Abstract
Reactive astrocytes and activated microglia are the key players in several pathophysiologic modifications of the central nervous system. We used the spared nerve injury (SNI) of the sciatic nerve to induce glial maladaptive response in the ventral horn of lumbar spinal cord and examine its role in the remodeling of the tripartite synapse plasticity. Imaging the ventral horn revealed that SNI was associated with both an early microglial and astrocytic activation, assessed, respectively, by analysis of Iba1 and GFAP expression. Microglia, in particular, localized peculiarly surrounding the motor neurons somata. Perineuronal astrocytes, which play a key role in maintaining the homeostasis of neuronal circuitry, underwent a substantial phenotypic change following peripheral axotomy, producing reactive gliosis. The gliosis was associated with the reduction of glial aminoacid transporters (GLT1 and GlyT1) and increase of neuronal glutamate transporter EAAC1. Although the expression of GABAergic neuronal marker GAD65/67 showed no change, glutamate increase, as demonstrated by HPLC analysis, shifted the excitatory/inhibitory balance as showed by the net increase of the glutamate/GABA ratio. Moreover, endogenous NGF levels were altered in SNI animals and not restored by the intrathecal NGF administration. This treatment reverted phenotypic changes associated with reactive astrocytosis, but failed to modify microglia activation. These findings on one hand confirm the correlation between gliopathy and maladaptive plasticity of the spinal synaptic circuitry, on the other hand add new data concerning the complex peculiar behavior of different glial cells in neuronal degenerative processes, defining a special role of microglia in sustaining the inflammatory response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Axotomy and glial plasticity have been linked with changes in neurotrophins expression, neuronal growth genes, and several regulatory molecules (Cobianchi et al. 2013).
However, it is not fully unraveled how these different molecules can affect the central circuitry plasticity. In the ventral horn of the spinal cord, motoneurons are surrounded by perineuronal nets (PNNs) (Takahashi-Iwanaga et al. 1998) that regulate plasticity and preserve the synapses structure and functionality (Kwok et al. 2011). External synaptic stimulation reduces PNNs content, increases neuronal plasticity, and modulates the connectome of cerebral circuits (Corvetti and Rossi 2005; Foscarin et al. 2011). PNNs modification in the spinal cord can be differentially regulated by injury and physical activity (Wang et al. 2011). Following a peripheral nerve injury (PNI), the disconnection between axons and their targets is associated to critical changes in the spinal cord (Cirillo et al. 2011). Moreover, axotomized motoneurons show a severe loss of their central synapses, relatively to the muscle atrophy.
Neuronal and glial plasticity can occur over short timescales, through a variety of molecular pathways that are able to remove and modify the synaptic connections (Lüscher and Malenka 2012; Morris et al. 2013). Injuries to the central nervous system (CNS) have low recovery rate and limited healing capacities; therefore, large motor and sensory deficits persist following brain or spinal cord injuries (Raineteau and Schwab 2001). The most typical pathologic feature of axotomized motoneurons is the massive synaptic stripping, more conspicuous on the cell soma than on dendrites; this feature may become permanent if target reinnervation does not occur (Novikov et al. 2000). In the spinal cord, following an injury, the synaptic circuit asset might change due to either modification of the synaptic strength (functional plasticity), or addition of new nodes through anatomical remodeling and sprouting of axonal and dendritic branches (Raineteau and Schwab 2001). Fiber sprouting of unaffected systems and formation of collateral circuits with indirect pathways are currently emerging as crucial mechanisms to promote the functional recovery even when the anatomical integrity is permanently compromised (Schwab and Strittmatter 2014).
Damaged motoneuron axons reinnervate and partially regenerate their synaptic connections, but muscle spindle Ia excitatory synapses never recover baseline levels even when the muscle spindle and the muscle are correctly reinnervated. Therefore, other multiple candidate mechanisms, that centrally control neural circuits, may actively modify information encoded by regenerated proprioceptive afferents to prevent recovery (Haftel et al. 2005).
Neurotrophic support is determinant for the appropriate activity of the neural circuitry especially after traumatic injuries. Neurotrophins, exogenously delivered, fine-tune the synaptic plasticity by preventing the synaptic stripping of axotomized motoneurons, and promoting the formation and the density of synapses on motoneurons (Davis-López de Carrizosa et al. 2009; Colangelo et al. 2012; Cirillo et al. 2012).
The detailed understanding of the plastic changes faced by the two main glial populations (microglia and astrocytes) and neurons, after axotomy, is the key to further define the adaptive/maladaptive response following CNS lesions.
Trophic factors supply may eventually help to prevent these changes. Hence, this study aimed to analyze the molecular and morphological changes that axotomized motoneurons suffer after PNI, in parallel with the immune reactive phenotypic and functional changes of the two main glial populations. Furthermore, we investigated whether intrathecal (i.t.) administration of the trophic factor NGF could modulate these changes.
Elucidation of pathophysiological processes underlying the adaptive response to the spinal cord injury would support translational approach to improve functional recovery after PNI.
Materials and Methods
Animals
We used adult (250–300 g; Charles River, Calco, Italy) male Sprague–Dawley rats (n = 45). Rats were maintained on a 12/12-h light/dark cycle and allowed free access to food and water. Each animal was housed under specific pathogen-free conditions in iron-sheet cages with solid floors covered with 4–6 cm of sawdust. All surgery and experimental procedures were performed during the light cycle and approved by the Animal Ethics Committee of The Second University of Naples. Animal care was in compliance with Italian (D.L. 116/92) and EC (O.J. of E.C. L358/1 18/12/86) regulations on the care of laboratory animals. All efforts were made to reduce animal numbers.
SNI Model
Spared sciatic nerve injury was made according to the methods of Decosterd and Woolf (2000). Briefly, each rat was anesthetized with chlorhydrate tiletamine (40 mg/kg) during surgery. The skin on the lateral surface of the thigh was incised and a section was made directly through the biceps femoris muscle exposing the sciatic nerve and its three terminal branches: the sural, common peroneal, and tibial nerves. The spared nerve injury (SNI) procedure comprised axotomy and ligation of the tibial and common peroneal nerves leaving the sural nerve intact. The common peroneal and the tibial nerves were then tight-ligated with 5.0 silk and sectioned distal to the ligation. For the sham-operated control group (SHAM), nerves were exposed but not truncated. Great care was taken to avoid any contact with or stretching of the intact sural nerve. Muscle and skin were closed in two layers.
Drug Delivery
To reduce the bias of discomfort caused by lumbar spinal catheter, the chronic i.t. lumbar spinal catheter was positioned during the SNI surgery, according to the method described previously (Coderre and Melzack 1992). Briefly, a small opening was made at the laminas of the lumbar tract of the spine and a catheter (polyethylene (PE) 10 tubing attached to PE 60 tubing for connection to an osmotic pump) was inserted into the subarachnoid space and directed to the lumbar enlargement of the spinal cord. After anchoring the catheter across the careful apposition of a glass ionomer luting cement triple pack (Ketac Cem radiopaque; 3 M ESPE, Seefeld, Germany), the wound was irrigated with saline and closed in two layers with 3–0 silk (fascial plane) ands surgical skin staples. On recovery from surgery, lower body paralysis was induced by i.t. lidocaine (2 %) injection to confirm proper catheter localization.
Each rat was placed on a table, and the gait and posture of the affected hind paw were carefully observed for 2 min. Only animals exhibiting appropriate, transient paralysis to lidocaine, as well as a lack of motor deficits, were used for treatments (β-NGF or artificial CSF (ACSF) infusion; n = 15/each group). Three days after injury, rats were anesthetized by intraperitoneal (i.p.) chlorhydrate tiletamine (30 mg/kg), the free extremity of the catheter was connected to an osmotic minipump, and the pump implanted subcutaneously. Osmotic pumps attached to i.t. lumbar spinal catheters were filled with rat recombinant β-NGF (Sigma, Italy) (125 ng/μl) in a solution of ACSF containing 1 mg/ml rat serum albumin (Sigma, Italy), or vehicle only (ACSF). The osmotic pumps were model 2001 Alzet (Cupertino, CA) pumps, which pumped at a rate of 1 μl/h for 7 days. This rate produced an i.t. infusion dose of 125 ng/h of NGF corresponding to 12 μg/kg body weight/day.
Tissue Preparation
At the end of treatments, rats were deeply anesthetized by i.p. injection of chloral hydrate (300 mg/kg body weight) and perfused transcardially with saline solution (Tris–HCl 0.1 M/EDTA10 mM) followed by 4 % paraformaldehyde added to 0.1 % glutaraldehyde in 0.01 M phosphate buffer (PB), pH 7.4 at 4 °C. For light microscopy, spinal cords were removed and post-fixed 2 h in the same fixative, then soaked in 30 % sucrose PBS and frozen in chilled isopentane on dry ice. Serial sections were cut at the slide microtome (25 μm thickness) and collected in cold PBS for immunohistochemistry (IHC).
Antibodies
The following antibodies have been used for immunodetection: mouse antibodies directed against Glial Fibrillary Acidic Protein (GFAP; 1:400; Sigma-Aldrich Milano, Italy); rabbit antibodies to ionized calcium-binding adaptor molecule 1 (Iba1; 1:500; Wako Chemicals, USA); guinea pig antibodies to glutamate transporter (GLT1; 1:2000; Chemicon Inc. Temecula, CA, USA); goat antibodies to glycine transporter 1 (GlyT1; 1:10,000; Chemicon Inc. Temecula, CA, USA); mouse antibodies to Neuronal Nuclei (NeuN; 1:2000; Chemicon Inc. Temecula, CA, USA); rabbit antibodies against glutamic acid decarboxylase 65/67(GAD65/67; 1:1000; Sigma-Aldrich, Milano, Italy); goat antibodies to neuronal glutamate transporter EAAC1 (1:4000; Chemicon Inc. Temecula, CA, USA); and rabbit antibodies against NGF (1:250; Chemicon Inc. Temecula, CA, USA).
Spinal Cord IHC
Lumbar (L4–L6) spinal cord sections were blocked in 10 % normal serum (from animal species different from the species origin of the primary antibody used) in 0.01 M PBS/0.25 % Triton for 1 h at room temperature (RT). Each primary antibody (GFAP, Iba1, GLT1, GlyT1, GAD65/67, EAAC1, NGF, NeuN) was diluted in 0.01 M PBS containing 10 % normal serum and 0.25 % Triton. Following incubation for 48 h at 4 °C, sections were washed six times (10 min each) in PBS and incubated with the appropriate biotinylated secondary antibody (Vector Labs Inc., Burlingame, CA, USA; 1:200) for 90 min at RT, washed in PBS, and processed using the Vectastain avidin–biotin peroxidase kit (Vector LabsInc., Burlingame, CA, USA) for 90 min, at RT. Sections were then washed in 0.05 M Tris–HCl and reacted with 3.3-diaminobenzidine tetrahydrochloride (DAB; Sigma, 0.5 mg/ml in Tris–HCl) and 0.01 % hydrogenperoxide. Sections were mounted on chrome-alume gelatine-coated slides, dehydrated, and coverslipped.
HPLC Analysis of Amino Acids
Amino acids levels were analyzed by RP-HPLC analysis using an Agilent 1200 Series Liquid Chromatograph, equipped with a binary pump delivery system, robotic autosampler, column thermostat, and multi-wavelength detector. Briefly, tissues samples were diluted in borate buffer (0.15 M, pH 10.2) followed by addition of the derivatization solution (o-phthalaldehyde (OPA) (10 mg/ml), β-mercaptoethanol (10 mg/ml)), and diluent solution (mobile phase A: 1.5 % v/v H3PO4). After derivatization, the mixture (20 μl) was injected on a reverse-phase Jupiter 5 μm C18 300 Å (250 × 4.6 mm2) column at 40 °C, and derivatives absorption was detected at 338 nm. Separation was obtained at a flow rate of 1 ml/min with a gradient of the mobile phase A (Na2HPO4 (10 mM)/Na2B4O7·10H2O (10 mM)), and phase B (methanol/acetonitrile/water (9:9:2, v/v/v)). Spinal cord samples were automatically derivatized by the robotic autosampler and analyzed blindly using amino acid standard samples (glutamic acid, glutamine, glycine, and gamma-aminobutyric acid (GABA), 0.25 mM each). Method reproducibility was assessed by analyzing all samples five consecutive times. Amino acid concentrations were expressed as the mean of the peak areas, and the standard error of the mean (SEM) and coefficient of variation (% CV) were calculated. Results were reported as the ratio between the percentage of the areas and the total area of the investigated amino acids.
Measurements and Statistical Analysis
Slides were imaged with a Zeiss Axioskope 2 light microscope equipped with high-resolution digital camera (C4742-95, Hamamatsu Photonics, Italia). Measurements of markers in the whole-ventral horn of spinal cord were accomplished using computer-assisted image analysis system (MCID 7.0; Imaging Res. Inc, Canada). For glial markers, a morphometric approach was preferred because of the perfect visualization of single positive elements. Therefore, values of GFAP and Iba1, markers for astrocytes and microglia, respectively, were expressed as proportional areas: number of positive elements relative to the scanned area (5 × 5 cm2). The densitometric values of GLT1, GlyT1, EAAC1, and NGF were expressed as the total target measured area relative to the scanned area (5 × 5 cm2). Averages were obtained from five randomly selected spinal cord sections for each animal, and comparisons were made between treatment (NGF) and control groups (ACSF/SHAM). Data were exported and converted to frequency distribution histograms by using the Sigma-Plot 10.0 program (SPSS Erkrath, Germany). Data of all quantitative analyses were analyzed by one-way ANOVA using all pairwise Holm–Sidak method for multiple comparisons (*p ≤ 0.01; **p ≤ 0.001). All data shown were presented as the mean ± SEM. Single images of control and treated rats were assembled and then the same adjustments were made for brightness, contrast, and sharpness using Adobe Photoshop (Adobe Systems, San Jose, CA).
Results
Microglia Activation and Reactive Gliosis in Ventral Horn of Lumbar Spinal Cord
Complex mechanisms underlying neuron–glia interaction in synapse formation and function are still largely unclear. We have previously reported that PNI correlates with changes of fiber density in the dorsal horn of lumbar spinal cord and increase of glial markers indicative of reactive astrogliosis (Cirillo et al. 2010, 2011; Colangelo et al. 2008). The neuro-glial network rearrangement in spinal circuitry was studied with the SNI model of PNI to induce early and persistent reactive gliosis, disrupte the PNNs, and investigate its role in perturbing synaptic homeostasis.
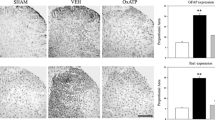
We analyzed ventral horns of lumbar spinal cord for expression levels of glial markers of astrocyte and microglial components. Indeed, IHC analysis of ventral horn of lumbar spinal cord sections revealed the presence of marked gliosis at the end of treatment, as demonstrated by the strong increase of GFAP staining (3.3 ± 1.5) (Fig. 1a, b) and Iba1 levels (30.4 ± 7.0) (Fig. 1c, d) in the ACSF-treated/SNI-injured animals, as compared to the SHAM group (1.6 ± 0.3 and 1.1 ± 0.5 for GFAP and Iba1, respectively; p ≤ 0.001).
Evaluation of glial markers in the ventral horns of spinal cord. Sections (a, c) and densitometric quantitation (b, d) of ventral horns of lumbar spinal cords from SHAM- and SNI-operated animals treated for 7 days with β-NGF (125 ng/μl/h) or ACSF (vehicle) and immunostained for GFAP (a, b) or Iba1 (c, d). Data are the mean ± SEM (**p ≤ 0.001, ACSF vs. SHAM/NGF; ANOVA and Holm–Sidak test). Scale bar 50 μm
Particularly, relevant was the observation that Iba1 expression could be easily matched with NeuN staining of motoneurons nuclei in the anterior horn (Fig. 2), displaying a dramatic change of the neuropil surrounding the neuronal somata.
Iba1 and NeuN immunostaining. Sections of ventral horns of lumbar spinal cord from SHAM and SNI animals treated for 7 days with ACSF (vehicle) or β-NGF (125 ng/μl/h). Microglial cells identified by Iba1 immunostaining following PNI proliferate and migrate toward the axotomized motoneuron somata identified by NeuN immunostaining. The process was not affected by NGF administration
To further understand its putative role in modulating reactive gliosis, we examined the effect of exogenous NGF supply. We found that i.t. treatment with rat recombinant β-NGF for 7 days restored GFAP levels to 1.8 ± 0.6 (Fig. 1a, b), while the neurotrophin did not affect the Iba1 expression (Fig. 1c, d) (30.0 ± 8.0; p ≤ 0.001). I.t. NGF supply inhibited the astrocytic reaction, but had no effect on microglia activation, proliferation, migration, and PNNs alteration.
These data, while confirming the role of NGF in inhibiting mechanisms of reactive astrocytosis, also indicate that the microglial population has probably different mechanisms of modulation associated with neuronal damage.
IHC analyses of NGF expression revealed that endogenous NGF levels in the ventral horns were slightly reduced in SNI animals (12.1 ± 0.9, ACSF group), compared to SHAM-operated animals (16.0 ± 2.1). NGF levels were not restored following i.t. NGF administration (12.4 ± 1.4) (Fig. 3a, b), maybe due to self-sustaining axonal degeneration and PNNs microglial disruption.
Endogenous NGF expression in the ventral horns of lumbar spinal cord. a Sections of lumbar spinal cord prepared from SHAM and SNI animals treated for 7 days with ACSF (vehicle) or β-NGF (125 ng/μl/h) and immunostained with NGF antibody. Scale bar 50 μm. b Densitometric quantitation of NGF levels. Data are expressed as the mean ± SEM (**p ≤ 0.001, ACSF vs. SHAM/NGF; ANOVA and Holm–Sidak test). Scale bar 50 μm
Gliotransmission Response in The Ventral Horn Following SNI and i.t. NGF
Glutamate/GABA balance is the cornerstone of the synaptic transmission. In the spinal cord ventral horn, the modulation of excitatory and inhibitory balance is crucial to prevent homeostasis bias and eventually neuronal loss (Meisner et al. 2010). The central role played by astrocytes in glutamate metabolism and maintenance of synaptic homeostasis has clearly established (Hua et al. 2004).
Therefore, we examined the correlation between reactive gliosis and the alteration of GLT1, the main astrocytic glutamate transporter. IHC analyses of the ventral horns revealed a decrease of GLT1 expression levels, as shown by the optical density of GLT1 staining in SNI animals (ACSF group) (9.0 ± 1.8), as compared to the SHAM group (11.6 ± 3.4) (Fig. 4a, b). The decrease of GLT1 levels in the ACSF group was paralleled by a similar reduction of the glycine transporter (GlyT1) staining (21.1 ± 1.9), about 33 % lower than that found in SHAM animals (28.1 ± 1.9) (Fig. 4c, d). Both GLT1 and GlyT1 expression were restored by NGF treatment (13.0 ± 1.5 and 26.2 ± 2.6, respectively) (Fig. 4a–d). The glutamate decarboxylase 65/67 (GAD 65/67) positive elements, instead, were not significantly affected in the ventral horns of SNI animals, compared to the SHAM group (Fig. 4g, h). These data indicate that neuropil disruption, microglia activation, and reactive gliosis modify the subtle equilibrium of synaptic transmission. Moreover, we found that the reduction of glial glutamate transporters (gGTs) in SNI animals was counterbalanced by a parallel increase of the neuronal glutamate transporter EAAC1. In fact, IHC analyses revealed that the EAAC1 expression was enhanced in ACSF rats (20.4 ± 2.0), as compared to SHAM animals (11.9 ± 2.7), and fully reverted (8.6 ± 1.0) by i.t. NGF administration (Fig. 4e, f).
Excitatory/inhibitory balance, amino acid transporters, and glutamate decarboxylase expression. Sections of ventral horns of lumbar spinal cord from SHAM and SNI animals treated for 7 days with β-NGF (125 ng/μl/h) or ACSF (vehicle) and immunostained for glial glutamate (a, b) or glycine (c, d) transporters, or the neuronal glutamate transporter EAAC1 (e, f) or the glutamate decarboxylase GAD (g, h). Data are expressed as the mean ± SEM (**p ≤ 0.001, ACSF vs. SHAM/NGF; ANOVA and Holm–Sidak test). Scale bar 50 μm
These data strongly implicate the beneficial role of NGF in restoring some key players of the synaptic network in the ventral horns of SNI-injured rats.
These data were further confirmed by HPLC analysis of neurotransmitter levels. As shown in Fig. 5, a significant increase of the glutamate/GABA ratio (1.37 ± 0.03) was evident in injured ACSF-treated animals compared to SHAM animals (0.65 ± 0.08). Clearly, the increase of glutamate/GABA ratio was significantly reduced by i.t. NGF treatment to 0.94 ± 0.03 (p ≤ 0.001), thus strongly supporting the essential role of NGF in maintaining the synaptic homeostasis through modulation of glial glutamate/GABA transporters and neurotransmitters levels at the synaptic cleft.
HPLC analysis of Glutamate/GABA ratio. Amino acid levels were measured by HPLC in the ventral horns of lumbar spinal cord dissected from SHAM and SNI animals treated for 7 days with β-NGF (125 ng/μl/h) or ACSF (vehicle). The Glutamate/GABA ratio was calculated as described in M&M. Data are expressed as the mean ± SEM (**p ≤ 0.001, ACSF vs. SHAM/NGF; ANOVA and Holm–Sidak test)
Discussion
In this study, we analyzed the ventral horns of lumbar spinal cord to examine the molecular and morphological changes in both glial and neuronal compartment associated to motoneurons distress following PNI. We report that peripheral axotomy induced: (i) microglia activation, (ii) reactive astrocytosis (Fig. 1), (iii) a breakdown of neuropil surrounding the motoneurons, and (iv) increased glutamatergic tone accompanied by lowered endogenous NGF levels.
In our previous studies, focused on the dorsal horn of the spinal cord, we described that i.t. NGF or NGF-like peptide administration was able to reverse glial and neuronal changes of synaptic components (Cirillo et al. 2011). We here show that exogenous i.t. NGF supply was also able to partially restore synaptic changes in the efferent compartment of the spinal cord without affecting the microglial reaction.
By measuring the IHC density in the ventral horns, we examined the expression of different molecular determinants of the tripartite synapse (Cirillo et al. 2014). The synaptic detachment suffered by motoneurons following axotomy is a degenerative process, determining a dramatic change of neuronal behavior: from the computation and distribution of information role to a stand by mode where only the pathways of survival and repair are activated and warranted (Gordon et al. 2015). The synaptic stripping has been related with the activation of microglial cells characterized by the withdrawal of their processes, proliferation, and migration toward the axotomized motoneuron somata (Svensson et al. 1993). In our study, we found a marked increase of microglial reaction surrounding axotomized motoneurons already at 1 week after PNI (Fig. 2). In parallel to the glutamate increase (Fig. 5), we observed a steady-state GABAergic tone and the reduction of the glial transporters (Fig. 4), thus affecting the integrity of synaptic circuitry, unbalancing the transmission toward the excitatory pathway.
Long-term glial changes have been described as relevant to the establishment of morphological and molecular features underlying several neurological disorders (Papa et al. 2014). In the spinal cord, signal processing and transmission rely on modulation and integration of a complex network involving glutamate transmission of pre-/postsynaptic neurons, and local inhibitory GABAergic interneurons (Meisner et al. 2010). Interestingly, we have found that the onset of reactive gliosis, as demonstrated by the strong increase of microglial and astroglial markers (Iba1 and GFAP) (Fig. 1), was paralleled by remarkable changes in the expression of glial and neuronal neurotransmitter transporters responsible for synaptic homeostasis (Fig. 4). In particular, we demonstrated the decrease of glial glutamate and glycine transporters and the increase of neuronal glutamate transporter EAAC1 (Fig. 4). The decrease of glial GLT1 has been shown to be due to calpain-mediated proteolysis (Cavaliere et al. 2007), and considered as the first direct consequences of glial activation, while the increase of EAAC1 might be the “desperate” reaction of neurons, ineffectively trying to lower glutamate levels and reduce excitatory glutamate transmission and excitotoxicity. In agreement with this hypothesis, our data indicate a net increase of the glutamate/GABA ratio not counterbalanced by an increase of GAD65/67 positive elements (Fig. 4).
Astrocytes play a central role in the extracellular homeostasis of neurotransmitters and predominantly of glutamate. Glutamate, is the main CNS excitatory molecule, but as a matter of fact its excess in the extracellular spaces triggers excitotoxic neuronal death. Astrocytes are the main sink of glutamate in the brain; from the bulk of glutamate released during synaptic transmission, about 20 % is accumulated into postsynaptic neurons and the remaining 80 % is taken up by perisynaptic astrocytes (Verkhratsky and Kirchhoff 2007).
Another important outcome of these experiments pertains to the role of NGF in modulating/restoring microglial phenotype and astroglial mechanisms involved in maintenance of synaptic homeostasis and of PNNs integrity. Increased plasticity of the nervous system has been related with PNNs reduction. Enzymatic degradation of PNNs enhances functional recovery after spinal cord injury (Massey et al. 2006) by facilitating plasticity of the circuitry. However, there are few experimental reports evaluating the fate of spinal motoneuron PNNs after PNI and following neurotrophic factors administration.
In a spinal cord injury model, it has been described that rehabilitation increased motoneuron PNNs (Wang et al. 2011). After PNI-induced synaptic detachment, microglia surrounded injured motoneurons potentially disrupting the PNNs. These nets contribute to stabilize synapses and are affected by the process of synaptic stripping, while in other neurons of the CNS, such as those of the visual (Liu et al. 2013) or the somatosensory system (McRae et al. 2007) or the cerebellum (Carulli et al. 2013), their formation and maintenance is inversely proportional to the afferent neuronal activity, with sensory inputs reducing PNNs.
Following PNI, sensory inputs from Ia afferents are injured and disconnected from the muscle spindle, their target organ, while motoneurons switch to an unstable regenerative state that includes changes of their dendrites. In particular, it seems that motoneuron PNNs depend on increased input activity mediated by sensory afferents (Molteni et al. 2004).
Although it has been proposed that reduction of microglia reactivity can prevent neuronal loss (Milligan and Watkins 2009), others suggest that activated microglia play a predominantly protective role (Cullheim and Thams 2007) leaving an open debate on the adaptive/maladaptive microglial activity. Here we show that i.t. administration of NGF was able to reduce GFAP (Fig. 1) and restore glial and neuronal amino acid transporters (Fig. 4), but was totally ineffective toward Iba1 levels (Fig. 1). The relevance of this neurotrophin in reverting the morphological and biochemical alterations linked to reactive gliosis and modification of synaptic circuits was confirmed by the observation that endogenous NGF levels were reduced following SNI lesion (Fig. 2) chiefly through MMPs proteolytic activity (Cirillo et al. 2012) that would suggest a therapeutic approach by inhibiting these enzymes (Bruno and Cuello 2006).
These data suggest that delivery of NGF into the spinal cord could be a more effective method to reverse synaptic stripping, but it will only partially restore the system homeostasis. Perhaps, the injection of NGF to the proximal stump of the nerve could help, quenching the self-sustaining process developed after axotomy, mimicking the trophic support normally received from the target. Even if the application at the injury site could be a good strategy, since it might be performed in conjunction with surgical repair, other beneficial effects can be achieved only by a central delivery. Continuous delivery of NGF and other neurotrophins by peptidomimetics, microparticles injection, grafting of neural progenitor cells or by viral vector-mediated transfer (Blits et al. 2003; Su et al. 2009) may guarantee a trophic support for longer periods of time. A combined approach (central and peripheral delivery) might possibly give better results. Furthermore, other trophic factors may be important in spinal motoneurons to maintain the synaptic contacts. Given the variety of cellular and inter-cellular molecular pathways underlying PNI and the various therapeutics scenarios, combining pharmacological and molecular therapies with novel surgical intervention will be required.
Nerve engineering together with molecular targeting strategies, with scaffold materials to deliver drug may represent an intriguing perspective. Since inflammatory mediators released by activated microglia may activate astrocytes (Murdock et al. 2015), we also speculate that an immunomodulatory approach could be effective, preventing neural damage. New strategies should eventually focus not just on axonal regeneration with a central administration of neurotrophins but also target the distal stump and the molecular reactions triggered in the denervated muscle. Hence, given the actual knowledge, acquiring the ideal combination of factors, the appropriate dose and timing pattern to reverse the maladaptive changes are the next challenge.
Conclusions
In conclusion, our findings focused on the importance of phenotypic microglial changes and astrocytic reaction in determining maladaptive plasticity in the ventral horn of the spinal cord following PNI, consisting in (i) reduction of gGTs and perturbation of synaptic circuitry homeostasis; (ii) reduction of glutamate and glycine uptake; (iii) decrease of neuroprotection by endogenous NGF; and (iv) microglia invading the PNNs.
On the other hand, our data strongly support the relevance of NGF in modulating glial function and its role in (i) maintaining the synaptic circuitry through modulation of glutamatergic components and (ii) neuroprotection through the control of synaptic glutamate levels.
However, NGF did not affect microglia activation, probably due to a combined inflammatory and degenerative, self-sustaining process. These new data revealed many aspects of maladaptive plasticity following PNI, particularly shedding some light on the motor system.
Glial components (astrocytes and microglia), as key players in the synaptic stability, are essential for the physiology of the connectome and its pathological changes.
References
Blits B, Oudega M, Boer GJ, Bartlett Bunge M, Verhaagen J (2003) Adeno-associated viral vector-mediated neurotrophin gene transfer in the injured adult rat spinal cord improves hind-limb function. Neuroscience 118(1):271–281
Bruno MA, Cuello AC (2006) Activity-dependent release of precursor nerve growth factor, conversion to mature nerve growth factor, and its degradation by a protease cascade. Proc Natl Acad Sci USA 103(17):6735–6740
Carulli D, Foscarin S, Faralli A, Pajaj E, Rossi F (2013) Modulation of semaphorin3A in perineuronal nets during structural plasticity in the adult cerebellum. Mol Cell Neurosci 57:10–22
Cavaliere C, Cirillo G, Rosaria Bianco M, Rossi F, De Novellis V, Maione S, Papa M (2007) Gliosis alters expression and uptake of spinal glial amino acid transporters in a mouse neuropathic pain model. Neuron Glia Biol 3(2):141–153
Cirillo G, Cavaliere C, Bianco MR, De Simone A, Colangelo AM, Sellitti S, Alberghina L, Papa M (2010) Intrathecal NGF administration reduces reactive astrocytosis and changes neurotrophin receptors expression pattern in a rat model of neuropathic pain. Cell Mol Neurobiol 30(1):51–62
Cirillo G, Bianco MR, Colangelo AM, Cavaliere C, de Daniele L, Zaccaro L, Alberghina L, Papa M (2011) Reactive astrocytosis-induced perturbation of synaptic homeostasis is restored by nerve growth factor. Neurobiol Dis 41(3):630–639
Cirillo G, Colangelo AM, Bianco MR, Cavaliere C, Zaccaro L, Sarmientos P, Alberghina L, Papa M (2012) BB14, a Nerve Growth Factor (NGF)-like peptide shown to be effective in reducing reactive astrogliosis and restoring synaptic homeostasis in a rat model of peripheral nerve injury. Biotechnol Adv 30(1):223–232
Cirillo G, Colangelo AM, Berbenni M, Ippolito VM, De Luca C, Verdesca F, Savarese L, Alberghina L, Maggio N, Papa M (2014) Purinergic modulation of spinal neuroglial maladaptive plasticity following peripheral nerve injury. Mol Neurobiol. doi:10.1007/s12035-014-8943-y
Cobianchi S, Casals-Diaz L, Jaramillo J, Navarro X (2013) Differential effects of activity dependent treatments on axonal regeneration and neuropathic pain after peripheral nerve injury. Exp Neurol 240:157–167
Coderre TJ, Melzack R (1992) The contribution of excitatory amino acids to central sensitization and persistent nociception after formalin-induced tissue injury. J Neurosci 12(9):3665–3670
Colangelo AM, Bianco MR, Vitagliano L, Cavaliere C, Cirillo G, De Gioia L, Diana D, Colombo D, Redaelli C, Zaccaro L, Morelli G, Papa M, Sarmientos P, Alberghina L, Martegani E (2008) A new nerve growth factor-mimetic peptide active on neuropathic pain in rats. J Neurosci 28(11):2698–2709
Colangelo AM, Cirillo G, Lavitrano ML, Alberghina L, Papa M (2012) Targeting reactive astrogliosis by novel biotechnological strategies. Biotechnol Adv 30(1):261–271
Corvetti L, Rossi F (2005) Degradation of chondroitin sulfate proteoglycans induces sprouting of intact purkinje axons in the cerebellum of the adult rat. J Neurosci 25(31):7150–7158
Cullheim S, Thams S (2007) The microglial networks of the brain and their role in neuronal network plasticity after lesion. Brain Res Rev 55(1):89–96
Davis-López de Carrizosa MA, Morado-Díaz CJ, Tena JJ, Benítez-Temiño B, Pecero ML, Morcuende SR, de la Cruz RR, Pastor AM (2009) Complementary actions of BDNF and neurotrophin-3 on the firing patterns and synaptic composition of motoneurons. J Neurosci 29(2):575–587
Decosterd I, Woolf CJ (2000) Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 87(2):149–158
Foscarin S, Ponchione D, Pajaj E, Leto K, Gawlak M, Wilczynski GM, Rossi F, Carulli D (2011) Experience-dependent plasticity and modulation of growth regulatory molecules at central synapses. PLoS One 6(1):e16666
Gordon T, You S, Cassar SL, Tetzlaff W (2015) Reduced expression of regeneration associated genes in chronically axotomized facial motoneurons. Exp Neurol 264:26–32
Haftel VK, Bichler EK, Wang QB, Prather JF, Pinter MJ, Cope TC (2005) Central suppression of regenerated proprioceptive afferents. J Neurosci 25(19):4733–4742
Hua X, Malarkey EB, Sunjara V, Rosenwald SE, Li WH, Parpura V (2004) Ca(2+)-dependent glutamate release involves two classes of endoplasmic reticulum Ca(2+) stores in astrocytes. J Neurosci Res 76:86–97
Kwok JC, Dick G, Wang D, Fawcett JW (2011) Extracellular matrix and perineuronal nets in CNS repair. Dev Neurobiol 71(11):1073–1089
Liu H, Xu H, Yu T, Yao J, Zhao C, Yin ZQ (2013) Expression of perineuronal nets, parvalbumin and protein tyrosine phosphatase σ in the rat visual cortex during development and after BFD. Curr Eye Res 38(10):1083–1094
Lüscher C, Malenka RC (2012) NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb Perspect Biol 4(6):a005710. doi:10.1101/cshperspect.a005710
Massey JM, Hubscher CH, Wagoner MR, Decker JA, Amps J, Silver J, Onifer SM (2006) Chondroitinase ABC digestion of the perineuronal net promotes functional collateral sprouting in the cuneate nucleus after cervical spinal cord injury. J Neurosci 26(16):4406–4414
McRae PA, Rocco MM, Kelly G, Brumberg JC, Matthews RT (2007) Sensory deprivation alters aggrecan and perineuronal net expression in the mouse barrel cortex. J Neurosci 27(20):5405–5413
Meisner JG, Marsh AD, Marsh DR (2010) Loss of GABAergic interneurons in laminae I–III of the spinal cord dorsal horn contributes to reduced GABAergic tone and neuropathic pain after spinal cord injury. J Neurotrauma 27(4):729–737
Milligan ED, Watkins LR (2009) Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci 10(1):23–36
Molteni R, Zheng JQ, Ying Z, Gómez-Pinilla F, Twiss JL (2004) Voluntary exercise increases axonal regeneration from sensory neurons. Proc Natl Acad Sci USA 101(22):8473–8478
Morris GP, Clark IA, Zinn R, Vissel B (2013) Microglia: a new frontier for synaptic plasticity, learning and memory, and neurodegenerative disease research. Neurobiol Learn Mem 105:40–53
Murdock BJ, Bender DE, Segal BM, Feldman EL (2015) The dual roles of immunity in ALS: injury overrides protection. Neurobiol Dis 77:1–12
Novikov LN, Novikova LN, Holmberg P, Kellerth J (2000) Exogenous brain-derived neurotrophic factor regulates the synaptic composition of axonally lesioned and normal adult rat motoneurons. Neuroscience 100(1):171–181
Papa M, De Luca C, Petta F, Alberghina L, Cirillo G (2014) Astrocyte-neuron interplay in maladaptive plasticity. Neurosci Biobehav Rev 42:35–54
Raineteau O, Schwab ME (2001) Plasticity of motor systems after incomplete spinal cord injury. Nat Rev Neurosci 2(4):263–273
Schwab ME, Strittmatter SM (2014) Nogo limits neural plasticity and recovery from injury. Curr Opin Neurobiol 27:53–60
Su H, Zhang W, Guo J, Guo A, Yuan Q, Wu W (2009) Neural progenitor cells enhance the survival and axonal regeneration of injured motoneurons after transplantation into the avulsed ventral horn of adult rats. J Neurotrauma 26(1):67–80
Svensson M, Eriksson P, Persson JK, Molander C, Arvidsson J, Aldskogius H (1993) The response of central glia to peripheral nerve injury. Brain Res Bull 30(3–4):499–506
Takahashi-Iwanaga H, Murakami T, Abe K (1998) Three-dimensional microanatomy of perineuronal proteoglycan nets enveloping motor neurons in the rat spinal cord. J Neurocytol 27(11):817–827
Verkhratsky A, Kirchhoff F (2007) Glutamate-mediated neuronal-glial transmission. J Anat 210(6):651–660
Wang D, Ichiyama RM, Zhao R, Andrews MR, Fawcett JW (2011) Chondroitinase combined with rehabilitation promotes recovery of forelimb function in rats with chronic spinal cord injury. J Neurosci 31(25):9332–9344
Acknowledgments
This work was supported by Grants from the Italian Minister of University and Research (PRIN2007 to M.P. and to A.M.C.); SYSBIONET—Italian ROADMAP ESFRI Infrastructures to L.A., A.M.C., M.P. (fellowship to L.S.); FIRB-ITALBIONET and NEDD to L.A.; Blueprint Pharma s.r.l.; PRIMM, s.r.l.. Research work in authors’ laboratory was funded by Grants from Regione Campania (L.R. N.5 Bando 2003 to M.P.), Regione Campania (Prog. Spec art 12 E.F. 2000 to M.P.), CNR (Neurobiotecnologie 2003 to M.P.), and Associazione Levi-Montalcini (fellowships to G.C.).
Conflict of interest
The authors declare that this article has no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De Luca, C., Savarese, L., Colangelo, A.M. et al. Astrocytes and Microglia-Mediated Immune Response in Maladaptive Plasticity is Differently Modulated by NGF in the Ventral Horn of the Spinal Cord Following Peripheral Nerve Injury. Cell Mol Neurobiol 36, 37–46 (2016). https://doi.org/10.1007/s10571-015-0218-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-015-0218-2