Abstract
Purpose
To assess the real-world 5-year treatment outcomes of ranibizumab therapy in Japanese patients with neovascular age-related macular degeneration (AMD).
Methods
This was a retrospective, observational, and open-label effectiveness study that included 295 eyes. The participants were patients with treatment-naïve neovascular AMD who received intravitreal ranibizumab (IVR) monthly injection at least three times as the loading phase, followed by further injections as needed (pro re nata (PRN)) and follow-up assessments for 5 years. Outcomes were determined at least 5 years after the first ranibizumab injection.
Results
Mean logMAR best-corrected visual acuity (BCVA) at baseline was 0.52. The mean BCVA significantly improved after three loading injections; however, it declined gradually. The BCVA at 1 year was significantly better than the baseline BCVA, whereas the 3-year, 4-year, and 5-year BCVA values were significantly lower than the baseline values. The average central foveal thickness improved significantly from 366 ± 125 μm to 268 ± 134 μm (p < 0.0001). Macular atrophy was significantly more likely to occur in cases with classic choroidal neovascularization (CNV) than in cases with other AMD (p = 0.01).
Conclusions
IVR is well tolerated in eyes with AMD. However, a PRN regimen for AMD may have limited real-world effectiveness for long-term maintenance of improved visual acuity. Macular atrophy may occur more frequently in classic CNV. To maintain good vision, IVR treatment should be started earlier and performed continuously.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Age-related macular degeneration (AMD) is a progressive, degenerative disease of the retina that causes vision loss and irreversible blindness in elderly populations around the world. AMD is characterized by the appearance of drusen in the macula followed by choroidal neovascularization (CNV) or macular atrophy (MA) [1]. The pathology of AMD is not clear, but environmental factors, lipid transport, metabolism, the complement cascade, remodeling of the retinal extracellular collagen matrix, and the angiogenesis pathway are considered to contribute to its development [2].
AMD is classified as dry or neovascular (wet) based on the absence or presence of new choroidal blood vessels that invade the retina, respectively [3]. Neovascular AMD causes severe vision loss as a result of CNV and the associated retinal edema. The high incidence of neovascular AMD among Japanese patients is well known. The treatments for CNV and associated edema include laser ablation, photodynamic therapy with verteporfin, and anti-vascular endothelial growth factor (VEGF) injections [4, 5]. However, at present, anti-VEGF therapy is the first choice for neovascular AMD.
Ranibizumab is an anti-VEGF drug and a humanized monoclonal antibody fragment that blocks VEGF. It can prevent vision loss in most patients and can also lead to significant visual gain in approximately one-third of the patients. Regular monthly injections of ranibizumab have been established as the gold standard treatment for neovascular AMD [6,7,8].
Among the previous large-scale clinical trials, the MARINA and ANCHOR studies also showed that monthly injections of ranibizumab improved visual acuity (VA) [6, 7, 9]. However, the more recent SEVEN-UP study showed that the switch to “as needed” (pro re nata (PRN)) dosing regimens after monthly ranibizumab injections in the MARINA and ANCHOR studies caused eventual visual loss [10,11,12,13,14]. Moreover, MA of the retinal pigment epithelium (RPE) is a frequent cause of severe visual loss in patients with neovascular AMD. This degenerative disease presents with progressive loss of areas of the RPE, photoreceptors, and the underlying choriocapillaris [15,16,17,18,19,20]. However, the pathophysiologic mechanisms underlying this condition are poorly understood at present.
Therefore, this study is aimed at investigating the real-world outcomes of 5-year ranibizumab treatment for Japanese neovascular AMD patients. In addition, anti-VEGF therapy for AMD is associated with atrophic changes such as MA. Therefore, this study also examined whether MA occurred 5 years after treatment and assessed the association of MA occurrence with patient and drug characteristics.
Methods
This study was a retrospective, observational, open-label effectiveness study conducted using the medical records of patients treated at Kyushu University Hospital in Japan.
Patient population
This retrospective study included 295 eyes of 288 patients with neovascular AMD and abnormal type who were followed up for 5 years after the first ranibizumab injection, including cases of dropout and changes to other anti-VEGF drugs at Kyushu University Hospital between April 2009 and March 2016. There were 186 males and 102 females, averaging 74.4 years of age.
Intravitreal treatment
This chart review study included patients with neovascular AMD in whom intravitreal ranibizumab (0.5 mg) treatment was initiated between April 2009 and March 2016. Consecutive intravitreal ranibizumab injections were administered monthly at least three times as the loading phase, and further injections were administered on a consecutive PRN basis at monthly follow-up visits. Patients who showed multiple recurrences or resistance while on ranibizumab, injections observed during monthly monitoring, were switched to aflibercept treatment.
Method of administration
Intravitreal ranibizumab (0.5 mg) (IVR) was administered in three loading doses at months 0, 1, and 2, followed by further injections on a PRN basis. Recurrence was assessed based on new bleeding of the fundus and the presence or absence of exudative changes in spectral-domain optical coherent tomography (OCT). Informed consent was obtained from all AMD patients. This study was approved by the Institutional Review Board at Kyushu University Hospital, and all experiments were performed in accordance with the Declaration of Helsinki for research involving human subjects.
Ophthalmic examination
At each follow-up visit, the patients underwent a complete ophthalmic examination, which included an assessment of best-corrected visual acuity (BCVA), slit-lamp biomicroscopy, dilated funduscopy, fundus photography, and OCT. The decimal BCVA was measured using the Landolt chart and was expressed as the logarithm of minimal angle of resolution (logMAR). Central subfield macular thickness (CMT) was measured via OCT as the average thickness of the central 1-mm thickness map measurement area. Angiography was performed with both fluorescein (FA) and indocyanine green (ICGA) at baseline.
Macular atrophy
This study also examined whether MA occurred 5 years after treatment using funduscopy and OCT images, in accordance with a previous study [12, 21]. The characteristics of neovascular patients with MA were also examined. The characteristics assessed were age, sex, FA classification (predominantly, minimally classic, and occult), VA noted pretreatment and 5 years after treatment, the number of medical treatments required, and the number of times treatment was switched from ranibizumab to the other anti-VEGF drug, aflibercept.
Characteristics
This study included assessments of the following characteristics to determine their associations with neovascular AMD: age, sex, VA before treatment, hypertension, diabetes mellitus, history of smoking, pretreatment CMT, pretreatment greatest linear dimension (GLD), number of injections, switching to aflibercept from ranibizumab, FA classification (predominantly, minimally classic, and occult) at baseline, and neovascular AMD classification (typical exudative AMD (tAMD), polypoidal choroidal vasculopathy (PCV), and retinal angiomatous proliferation (RAP)). Information on hypertension, diabetes, and history of smoking was obtained using a questionnaire by trained doctors during the initial examination on the same day as the neovascular AMD detection.
Statistical methods
All statistical analyses were performed using a commercial software package (JMP pro software, version 12.0; SAS, Inc., Cary, NC). Descriptive statistics included mean, standard deviation (SD), median, range, and percentages where appropriate. Correlations between any two of the following variables were analyzed using the Dunnett test, Fisher exact test, ANOVA test, chi-square test, multiple logistic regression analysis, and t-test: sex, age, BCVA, anamnesis, life history, CMT, GLD, changes in the BCVA and OCT parameters, and drug history. All tests of associations were considered statistically significant if p < 0.05.
Results
Baseline characteristics
The participants’ baseline characteristics are shown in Table 1. Of the 295 patients in this study, 189 (64%) were men and 106 (36%) were women. The average age of the patients was 74.3 ± 8.3 years (range, 51–96 years), the BCVA was 0.40 ± 0.26, CMT was 366 ± 129 μm, and GLD was 4139 ± 2299 μm. On the basis of FA, ICGA, and OCT images, the CNV lesion was classified as typical AMD in 167 eyes, PCV in 119 eyes, and RAP in 9 eyes. Forty-three (17%) patients had diabetes and 117 (46%) had hypertension. Moreover, 39 (17%) patients had a smoking habit. Among the analyzed characteristics, however, only the BCVA and GLD showed significant differences at baseline among the different types of AMD. Patients with PCV showed significantly better VA before treatment in comparison with the others. In addition, pretreatment GLD in RAP patients was significantly smaller than that in the others.
Administration outcomes
The mean number of injections was 11.4 for 5 years. There is no significant difference in the mean number of injections between typical AMD and PCV. In this study, 63 (36%) patients were switched over to aflibercept because of the low efficacy of ranibizumab observed in the patients.
Visual acuity outcomes
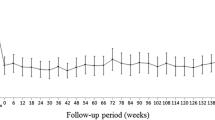
Among the 295 patients enrolled in this study, 177 received monitoring and treatment over the 5-year follow-up period. Although the VA in all cases with AMD (including RAP), typical AMD, and PCV significantly improved until the first year after treatment, subsequently, it declined gradually. The mean BCVA in all patients significantly declined from 0.48 ± 0.35 logMAR at baseline to 0.66 ± 0.53 logMAR at 5 years (p < 0.0001). The mean BCVA also declined significantly by the end of year 5 in both types of AMD. Briefly, the mean BCVA change was 0.19 logMAR for typical AMD (p < 0.0001) and 0.16 logMAR for PCV (p < 0.0001) (Fig. 1).
The mean changes in best-corrected visual acuity (BCVA) scores. Changes in BCVA were calculated for each patient, and the means are shown at various time points. The type of AMD included total AMD, typical AMD, and PCV. A total of 177 patients with AMD were observed and treated over a 5-year follow-up period. After loading injections for 3 months, the visual acuity in the total AMD, typical AMD, and PCV groups significantly improved, but it gradually declined by the end of year 5
Furthermore, a BCVA change of ≥ 0.3 logMAR was considered significant. When the decline in BCVA was defined as a decrease of > 0.3 logMAR, 64% (114 eyes) of the patients showed improvement or maintained their BCVA at 5 years. The 5-year BCVA change did not differ significantly between each type of AMD (Fig. 2). Moreover, there was no significant difference in VA at the end of this study based on the number of injections or treatment switch.
The distribution of changes in the best-corrected visual acuity (BCVA) score. The diagram shows the distribution of visual acuity (VA) changes from previous time points in the total AMD, typical AMD, PCV, and RAP groups. The 5-year BCVA changes did not show significant differences between each type of AMD
Anatomical outcomes
The mean CMT as measured using OCT significantly decreased in the total AMD (including RAP), typical AMD, and PCV groups over the entire observation period. The mean CMT in total AMD significantly decreased from 366 ± 125 μm at baseline to 287 ± 96.8 μm at 1 year (p < 0.0001), 290 ± 114 μm at 2 years, 270 ± 112 μm at 3 years (p < 0.0001), 257 ± 92 μm at 4 years (p < 0.0001), and 268 ± 134 μm at 5 years (p < 0.0001, Fig. 3). In terms of the type of AMD, the mean CMT significantly decreased for 5 years in both typical AMD and PCV (p < 0.0001). Moreover, there was no significant difference in the CMT based on the number of injections or treatment switch at the end of this study. In addition, the decrease in the mean CMT with atrophy was significantly more than that without atrophy after 5 years in both typical AMD and PCV (p < 0.01).
The mean change in central macular thickness (CMT). Changes in the CMT were examined for each patient by OCT, and the mean values for various time points are shown. The type of AMD included total AMD, typical AMD, and PCV. A total of 177 patients with AMD were observed and treated over a 5-year follow-up period
Predictive factors for better visual acuity
This study defined the group with a 5-year reduction in VA of less than 0.3 logMAR as the “better” VA group and analyzed the predictive factors for better VA after 5 years of treatment. The background factors investigated are as follows: age, sex, neovascular AMD type (tAMD, PCV, RAP), FA classification (predominantly, minimally classic, occult), medical history (hypertension, diabetes), smoking habit, pretreatment GLD, pretreatment CMT, the number of anti-VEGF treatments, and switching of the agent. The better and worse BCVA groups showed significant differences in age (p = 0.004), GLD (p < 0.0001), and baseline BCVA (p < 0.0001) in univariate analysis (Table 2). Multivariable analysis revealed that baseline GLD and BCVA were independent risk factors for better vision after 5 years of treatment. Briefly, patients with smaller pretreatment GLD and better pretreatment VA showed better final VA after the 5-year treatment (Table 3).
Analysis for retinal atrophy
The occurrence of MA could be assessed using funduscopy and OCT images in 150 of the 177 patients in this study. MA occurred in 65 images (43%) after the 5-year treatment. MA was significantly more likely to occur in classic CNV (i.e., predominantly) than in the other AMD type (p = 0.01) and in cases with a worse average BCVA both before (p = 0.0004) and after treatment (p = 0.0002). However, age, sex, the number of medical treatments, and treatment switching from IVR to intravitreal aflibercept (IVA) were not associated with MA occurrence (Table 4).
Discussion
The results of this retrospective study demonstrate the long-term effectiveness of IVR monotherapy in treating neovascular AMD in Japanese patients. The assessment of patients with neovascular AMD treated using IVR monotherapy revealed that three monthly injections followed by the PRN regimen for subsequent injections resulted in significantly improved VA by the end of the first year, but VA showed a continuous decline to a level below baseline subsequently. The BCVA improved significantly from baseline during the first year, with a maximum increase of 0.14 logMAR at 3 months and 0.077 logMAR at 1 year. Subsequently, there was a continuous BCVA decline. At 5 years, there was significant loss of 0.18 logMAR compared with the baseline values. Eyes with typical AMD and PCV showed similar improvement and decline in vision over 5 years.
Although a direct comparison with other major studies is difficult due to differences in the baseline characteristics, measurements of VA, and reinjection criteria, the 5-year outcomes in the CATT study revealed a decrease in the mean BCVA with 3 ETDRS letters below the baseline [12]. One previous large-scale clinical trial (SEVEN-UP study) assessed the switch to PRN dosing regimens after the monthly ranibizumab injections in the MARINA and ANCHOR studies [10]. That study showed a decline of 8.6 ETDRS letters compared with the baseline after 7 years of treatment. Wecker et al. reported the five-year visual outcomes in patients receiving a PRN regimen for AMD. They found that the maximum mean VA gain in year 1 was 5.2 ETDRS letters, and the gain declined after that. By year 5, 34% of the patients with AMD had experienced VA loss of more than 15 letters [22]. In our study, the proportion of patients showing VA loss of more than 0.3 logMAR after the 5-year treatment was 35.6%, which was comparable with the results of other studies.
Several factors have been proposed to explain this vision decline, including the type of AMD, the number of injections, residual exudation, and MA or scarring [23, 24]. Residual subretinal fluid and insufficient injections are known to be important factors for vision decline [11]. The mean injection number in our study was 14.8 over 5 years, and the mean number of ranibizumab injections administered each year was as follows: 5.1 injections during year 1, 2.1 injections during year 2, 2.6 injections during year 3, 2.5 injections during year 4, and 2.6 injections during year 5. One of the reasons for the vision decline in our patients could be undertreatment due to fewer injections. Many studies have revealed the risk of MA during anti-VEGF therapy for AMD patients. Maguire et al. reported in the CATT study that MA was found in 41% of subjects after 5 years of treatment [12], and Martin et al. also used two-year data from the CATT study to report a higher risk of MA development in eyes treated monthly than in eyes treated PRN [11]. In the SEVEN-UP study, MA was found in 98% of the eyes during 7.3 years [10], and the area of MA was correlated with worsening VA [25]. Munk et al. reported MA in 73.5% of the eyes in their retrospective study treated with ranibizumab or aflibercept PRN regimen. The mean treatment period was 6.2 years and the mean number of anti-VEGF injections was 48. They revealed that the presence of intraretinal cysts was a significant factor for MA development, so they recommended undertreatment rather than overtreatment as a possible cause of formation and development of MA [26]. In our study, MA was found in 43% of the patients after 5 years of treatment, and multivariable analysis revealed that similar to intraretinal pathological lesions, predominantly classic CNV was an independent risk factor for MA formation. Our findings also suggest that undertreatment, rather than overtreatment, is a risk factor for MA.
To identify other factors for the declining VA, we performed multivariable analysis to detect independent risk factors for improvements or reduction in visual acuity. The better and worse BCVA groups showed significant differences in age, GLD, and baseline BCVA in univariate analysis. Subsequent multivariable analysis revealed that baseline GLD and BCVA were independent risk factors for better vision after 5-year treatment. In short, this result suggested that smaller GLD and better VA at baseline were risk factors to maintain good VA after 5-year treatment.
Our study included a high proportion of eyes with PCV (40%), as expected for a Japanese population. Although the percentage of eyes with PCV has not been reported in the CATT and SEVEN-UP studies, or other long-term studies from the USA or Europe, this percentage is speculated to be low because the prevalence of PCV in western countries is lower than that in Asian countries. In a multicenter prospective randomized trial for eyes with PCV (EVEREST II study), Koh et al. found a significantly higher rate of polyp regression in the ranibizumab/PDT combination group (69.3%) than the ranibizumab monotherapy group (34%) and that BCVA change from baseline was significantly better in the ranibizumab/PDT combination group than in the ranibizumab monotherapy group [27]. We also reported the efficacy of aflibercept monotherapy for eyes with PCV in a multicenter, prospective study (APOLLO study) that shows a higher polyp regression rate (72.5%) with a 3 plus bimonthly regimen [28]. Therefore, a combination of anti-VEGF therapy and PDT or aflibercept monotherapy has recently been preferred for PCV treatment. In this study, we performed ranibizumab monotherapy or switched to aflibercept for eyes with PCV under a PRN regimen, but the BCVA declined, like in typical AMD. We speculate that if we had chosen the proactive regimen, treat and extend, or fixed dosing, VA could have been maintained longer than this study for both PCV and typical AMD. The combination of anti-VEGF drug and PDT or aflibercept monotherapy could maintain VA for PCV patients.
This study has limitations due to its retrospective nature. During the 5-year study period, 40% of the patients were dropped out from this study. The study included all patients for whom treatment was initiated with ranibizumab with the PRN protocol, but switched to aflibercept (36%) because of the low efficacy of ranibizumab. There was no comparison group of untreated patients, patients treated only with other products, or different regimens of ranibizumab, and safety was not an objective of our study. Our study provides the long-term real-world outcome data on VA and anatomical findings of Japanese AMD patients.
In conclusion, in Japanese eyes with exudative AMD, ranibizumab monotherapy performed in the form of a 3-month induction phase followed by a PRN regimen resulted in a decline in VA and decreased CMT with a mean of 14 injections performed for 5 years. A smaller GLD was independently associated with better VA, and classic CNV was independently associated with MA that was related to poor vision.
References
Gheorghe A, Mahdi L, Musat O (2015) Age-related macular degeneration. Rom J Ophthalmol 59:74–77
Francis PJ, Klein ML (2011) Update on the role of genetics in the onset of age-related macular degeneration. Clin Ophthalmol 5:1127–1133. https://doi.org/10.2147/OPTH.S11627
Ambati J, Fowler BJ (2012) Mechanisms of age-related macular degeneration. Neuron 75:26–39. https://doi.org/10.1016/j.neuron.2012.06.018
Macular Photocoagulation Study Group (1991) Laser photocoagulation of subfoveal neovascular lesions in age-related macular degeneration. Results of a randomized clinical trial. Arch Ophthalmol 109:1220–1231
Treatment of age-related macular degeneration with photodynamic therapy (TAP) Study Group (1999) Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin one-year results of 2 randomized clinical trials—TAP report. Arch Ophthalmol 117:1329–1345
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, Group MS (2006) Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 355:1419–1431. https://doi.org/10.1056/NEJMoa054481
Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP, Schneider S, Group AS (2006) Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 355:1432–1444. https://doi.org/10.1056/NEJMoa062655
Brown DM, Heier JS, Ciulla T, Benz M, Abraham P, Yancopoulos G, Stahl N, Ingerman A, Vitti R, Berliner AJ, Yang K, Nguyen QD, Investigators C-I (2011) Primary endpoint results of a phase II study of vascular endothelial growth factor trap-eye in wet age-related macular degeneration. Ophthalmology 118:1089–1097. https://doi.org/10.1016/j.ophtha.2011.02.039
Singer MA, Awh CC, Sadda S, Freeman WR, Antoszyk AN, Wong P, Tuomi L (2012) HORIZON: an open-label extension trial of ranibizumab for choroidal neovascularization secondary to age-related macular degeneration. Ophthalmology 119:1175–1183. https://doi.org/10.1016/j.ophtha.2011.12.016
Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K, Group S-US (2013) Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology 120:2292–2299. https://doi.org/10.1016/j.ophtha.2013.03.046
Comparison of Age-related Macular Degeneration Treatments Trials Research G, Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, Toth C, Redford M, Ferris FL 3rd (2012) Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 119:1388–1398. https://doi.org/10.1016/j.ophtha.2012.03.053
Comparison of Age-related Macular Degeneration Treatments Trials Research G, Maguire MG, Martin DF, Ying GS, Jaffe GJ, Daniel E, Grunwald JE, Toth CA, Ferris FL 3rd, Fine SL (2016) Five-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration: the comparison of age-related macular degeneration treatments trials. Ophthalmology 123:1751–1761. https://doi.org/10.1016/j.ophtha.2016.03.045
Boulanger-Scemama E, Sayag D, Ha Chau Tran T, Quaranta-El Maftouhi M, Rumen F, Creuzot-Garcher C, Blanco Garavito R, Jung C, Souied E (2016) Ranibizumab and exudative age-related macular degeneration: 5-year multicentric functional and anatomical results in real-life practice. J Fr Ophtalmol 39:668–674. https://doi.org/10.1016/j.jfo.2016.06.001
Heimes B, Lommatzsch A, Zeimer M, Gutfleisch M, Spital G, Dietzel M, Pauleikhoff D (2011) Long-term visual course after anti-VEGF therapy for exudative AMD in clinical practice evaluation of the German reinjection scheme. Graefes Arch Clin Exp Ophthalmol 249:639–644. https://doi.org/10.1007/s00417-010-1524-5
Maguire P, Vine AK (1986) Geographic atrophy of the retinal pigment epithelium. Am J Ophthalmol 102:621–625
Holz FG, Wolfensberger TJ, Piguet B, Gross-Jendroska M, Wells JA, Minassian DC, Chisholm IH, Bird AC (1994) Bilateral macular drusen in age-related macular degeneration: prognosis and risk factors. Ophthalmology 101:1522–1528
Sunness JS, Gonzalez-Baron J, Applegate CA, Bressler NM, Tian Y, Hawkins B, Barron Y, Bergman A (1999) Enlargement of atrophy and visual acuity loss in the geographic atrophy form of age-related macular degeneration. Ophthalmology 106:1768–1779. https://doi.org/10.1016/S0161-6420(99)90340-8
Grunwald JE, Pistilli M, Ying GS, Maguire MG, Daniel E, Martin DF, Comparison of Age-related Macular Degeneration Treatments Trials Research G (2015) Growth of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology 122:809–816. https://doi.org/10.1016/j.ophtha.2014.11.007
Grunwald JE, Pistilli M, Daniel E, Ying GS, Pan W, Jaffe GJ, Toth CA, Hagstrom SA, Maguire MG, Martin DF, Comparison of Age-Related Macular Degeneration Treatments Trials Research G (2017) Incidence and growth of geographic atrophy during 5 years of comparison of age-related macular degeneration treatments trials. Ophthalmology 124:97–104. https://doi.org/10.1016/j.ophtha.2016.09.012
Holz FG, Strauss EC, Schmitz-Valckenberg S, van Lookeren Campagne M (2014) Geographic atrophy: clinical features and potential therapeutic approaches. Ophthalmology 121:1079–1091. https://doi.org/10.1016/j.ophtha.2013.11.023
Takahashi A, Ooto S, Yamashiro K, Oishi A, Tamura H, Nakanishi H, Ueda-Arakawa N, Tsujikawa A, Yoshimura N (2016) Photoreceptor damage and reduction of retinal sensitivity surrounding geographic atrophy in age-related macular degeneration. Am J Ophthalmol 168:260–268. https://doi.org/10.1016/j.ajo.2016.06.006
Wecker T, Ehlken C, Buhler A, Lange C, Agostini H, Bohringer D, Stahl A (2017) Five-year visual acuity outcomes and injection patterns in patients with pro-re-nata treatments for AMD, DME, RVO and myopic CNV. Br J Ophthalmol 101:353–359. https://doi.org/10.1136/bjophthalmol-2016-308668
Takahashi Y, Koizumi H, Hasegawa T, Izumi T, Maruko I, Sonoda S, Sakamoto T, Iida T (2018) Comparison of subfoveal choroidal structures in typical neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Jpn J Ophthalmol 62:576–583. https://doi.org/10.1007/s10384-018-0615-4
Ogasawara M, Koizumi H, Yamamoto A, Itagaki K, Saito M, Maruko I, Okada AA, Iida T, Sekiryu T (2018) Prognostic factors after aflibercept therapy for typical age-related macular degeneration and polypoidal choroidal vasculopathy. Jpn J Ophthalmol 62:584–591. https://doi.org/10.1007/s10384-018-0605-6
Bhisitkul RB, Mendes TS, Rofagha S, Enanoria W, Boyer DS, Sadda SR, Zhang K (2015) Macular atrophy progression and 7-year vision outcomes in subjects from the ANCHOR, MARINA, and HORIZON studies: the SEVEN-UP study. Am J Ophthalmol 159:915–924 e912. https://doi.org/10.1016/j.ajo.2015.01.032
Munk MR, Ceklic L, Ebneter A, Huf W, Wolf S, Zinkernagel MS (2016) Macular atrophy in patients with long-term anti-VEGF treatment for neovascular age-related macular degeneration. Acta Ophthalmol 94:e757–e764. https://doi.org/10.1111/aos.13157
Koh A, Lai TYY, Takahashi K, Wong TY, Chen LJ, Ruamviboonsuk P, Tan CS, Feller C, Margaron P, Lim TH, Lee WK, group EIs (2017) Efficacy and safety of ranibizumab with or without verteporfin photodynamic therapy for polypoidal choroidal vasculopathy: a randomized clinical trial. JAMA Ophthalmol 135:1206–1213. https://doi.org/10.1001/jamaophthalmol.2017.4030
Oshima Y, Kimoto K, Yoshida N, Fujisawa K, Sonoda S, Kubota T, Murata T, Sakamoto T, Yoshida S, Sonoda KH, Ishibashi T (2017) One-year outcomes following intravitreal aflibercept for polypoidal choroidal vasculopathy in Japanese patients: the APOLLO study. Ophthalmologica 238:163–171. https://doi.org/10.1159/000477448
Funding
This study was funded by the JSPS KAKENHI Grant Number (Kiban C 17K11454 (to Y.O.)).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author T.I. has received research grants from Bayer Yakuhin, Ltd., and Santen Pharmaceutical Co., Ltd. Author K.S. has received research grants from Santen Pharmaceutical Co., Ltd.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all the participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wada, I., Oshima, Y., Shiose, S. et al. Five-year treatment outcomes following intravitreal ranibizumab injections for neovascular age-related macular degeneration in Japanese patients. Graefes Arch Clin Exp Ophthalmol 257, 1411–1418 (2019). https://doi.org/10.1007/s00417-019-04361-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-019-04361-8