuroglial network’s early response to noxious stimuli involves both glial cells and the neural extracellular matrix (nECM), the matrisome (De Luca et al. 2020a, b). Indeed, the role of astrocytosis and microglial activation in central nervous system (CNS) pathology has been extensively related to maladaptive changes in synaptic homeostasis, hyper-oxidation, cytokine release, and enhanced activation of matrix metalloproteinases (MMPs) (Cavaliere et al. 2007; Cirillo et al. 2012, 2015; Virtuoso et al. 2021), pivotal enzymes for nECM formation, adaptability, and pathological remodeling (De Luca et al. 2017; Visse and Nagase 2003). Changes in these components first affect the synaptic transmission, causing a reversible loss of function, and later involve neuronal viability resulting in maladaptive system rewiring and permanent damage (Inoue and Tsuda 2012).
Evidence suggests that the purinergic system, involved in many neural processes including sensory transmission and chronic neuropathic pain, prompts reactive gliosis (Cirillo et al. 2015; Inoue and Tsuda 2012) and modulate MMP expression (Virtuoso et al. 2020).
The release of adenosine 5′ triphosphate (ATP) from glial cells exerts a wide range of effects through the ionotropic P2X receptors (P2XRs) (Sivaramakrishnan and Fountain 2012), being involved in modulation of intracellular Ca2+ currents, neuronal and glial transmitters release, glial fibrillary acidic protein (GFAP) upregulation, and inflammatory microglial invasion (Cirillo et al. 2012; Sivaramakrishnan and Fountain 2012). Spinal cord neurons and glial cells mainly express P2X7 and P2X4 receptors, which can mediate Ca2+ signaling, cellular apoptosis, cytokine release, and in turn, MMP activation, among other functions (Gu and Wiley 2006; Cirillo et al. 2016). Overexpression of purinergic receptors during pathological conditions affects lesion remodeling in combination with neurotrophic factors (Mishra 2017; de Rivero Vaccari et al. 2012). Spared nerve injury (SNI) of the sciatic nerve provided a powerful tool to investigate neuropathic pain-like behavior and neuroglial changes in the spinal cord and supraspinal centers (Marcello et al. 2013). The pharmacological interference with neurotrophins, purinergic antagonism, and the MMP inhibitors produced partial yet promising results in both neuropathic behavior and reactive gliosis (Cirillo et al. 2012, 2015). More interestingly, the SNI model can be used to assess the plasticity of CNS circuitry without violating its integrity: through a lesion of a peripheral nerve, it induces reproducible morpho-functional changes in the spinal cord dorsal and ventral horns networks (Cirillo et al. 2011, 2012, 2016; De Luca et al. 2016).
Based on our previous research, we aimed to use SNI to further study how purinergic and MMP antagonism could interfere with maladaptive plasticity in the spinal cord, with a focus on glial reactivity and neurotrophin receptor expression. To achieve this goal we injected intraperitoneally (i.p.) the oxidized ATP (oxATP), a nonselective P2XR antagonist, alone or in combination with intrathecal (i.t.) infusion of GM6001, a broad-spectrum MMP inhibitor. Finally, to consider the role of transcription and histone modifications that could be modulated by both treatments (Wang et al. 2011; Lu et al. 2013), we also evaluated the expression of histone deacetylases (HDACs) involved in the alteration of chromatin accessibility (Kaminska et al., 2016; Halsall and Turner 2016).
Materials and methods
Animals
Adult (250–300 g; Charles River, Calco, Italy) Sprague Dawley rats (n = 58) were used. This study was performed in accordance with Italian and European guidelines for the care and use of laboratory animals (EU Directive 2010/63). All protocols were approved by the Animal Care and Use Committees of the University of Campania “Luigi Vanvitelli” (auth. 153/2018-PR). Each animal was allowed free access to food and water, under a 12/12 h light/dark cycle. Animals were kept in pathogen-free iron-sheet cages with a solid floor covered with 4–6 cm of sawdust. We did not use cages with thin-plate floors in order to avoid exacerbation of the discomfort from the affected hind paw (Bai et al. 1999).
SNI model
Sciatic SNI was induced according to Decosterd and Woolf (Decosterd and Woolf 2000). Briefly, each rat was anesthetized with chloral hydrate/tiletamine (30 mg/kg) during surgery. The sciatic nerve and its three terminal branches (the sural, common peroneal, and tibial nerves) were exposed on the lateral surface of the thigh. The SNI procedure comprised axotomy and tight ligation of the tibial and common peroneal nerves, leaving the sural nerve intact. For the sham-operated control (CTR) group (n = 10), nerves were exposed but not truncated. Muscle and skin were closed in layers. Great care was taken to avoid any contact with or stretching of the intact sural nerve.
Drug delivery
Drug delivery was performed according to our previous protocols (Cirillo et al. 2011, 2012, 2015, 2016; Virtuoso et al. 2020), starting from the day after SNI. Animals were treated with daily i.p. oxATP (6 mg/kg), dissolved in 100 µl of sterile distilled water (dH2O). For i.t. drug delivery of GM6001 or vehicle (artificial cerebrospinal fluid—ACSF), the lumbar spinal catheter was positioned during the SNI procedure to reduce the discomfort bias. Briefly, a small opening was made through the laminas of the lumbar spine and a catheter [polyethylene (PE) 10 tubing attached to PE 60 tubing for connection to an osmotic pump] was inserted into the subarachnoid space and directed to the lumbar enlargement of the spinal cord. After anchoring the catheter through the careful apposition of a glass ionomer luting cement triple pack (Ketac Cem radiopaque; 3M ESPE, Seefeld, Germany), the wound was irrigated with saline and closed in two layers with 3–0 silk (fascial plane) and surgical skin staples. On recovery from surgery, lower body paralysis was induced by intrathecal lidocaine (2%) injection to confirm proper catheter localization. Each rat was placed on a table, and the gait and posture of the affected hind paw were carefully observed for 2 min. Only animals exhibiting appropriate, transient paralysis to lidocaine, as well as a lack of motor deficits, were used for treatments; the free extremity of the catheter was connected to an osmotic minipump, and the pump was implanted subcutaneously. The osmotic pumps (Model 2001 ALZET, Cupertino, CA) pumped at a rate of 1 μl/h and were filled with GM6001 (Calbiochem, Germany) (180 μg/μl, corresponding to 100 mg/kg body weight/day) or ACSF.
Animals were divided into the following groups:
-
Group I (n = 8): SNI rats, i.p. oxATP for 3 days (from day 1 to 3), and i.t. ACSF
-
Group II (n = 8): SNI rats, i.p. oxATP for 8 days (from day 1 to 8), and i.t. ACSF
-
Group III (n = 8): SNI rats, i.p. oxATP and i.t. GM6001 for 3 days (from day 1 to 3)
-
Group IV (n = 8): SNI rats, i.p. oxATP and i.t. GM6001 for 8 days (from days 1 to 8)
-
Group V–VI: SNI rats, vehicles (i.p. dH2O and i.t. ACSF) for 3 or 8 days (n = 8 each)
-
Group VII: control (CTR) group, sham-operated animals (n = 10).
Tissue preparation
Rats were deeply anesthetized on days 3 and 8 after SNI with an i.p. injection of chloral hydrate (300 mg/kg body weight) and perfused transcardially with saline solution (Tris HCl 0.1 M/ EDTA 6 mM). Spinal cords for western blotting (n = 29) were removed whilst the samples for immunohistochemistry (n = 29) continued with fixation in 4% paraformaldehyde in 0.01 M phosphate-buffered saline (PBS), pH 7.4 at 4 °C. Spinal cords were then postfixed 2 h in the same fixative, then soaked in 30% sucrose phosphate-buffered saline (PBS), and frozen in chilled isopentane on dry ice, as described previously (Maggio et al. 2001). Serial sections were cut at the slide microtome (25 μm thickness) and collected in cold PBS.
Immunofluorescence (IF)
Immunofluorescence staining was performed according to our protocols (Papa et al. 2003). Sections were incubated with the primary antibody: GFAP (1:400) (Sigma-Aldrich, St. Louis, MO USA), Iba1 (1:500) (Wako Chemicals, VA, USA), TrkA (1:2000) (Chemicon Inc., Temecula, CA, USA), p75 NGF receptor (1:500) (Abcam, Cambridge, England) for 48 h at 4 °C. Following washes with PBS, sections were incubated with the appropriate secondary antibody (Alexa Fluor 488 anti-rabbit IgG, Alexa Fluor 546 anti-rabbit IgG; 1:200; Invitrogen, Carlsbad, CA, USA) for 2 h. Sections were mounted and cover-slipped with VECTASHIELD (Vector Laboratories). Negative controls were performed for each antibody using only the secondary antibodies with the same protocol on a tissue section (not shown).
Western blotting (WB)
Lumbar spinal cord samples were homogenized in 50 mM HEPES pH 7.5, 10% glycerol, 10 mM NaCl, 10 mM dithiothreitol, 1% SDS, 5 mM EDTA, and protease inhibitors (Sigma Aldrich). Lysates were loaded on a 0.75 mm sodium dodecyl sulfate (SDS) polyacrylamide minigel (8%–10%–12%), which was electrophoresed at 150 V for 90 min. The proteins were transferred overnight to nitrocellulose membranes at 30 V, 4 °C. After blocking of nonspecific sites by 5% milk in [20 mM Tris HCl (pH 7.4), 0.2% Tween 20 (TBST)], membranes were incubated overnight with primary antibody anti-GFAP (1:400), anti-Iba1 (1:250), anti-HDAC1 (1:500) and anti-HDAC2 (1:1000) (Sigma-Aldrich, St. Louis, MO, USA), anti-P2X4 (1:500) (Immunological Sciences, Rome, Italy), anti-TrkA (1:1000), anti p75 NGF receptor (1:500), and β-actin (1:2000) (Sigma-Aldrich, St. MO, Louis, USA). After washing in TBST, membranes were incubated with the appropriate biotinylated secondary antibody (Vector Labs, Inc.; 1:500) in blocking solution for 60 min at room temperature. Subsequently, they were washed in TBS and processed using the Vectastain avidin–biotin–peroxidase kit (Vector Labs, Inc.) for 30 min at room temperature. After washing in 0.05 M Tris HCl, they reacted with 3,3-diaminobenzidine tetrahydrochloride (DAB; Sigma Aldrich, 0.5 mg/ml Tris HCl) and 0.01% hydrogen peroxide. Each specific band was acquired and processed for density measurement with the computer-assisted imaging analysis system. To compare the differences between control and treatment groups, we first normalized the density of each specific band against the density of the corresponding internal loading band.
Measurements and statistical analysis
Slides were imaged with a Zeiss Axioskop 2 light microscope equipped with Filter Sets 45, 21, and 74, a high-resolution (1.37 million pixels) digital camera (C4742-95; Hamamatsu Photonics, Italy) with single-channel B/W image acquisition (multifluorescence not applicable). For glial markers, we preferred a morphometric approach for the optimal visualization of each positive element. Therefore, values of GFAP and Iba1—markers for astrocytes and microglia, respectively—were expressed as count density (number of positive elements relative to the scanned area). The densitometric values of the other markers were expressed as the total density measured per scanned area. We performed background subtraction and correction gamma LUT (set value 2) at every acquisition using HiPic 8.4.0 software. The magnification applied to each image is detailed in the figure caption. Measurements of markers in the whole lumbar spinal cord were accomplished using a computer-assisted image analysis system (MCID 7.1; Imaging Res. Inc., Canada). All data were collected in a blinded manner; the observer making the measurements was not aware of the group. Data were exported and converted to a density distribution histogram using the SigmaPlot 10.0 program (SPSS-Erkrath) and presented as the mean ± SEM for all quantitative analyses. Data were checked for normal distribution and homogeneity of variance by the Kolmogorov–Smirnov and Levene mean tests, respectively. Afterward, the data were statistically analyzed by using a one-way analysis of variance (ANOVA) for multiple comparisons. The ANOVA test was followed by all pairwise Holm-Šídák test to determine which group differences were statistically significant and to reduce the multiple comparisons bias. The level of significance was always set at p = 0.05 (*p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001). Individual images were assembled, and the same adjustments were made for brightness, contrast, and sharpness using Adobe Photoshop (Adobe Systems).
Results
Reactive glial populations are modulated by oxATP and GM6001 treatment
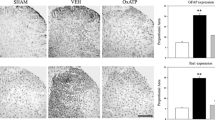
Analysis of the microglial marker Iba1 demonstrated microglial activation 8 days after SNI (89.2 ± 4.6) compared to the CTR group (39.2 ± 3.2). Both oxATP (64.3 ± 4.4) and oxATP + GM6001 treatments (46.18 ± 1.7) significantly reduced microglial activation (p ≤ 0.001) (Fig. 1a, b). Accordingly, WB analysis for Iba1 showed increased expression in SNI animals at day 3 (0.43 ± 0.12) and day 8 (0.68 ± 0.07) compared to the CTR group (day 3: 0.18 ± 0.04, day 8: 0.19 ± 0.02 p ≤ 0.01). Both therapeutic strategies significantly reduced Iba1 expression (oxATP 0.43 ± 0.06; oxATP + GM6001 0.28 ± 0.06, p ≤ 0.01) (Fig. 2a, b). No changes of Iba1 levels were detected after 3 days of treatments with oxATP/GM6001.
Spinal reactive gliosis following SNI and modulation by purinergic and MMP inhibition. a Low (×10) and high (×20) magnification representative images of immunofluorescence staining for Iba1 and GFAP in the dorsal horn of lumbar spinal cord in sham-operated (CTR) SNI animals, and after treatment with oxATP or oxATP + GM6001 (GM) for 8 days. Scale bar 50 μm. b, c Densitometric quantitation. Data shown as the mean ± SEM (*p ≤ 0.05; ***p ≤ 0.001; one-way ANOVA and post hoc Holm-Šídák correction for multiple pairwise comparisons)
a Representative immunoblot of Iba1, GFAP, and HDAC2 in the lumbar spinal cord from sham-operated (CTR) SNI animals treated with oxATP or oxATP + GM6001(GM) for 3 or 8 days. b−d Corresponding quantitation of Iba1, GFAP, and HDAC2 immunoblots, normalized in relation to β-actin. Data expressed as mean ± SEM (**p ≤ 0.01; ***p ≤ 0.001; one-way ANOVA followed by post hoc Holm-Šídák correction for multiple pairwise comparisons)
The astrocytic reaction was documented 8 days after SNI, as revealed by the intense staining for GFAP in SNI animals (51.8 ± 1.1) compared to CTR rats (27.0 ± 2.2) (p ≤ 0.001) and was modulated only by oxATP treatment (33.8 ± 1.4) (p ≤ 0.001) and not by oxATP + GM6001 treatment (41.0 ± 3.8) (p > 0.1) (Fig. 1 a–c).
WB analysis of GFAP revealed astrocytosis in SNI animals 3 days (1.3 ± 0.19) and 8 days (2.5 ± 0.04) after nerve injury compared to CTR (day 3: 0.5 ± 0.02, p ≤ 0.01; day 8: 0.48 ± 0.005, p ≤ 0.001), indicative of reactive gliosis. Treatment for 8 days with oxATP significantly reduced GFAP expression (1.6 ± 0.37, p ≤ 0.01); however, the combined treatment with oxATP + GM6001 did not significantly affect the astrocytic reaction (day 8: 2.3 ± 0.01; day 3:1.5 ± 0.1) (Fig. 2a–c).
Taken together, these data showed a different time course and response of activated glial cell populations, differently modulated by a purinergic or MMP system.
Class I HDACs are differentially modulated by SNI and purinergic/MMP systems
Here we have considered and analyzed the expression of HDAC1 and HDAC2, class I deacetylases, and key regulators of many epigenetic processes. HDAC1 levels at WB were not affected by the SNI procedure or treatments, and did not differ from CTR values at day 3 and day 8. HDAC2 levels were unchanged at day 3. However, on day 8, SNI significantly increased HDAC2 levels (1.17 ± 0.1) compared to CTR (0.32 ± 0.001, p ≤ 0.001). Treatment with oxATP alone or in combination with GM6001 downregulated HDAC2 levels (oxATP: 0.80 ± 0.12; oxATP + GM6001: 0.62 ± 0.08, p = 0.010) (Fig. 2a–d). These data suggest a specific role of the spinal HDACs, differently modulated by nerve injury and treatments with inhibition of purinergic receptors and MMPs.
OxATP and GM6001 downregulate P2X4R expression
Among purinergic receptors, P2X4R is the most widely expressed in the CNS, being localized on microglial cells and involved in the inflammatory response, spinal cord damage, and epigenetic modulation (de Rivero Vaccari et al. 2012; Jacobson and Müller 2016; Lu et al. 2013; Lee et al. 2012). In this study we found that P2X4R was increased 8 days after SNI (0.8 ± 0.04, p ≤ 0.001) compared to the CTR group (0.35 ± 0.06) and significantly reduced by treatments (oxATP: 0.59 ± 0.04; oxATP + GM6001: 0.52 ± 0.05, p ≤ 0.01) (Fig. 3a, b). No changes were detected on day 3.
Modulation of purinergic and NGFR expression following SNI and treatments with oxATP or oxATP + GM6001 (GM). a Representative immunoblot of P2X4R, TrkA, and p75 receptors in the lumbar spinal cord from sham-operated (CTR) SNI animals treated with oxATP or oxATP + GM6001 (GM) for 3 or 8 days. b−d Corresponding quantitation of P2X4, TrkA, and p75 receptor immunoblots normalized in relation to β-actin. Data expressed as mean ± SEM (*p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001; one-way ANOVA followed by post hoc Holm-Šídák correction for multiple pairwise comparisons)
These data support the anti-inflammatory effect of both P2XR inhibition and MMP modulation.
Different time-course and modulation of neurotrophin receptor expression
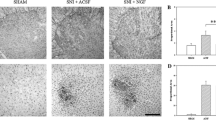
Neurotrophins strictly interact with the purinergic and MMP systems, and play a key role in spinal cord remodeling after lesions and maladaptive plasticity (Cirillo et al. 2010, 2012; Virtuoso et al. 2020; Mishra et al. 2006). We show here that the expression of TrkA, the NGF high-affinity receptor, was significantly increased 8 days following SNI (0.5 ± 0.06, p ≤ 0.01) and dramatically reduced after treatment with oxATP (0.16 ± 0.01) or oxATP + GM6001 (0.18 ± 0.04) (p ≤ 0.001) (Fig. 3a–c). No changes were detected on day 3.
WB analysis of p75 expression, the NGF low-affinity receptor, demonstrated two patterns: the first, at day 3, showing upregulation after SNI (0.39 ± 0.04), compared to CTR values (0.22 ± 0.02) p ≤ 0.01), and after treatment with oxATP for 3 days (0.58 ± 0.03, p ≤ 0.01) and downregulation after the combined treatment with oxATP and GM6001 (0.29 ± 0.05, p ≤ 0.05) (Fig. 3a–d). The second, at day 8, showed an increased expression after treatment with oxATP (0.69 ± 0.1) and oxATP + GM6001 (0.8 ± 0.1), compared to the values in the CTR (0.24 ± 0.07) and SNI groups (0.18 ± 0.04) (p ≤ 0.01) (Fig. 3a–d).
These data were confirmed by densitometric analysis of IF sections of the dorsal horn of the spinal cord (Fig. 4). The expression of TrkA was increased in SNI animals (2.64 ± 0.3) compared to the CTR group (1.20 ± 0.06, p ≤ 0.001) and downregulated to control values after treatment for 8 days with oxATP (1.45 ± 0.05) or oxATP + GM6001 (1.41 ± 0.07, p ≤ 0.001) (Fig. 4a, b). As showed by WB analysis, the expression of the p75 receptor, not conditioned by nerve injury (1.57 ± 0.05) and unchanged if compared to CTR animals (1.49 ± 0.08), was significantly upregulated by 8 days treatment with oxATP (2.0 ± 0.1) and oxATP + GM6001 (2.9 ± 0.2, p ≤ 0.001).
a Representative immunofluorescence pictures of TrkA/p75 receptor expression in the dorsal horn spinal cord after SNI and after treatment with oxATP or oxATP + GM6001 (GM) for 8 days. Scale bar 100 μm. b−c Densitometric quantitation. Data shown as mean ± SEM (*p ≤ 0.05; ***p ≤ 0.001; one-way ANOVA followed by post hoc Holm-Šídák correction for multiple pairwise comparisons)
Taken together, these findings highlight a peculiar time-course and prompt to hypothesize distinct roles of the high- and low-affinity NGF receptors in the spinal cord following nerve injury, differently modulated by the purinergic and MMP systems.
Discussion
Substantial modifications occur in the CNS following peripheral nerve injury, which exceeds the neuropathic behavior-phenotype and are strictly related to morpho-functional cell features, types of injury, and time course of response (Virtuoso et al. 2019, 2020; Weng et al. 2020). However, interactions between glial cells and neurons through the neural extracellular matrix drive the pathological changes after the nerve damage (Zhao et al. 2017).
In the present study, we used the SNI model to evaluate the spinal cord remodeling after nerve injury and after 3 or 8 days of treatment with oxATP alone (an antagonist of the purinergic system) or in combination with GM6001, an inhibitor of the MMPs. Modifications in the spinal cord are closely related to the time after injury, highlighting the relevance of the temporal intervals for therapeutical intervention (Weng et al. 2020; Kawasaki et al. 2008).
Anti-inflammatory and anti-reactive gliosis activity of oxATP and GM6001 treatment has been previously reported by our group (Cirillo et al. 2011, 2012, 2015, 2016; Virtuoso et al. 2020): here we aimed to examine the expression of key pivotal proteins in the neuroinflammatory and neurotrophic networks that could support their interactions. Reactive gliosis induced by SNI, starting from day 3 after injury, was reduced by oxATP injection for 8 days, as showed by Iba1 and GFAP expression. Interestingly, oxATP was ineffective in reducing glial activation after 3 days, even in combination with GM6001. While oxATP treatment reduced both microglial and astrocytic response, the combined treatment with GM6001 was only effective in reducing microglial but not astrocytic activation (Figs. 1, 2). In the early phases after SNI, glial activation seems not to be modulated by the treatment with both drugs, as we also previously demonstrated with GM6001 alone (Virtuoso et al. 2020). Moreover, the combination of the two compounds did not have the expected synergistic effects, but reduced the long-term efficacy of MMP inhibition on astrocytic reaction (Virtuoso et al. 2020). Altogether, these data suggest that the early modulation of glial activation is somehow independent by both ECM remodeling and purinergic signaling. The simultaneous administration of these two compounds has not shown the expected positive combinatory effects, even in later stages, being potentially detrimental. The latter evidence cannot be justified by the presented results but can depend on limiting factors that are common to the signaling pathways of both drugs. The similar activity of these two drugs on the expression of p75, TrkA, and HDAC2 that we observed in this and previous experiments (Virtuoso et al. 2020; Cirillo et al. 2012) could represent part of the explanation. Moreover, P2X4R has been mainly associated with the activation of microglial cells and their neuroinflammatory reaction following injury (Zhang et al. 2008; Tsuda et al. 2017). As shown in Figs. 2 and 3, activation of microglia (Iba1 increase) was documented 3 days after nerve injury, peaked at day 8, and is paralleled by an increase in P2X4R that contributes to further activation, proliferation, and/or migration of microglial cells from the closer laminae in the cord. Accordingly, we demonstrated a significant decrease in P2X4R expression after treatment for 8 days with oxATP and oxATP + GM6001 (Fig. 3). P2X4R levels, already measured with GM6001 interference alone, had the same characteristic course (Virtuoso et al. 2020). Interestingly, the administration of valproic acid, an HDAC inhibitor, also affects the levels and expression of this receptor (Lu et al. 2013; Lee et al. 2012). P2X7R, in contrast, was found to be modulated by OxATP treatment as well (Cirillo et al. 2015).
Morpho-functional, adaptive, and maladaptive changes to external insult and inflammation also drive epigenetic changes. In particular, the epigenetic regulation of transcriptional programs in the dorsal horn after peripheral nerve injury is cell-specific and depends on multiple factors. Among many other factors, histone deacetylases (HDACs) or demethylases (KDMs) of lysine residues regulate chromatin access to transcriptional factors with repression or activation of specific genes (Jih et al. 2017). While HDAC1 protein expression was not modified, HDAC2 was promptly upregulated after nerve injury, suggesting distinct roles in epigenetic changes (Gonneaud et al. 2019). Although the exact mechanisms are unknown, the increase in HDAC2 1 week after the nerve injury might be related to astrocytic response and may inhibit astrocytic gene expression in neuropathic pain states (Maiarù et al. 2016). Our group has demonstrated a link between NGF and MMPs, mediated by the cleavage of pro-NGF and the interactions with both the high- (TrkA) and the low-affinity (p75) receptors (Cirillo et al. 2012; Papa et al. 2014). Moreover, there was a modulatory effect of P2XR interference on the spinal cord neuroglial plasticity following SNI (Cirillo et al. 2015). The effects of MMP and purinergic inhibition pathways, as observed for GM6001 administration alone in a previous experiment (Virtuoso et al. 2020), could have common effects on HDAC modification. Here we found that HDAC2 expression was modulated by both purinergic (with oxATP) and MMP antagonism by GM6001 (Fig. 2). The modulation of histone acetylation is a common pathway of both treatments even if the putative intracellular signaling has not yet been elucidated (Virtuoso et al. 2020). Remarkably, administration of the HDAC inhibitor valproic acid significantly reduced not only P2X4R expression on microglia but also spinal MMP9 levels and improved rat recovery after spinal cord/peripheral nerve injury (Lu et al. 2013; Lee et al. 2012).
Neurotrophic support is determinant for the suitable activity of the neural circuitry, especially after injury (Inoue and Tsuda 2012; Martorana et al. 2018). While inhibition of MMPs changes the availability of neurotrophins modulating their activation (from precursors to the active forms) or degradation (Cirillo et al. 2011, 2016), P2XR modulation can prevent MMP activation or affect precursor neurotransmitter release and NGF signaling (Yang et al. 2016; D’Ambrosi et al. 2001; Sáez-Orellana et al. 2015). The two NGF receptors (TrkA and p75) have demonstrated opposite biological effects, among others, the first being involved in survival and the second in neural death (Blöchl and Blöchl 2007; Ritala et al. 2022; Chao 2003). Accordingly, expression analysis of NGF receptors showed upregulation of TrkA following SNI and downregulation after treatment with oxATP alone and in combination with GM6001 for 8 days. In contrast, p75 was upregulated early by SNI and oxATP treatment, whilst co-treatment with GM6001 reduced the expression at day 3 and increased its expression at day 8. We hypothesize a distinctive modulation of NGF receptor expression: TrkA decrease could be the adaptive response of NGF matrisome enrichment, while p75 can generate pro-survival or pro-apoptotic signals depending on co-receptors and different intracellular pathways in neuronal and glial cells (Blöchl and Blöchl 2007; Inoue and Tsuda 2012).
In conclusion, this work analyzed the expression of key molecules in the matrix-neuroglial network and the impact of the purinergic and MMP inhibition in the spinal cord following peripheral nerve injury (PNI). The resulting data suggest complex protein interconnections, which are not passively influenced by epigenetics but actively contribute to modifying the transcriptomics machinery. Purinergic and MMP inhibition affected cellular/matrix balance at various levels, suggesting a connection between the extracellular matrix and the chromatin rearrangement. The relations between P2XRs, MMPs, and their modulation in the spinal cord after SNI are summarized in Fig. 5 and need to be further studied in future experiments. These may help to identify targeted treatment in neurological diseases with benefits for neuroprotection, spinal plasticity, and functional recovery.
Data availability
The datasets and materials generated and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Adinolfi E, Giuliani AL, De Marchi E, Pegoraro A, Orioli E, Di Virgilio F (2018) The P2X7 receptor: a main player in inflammation. Biochem Pharmacol 151:234–244. https://doi.org/10.1016/j.bcp.2017.12.021
Bai YH, Takemitsu M, Atsuta Y, Matsuno T (1999) Peripheral mononeuropathy induced by loose ligation of the sciatic nerve in the rat: behavioral electrophysiological and histopathologic studies. Exp Anim 48(2):87–94. https://doi.org/10.1538/expanim.48.87
Bianco MR, Cirillo G, Petrosino V, Marcello L, Soleti A, Merizzi G, Cavaliere C, Papa M (2012) Neuropathic pain and reactive gliosis are reversed by dialdehydic compound in neuropathic pain rat models. Neurosci Lett 530:85–90. https://doi.org/10.1016/j.neulet.2012.08.088
Blöchl A, Blöchl R (2007) A cell-biological model of P75NTR signaling. J Neurochem 102(2):289–305. https://doi.org/10.1111/j.1471-4159.2007.04496.x
Cavaliere C, Cirillo G, Bianco MR, Rossi F, De Novellis V, Maione S, Papa M (2007) Gliosis alters expression and uptake of spinal glial amino acid transporters in a mouse neuropathic pain model. Neuron Glia Biol 3:141–153. https://doi.org/10.1017/S1740925X07000695
Chao MV (2003) Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci 4(4):299–309. https://doi.org/10.1038/nrn1078
Cirillo G, Cavaliere BMR, De Simone A, Colangelo AM, Sellitti S, Alberghina L, Papa M (2010) Intrathecal NGF administration reduces reactive astrocytosis and changes neurotrophin receptors expression pattern in a rat model of neuropathic pain. Cell Mol Neurobiol 30(1):51–62. https://doi.org/10.1007/s10571-009-9430-2
Cirillo G, Bianco MR, Colangelo AM, Cavaliere C, De Luca D, Zaccaro L, Alberghina L, Papa M (2011) Reactive astrocytosis-induced perturbation of synaptic homeostasis is restored by nerve growth factor. Neurobiol Dis 41(3):630–639. https://doi.org/10.1016/j.nbd.2010.11.012
Cirillo G, Colangelo AM, Bianco MR, Cavaliere C, Zaccaro L, Sarmientos P, Alberghina L, Papa M (2012) BB14, a nerve growth factor (NGF)-like peptide shown to be effective in reducing reactive astrogliosis and restoring synaptic homeostasis in a rat model of peripheral nerve injury. Biotechnol Adv 30:223–232. https://doi.org/10.1016/j.biotechadv.2011.05.008
Cirillo G, Colangelo AM, Berbenni M, Ippolito VM, De Luca C, Verdesca F, Savarese L, Alberghina L, Maggio N, Papa M (2015) Purinergic modulation of spinal neuroglial maladaptive plasticity following peripheral nerve injury. Mol Neurobiol 52(3):1440–1457. https://doi.org/10.1007/s12035-014-8943-y
Cirillo G, Colangelo AM, De Luca C, Savarese L, Barillari MR, Alberghina L, Papa M (2016) Modulation of matrix metalloproteinases activity in the ventral horn of the spinal cord re-stores neuroglial synaptic homeostasis and neurotrophic support following peripheral nerve injury. PLoS ONE 11(3):e015275. https://doi.org/10.1371/journal.pone.0152750
D’Ambrosi N, Murra B, Cavaliere F, Amadio S, Bernardi G, Burnstock G, Volonté C (2001) Interaction between ATP and nerve growth factor signalling in the survival and neuritic outgrowth from PC12 cells. Neuroscience 108(3):527–534. https://doi.org/10.1016/s0306-4522(01)00431-6
De Luca C, Papa M (2016) Looking Inside the matrix: perineuronal nets in plasticity, maladaptive plasticity and neurological disorders. Neurochem Res 41:1507–1515. https://doi.org/10.1007/s11064-016-1876-2
De Luca C, Papa M (2017) Matrix Metalloproteinases, Neural Extracellular Matrix, and Central Nervous System Pathology. Progress in Molecular Biology and Translational Science. Elsevier, USA, pp 167–202
De Luca C, Savarese L, Colangelo AM, Bianco MR, Cirillo G, Alberghina L, Papa M (2016) Astrocytes and microglia-mediated immune response in maladaptive plasticity is differently modulated by NGF in the ventral horn of the spinal cord following peripheral nerve injury. Cell Mol Neurobiol 36(1):37–46. https://doi.org/10.1007/s10571-015-0218-2
De Luca C, Colangelo AM, Virtuoso A, Alberghina L, Papa M (2020a) Neurons, glia, extracellular matrix and neurovascular unit: a systems biology approach to the complexity of synaptic plasticity in health and disease. IJMS 21:1539. https://doi.org/10.3390/ijms21041539
De Luca C, Virtuoso A, Maggio N, Izzo S, Papa M, Colangelo AM (2020b) Roadmap for stroke: challenging the role of the neuronal extracellular matrix. Int J Mol Sci 21(20):7554. https://doi.org/10.3390/ijms21207554
de Rivero Vaccari JP, Bastien D, Yurcisin G, Pineau I, Dietrich WD, De Koninck Y, Keane RW, Lacroix S (2012) P2X4 receptors influence inflammasome activation after spinal cord injury. J Neurosci 32:3058–3066. https://doi.org/10.1523/JNEUROSCI.4930-11.2012
Decosterd I, Woolf CJ (2000) Spared nerve injury: an animal model of persistent peripheral neuropathic. Pain 87:149–158. https://doi.org/10.1016/S0304-3959(00)00276-1
Dityatev A, Rusakov DA (2011) Molecular signals of plasticity at the tetrapartite synapse. Curr Opin Neurobiol 21:353–359. https://doi.org/10.1016/j.conb.2010.12.006
Gonneaud A, Turgeon N, Jones C, Couture C, Lévesque D, Boisvert FM, Boudreau F, Asselin C (2019) HDAC1 and HDAC2 independently regulate common and specific intrinsic responses in murine enteroids. Sci Rep 9(1):5363. https://doi.org/10.1038/s41598-019-41842-6
Gu BJ, Wiley JS (2006) Rapid ATP-induced release of matrix metalloproteinase 9 is mediated by the P2X7 receptor. Blood 107:4946–4953. https://doi.org/10.1182/blood-2005-07-2994
Halsall JA, Turner BM (2016) Histone deacetylase inhibitors for cancer therapy: an evolutionarily ancient resistance response may explain their limited success. BioEssays 38(11):1102–1110. https://doi.org/10.1002/bies.201600070
Inoue K, Tsuda M (2012) Purinergic systems, neuropathic pain and the role of microglia. Exp Neurol 234:293–301. https://doi.org/10.1016/j.expneurol.2011.09.016
Jacobson KA, Müller CE (2016) Medicinal chemistry of adenosine, P2Y and P2X receptors. Neuropharmacology 104:31–49. https://doi.org/10.1016/j.neuropharm.2015.12.001
Jih G, Iglesias N, Currie MA, Bhanu NV, Paulo JA, Gygi SP, Garcia BA, Moazed D (2017) Unique roles for histone H3K9me states in RNAi and heritable silencing of transcription. Nature 547(7664):463–467. https://doi.org/10.1038/nature23267
Jin XR, Chen XS, Xiao L (2017) MeCP2 Deficiency in Neuroglia: New Progress in the Pathogenesis of Rett Syndrome. Front Mol Neurosci 10:316. https://doi.org/10.3389/fnmol.2017.00316
Kaminska B, Mota M, Pizzi M (1862) Signal transduction and epigenetic mechanisms in the control of microglia activation during neuroinflammation. Biochem Biophys Acta 3:339–351. https://doi.org/10.1016/j.bbadis.2015.10.026
Kawasaki Y, Xu ZZ, Wang X, Park JY, Zhuang Z-Y, Tan P-H, Gao Y-J, Roy K, Corfas G, Lo EH, Ji R-R (2008) Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med 14(3):331–336. https://doi.org/10.1038/nm1723
Lee JY, Kim HS, Choi HY, Oh TH, Ju BG, Yune TY (2012) Valproic acid attenuates blood-spinal cord barrier disruption by inhibiting matrix metalloprotease-9 activity and improves functional recovery after spinal cord injury. J Neurochem 121(5):818–829. https://doi.org/10.1111/j.1471-4159.2012.07731.x
Lu WH, Wang CY, Chen PS, Wang JW, Chuang DM, Yang CS, Tzeng SF (2013) Valproic acid attenuates microgliosis in injured spinal cord and purinergic P2X 4 receptor expression in activated microglia. J Neurosci Res 91:694–705. https://doi.org/10.1002/jnr.23200
Maggio N, Sellitti S, Capano CP, Papa M (2001) Tissue-transglutaminase in rat and human brain: light and electron immunocytochemical analysis and in situ hybridization study. Brain Res Bull 56(3–4):173–182. https://doi.org/10.1016/s0361-9230(01)00649-9
Maiarù M, Morgan OB, Tochiki KK, Hobbiger EJ, Rajani K, Overington DWU, Géranton SM (2016) Complex regulation of the regulator of synaptic plasticity histone deacetylase 2 in the rodent dorsal horn after peripheral injury. J Neurochem 138(2):222–232. https://doi.org/10.1111/jnc.13621
Marcello L, Cavaliere C, Colangelo AM, Bianco MR, Cirillo G, Alberghina L, Papa M (2013) Remodelling of supraspinal neuroglial network in neuropathic pain is featured by a reactive gliosis of the nociceptive amygdala. Eur J Pain 17(6):799–810. https://doi.org/10.1002/j.1532-2149.2012.00255.x
Martorana F, Gaglio D, Bianco MR, Aprea F, Virtuoso A, Bonanomi M, Alberghina L, Papa M, Colangelo AM (2018) Differentiation by nerve growth factor (ngf) involves mechanisms of crosstalk between energy homeostasis and mitochondrial remodeling. Cell Death Dis 9(3):391. https://doi.org/10.1038/s41419-018-0429-9
Mishra A (2017) Binaural blood flow control by astrocytes: listening to synapses and the vasculature: binaural blood flow control by astrocytes. J Physiol 595:1885–1902. https://doi.org/10.1113/JP270979
Mishra SK, Braun N, Shukla V, Füllgrabe M, Schomerus C, Korf H-W, Gachet C, Ikehara Y, Sévigny J, Robson SC, Zimmermann H (2006) Extracelluar nucleotide signaling in adult neural stem cells: synergism with growth factor-mediated cellular proliferation. Development 133(4):675–684. https://doi.org/10.1242/dev.02233
Papa M, Canitano A, Boscia F, Castaldo P, Sellitti S, Porzig H, Taglialatela M, Annunziato L (2003) Differential expression of the Na+-Ca2+ exchanger transcripts and proteins in rat brain regions. J Comp Neurol 461(1):31–48. https://doi.org/10.1002/cne.10665
Papa M, De Luca C, Petta F, Alberghina L, Cirillo G (2014) Astrocyte-neuron interplay in maladaptive plasticity. Neurosci Biobehav Rev 42:35–54. https://doi.org/10.1016/j.neubiorev.2014.01.010
Ritala JF, Lyne SB, Sajanti A, Girard R, Koskimäki J (2022) Towards a comprehensive understanding of p75 neurotrophin receptor functions and interactions in the brain. Neural Regen Res 17(4):701–704. https://doi.org/10.4103/1673-5374.314291
Sáez-Orellana F, Godoy PA, Silva-Grecchi T, Barra KM, Fuentealba J (2015) Modulation of the neuronal network activity by P2X receptors and their involvement in neurological disorders. Pharmacol Res 101:109–115. https://doi.org/10.1016/j.phrs.2015.06.009
Sivaramakrishnan V, Fountain SJ (2012) A Mechanism of Intracellular P2X Receptor Activation. J Biol Chem 287:28315–28326. https://doi.org/10.1074/jbc.M112.372565
Tsuda M (2017) P2 receptors, microglial cytokines and chemokines, and neuropathic pain. J Neurosci Res 95(6):1319–1329. https://doi.org/10.1002/jnr.23816
Virtuoso A, Herrera-Rincon C, Papa M, Panetsos F (2019) Dependence of neuroprosthetic stimulation on the sensory modality of the trigeminal neurons following nerve injury implications in the design of future sensory neuroprostheses for correct perception and modulation of neuropathic pain. Front Neurosci 13:389. https://doi.org/10.3389/fnins.2019.00389
Virtuoso A, De Luca C, Gargano F, Colangelo AM, Papa M (2020) The spinal extracellular matrix modulates a multi-level protein net and epigenetic inducers following peripheral nerve injury. Neuroscience 451:216–225. https://doi.org/10.1016/j.neuroscience.2020.09.051
Virtuoso A, Colangelo AM, Korai SA, Izzo S, Todisco A, Giovannoni R, Lavitrano M, Papa M, Cirillo G (2021) Inhibition of plasminogen/plasmin system retrieves endogenous nerve growth factor and adaptive spinal synaptic plasticity following peripheral nerve injury. Neurochem Int 148:105113. https://doi.org/10.1016/j.neuint.2021.105113
Visse R, Nagase H (2003) Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92:827–839. https://doi.org/10.1161/01.RES.0000070112.80711.3D
Wang Z, Leng Y, Tsai LK, Leeds P, Chuang DM (2011) Valproic acid attenuates blood-brain barrier disruption in a rat model of transient focal cerebral ischemia: the roles of HDAC and MMP-9 inhibition. J Cereb Blood Flow Metab 31:52–57. https://doi.org/10.1038/jcbfm.2010.195
Weng J, Li DD, Jiang BG, Yin XF (2020) Temporal changes in the spinal cord transcriptome after peripheral nerve injury. Neural Regen Res 5(7):1360–1367. https://doi.org/10.4103/1673-5374.272618
Yang F, Zhao K, Zhang X, Zhang J, Xu B (2016) ATP induces disruption of tight junction proteins via IL-1 beta-dependent MMP-9 activation of human blood-brain barrier in vitro. Neural Plast 2016:8928530. https://doi.org/10.1155/2016/8928530
Zhang Z, Zy Z, Fauser U, Schluesener HJ (2008) Mechanical allodynia and spinal up-regulation of P2X4 receptor in experimental autoimmune neuritis rats. Neuroscience 152(2):495–501. https://doi.org/10.1016/j.neuroscience.2007.12.042
Zhao H, Alam A, Chen Q, Eusman MA, Pal A, Eguchi S, Wu L, Ma D (2017) The role of microglia in the pathobiology of neuropathic pain development: what do we know? Br J Anaesth 118(4):504–516. https://doi.org/10.1093/bja/aex006
Funding
This work was supported by grants from the Italian Minister of University and Research (MIUR) (PRIN2007 to A.M.C. and M.P.; SYSBIO-Italian ROADMAP ESFRI Infrastructures to M.P. and A.M.C.; PRIN 2015-2015TM24JS_009 to M.P; PRIN2017-2017XJ38A4_003 to G.C. and M.L.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Conceptualization, GC and MP; data curation, CDL and AV; formal analysis, AV; funding acquisition, AMC, ML, GC, and MP; investigation, CDL, AV, and GC; methodology, CDL and AV; project administration, MP; resources, MP; supervision, GC and MP; writing—original draft, CDL, AV, FG, and GC; writing—review & editing, MC, FG, AAC, ML, GC, and MP. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or nonfinancial interests to disclose.
Ethical approval
The study was conducted according to the guidelines of Italy and Europe for the Care and Use of Laboratory Animals (EU Directive 2010/63). All protocols were approved by the Animal Care and Use Committees of the University of Campania “Luigi Vanvitelli”(auth. 153/2018-PR).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
De Luca, C., Virtuoso, A., Cerasuolo, M. et al. Matrix metalloproteinases, purinergic signaling, and epigenetics: hubs in the spinal neuroglial network following peripheral nerve injury. Histochem Cell Biol 157, 557–567 (2022). https://doi.org/10.1007/s00418-022-02082-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-022-02082-4