Abstract
Purpose
To evaluate and compare the intrachoroidal structures of eyes with typical neovascular age-related macular degeneration (AMD) with those of eyes with polypoidal choroidal vasculopathy (PCV).
Study design
Retrospective and comparative case series.
Methods
Eighty-four treatment-naïve eyes of 84 patients (22 women and 62 men) with typical neovascular AMD or PCV located in the subfoveal region were studied. Cross-sectional images of the retina and choroid were obtained by swept-source optical coherence tomography (SS-OCT). The horizontal SS-OCT images were analyzed by a manual delineation technique and by a binarization method.
Results
Thirty-nine eyes with typical neovascular AMD and 45 eyes with PCV were studied. Although the subfoveal choroidal thickness (SCT) did not differ significantly between the 2 subtypes (255.1 ± 86.7 µm in typical neovascular AMD and 289.2 ± 116.5 µm in PCV, P = 0.29), the ratio of the large choroidal vessel layer (LCVL) thickness to the SCT was significantly larger in the eyes with PCV than in the eyes with typical neovascular AMD (0.863 ± 0.084 vs 0.803 ± 0.125, P = 0.023). The binarization method did not find significant differences in the choroidal structure between the 2 subtypes. Multivariate logistic regression analyses found the ratio of the LCVL thickness to the SCT to be the only significantly different factor between typical neovascular AMD and PCV (P = 0.035).
Conclusion
The intrachoroidal structures of typical neovascular AMD and PCV eyes differ significantly. In eyes with PCV, there seemed to be a greater dilation of the large choroidal vessels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Neovascular age-related macular degeneration (AMD) is one of the leading causes of visual reduction in older adults in developed countries [1]. The clinical characteristics of neovascular AMD vary among different ethnic groups. Polypoidal choroidal vasculopathy (PCV) may be a subtype of neovascular AMD and is quite common in the Asian population [2]. It is characterized by multiple recurrent serosanguineous detachments of the retina and retinal pigment epithelium (RPE) caused by leakage and bleeding from choroidal vascular lesions [3]. The precise pathophysiology of PCV has not been completely determined, and whether PCV represents a subtype of neovascular AMD has not been conclusively established [2].
Optical coherence tomography (OCT) has been used to noninvasively analyze the morphology of the retina and choroid in different types of macular disease. Recently, improvements in OCT devices such as enhanced depth image OCT (EDI-OCT) and swept-source OCT (SS-OCT) have allowed clinicians to obtain cross-sectional images of the retina and choroid [4, 5]. The results of several studies have shown that the subfoveal choroidal thickness (SCT) is significantly different among the different neovascular AMD subtypes. We and others have reported that the subfoveal choroid is thicker in eyes with PCV than in those with typical neovascular AMD [6,7,8]. However, differences in the intrachoroidal structures between the 2 subtypes have not been reported.
The purpose of this study was to analyze and compare the intrachoroidal structures in eyes with typical neovascular AMD with those in eyes with PCV. To accomplish this, we recorded cross-sectional images of the retina and choroid by SS-OCT and analyzed the structures of the choroid.
Materials and methods
This was a retrospective study of 84 treatment-naïve eyes of 84 consecutive patients (22 women and 62 men) with typical neovascular AMD or PCV who were examined at the Macula Service of Tokyo Women’s Medical University Hospital. Two of the 84 patients had bilateral PCV, and the right eye was selected for this study. All of the eyes had a neovascular membrane located at the subfoveal or juxtafoveal region. The study protocol conformed to the tenets of the Declaration of Helsinki, and the procedures were approved by the institutional review board of Tokyo Women’s Medical University.
Each patient underwent a comprehensive ophthalmic examination including measurements of the refractive errors and best-corrected visual acuity (BCVA) with a Landolt C chart. They also underwent slit-lamp biomicroscopy with contact or noncontact lenses, color fundus photography, fluorescein angiography (FA), indocyanine green angiography (ICGA) using confocal scanning laser ophthalmoscopy (HRA-2; Heidelberg Engineering) and SS-OCT (DRI-OCT; Topcon). ICGA was performed with an intravenous dose of 25 mg of indocyanine green in 1 mL of aqueous solvent. SS-OCT was performed with 12-mm horizontal and vertical scans centered on the fovea. Up to 96 B-scan images were averaged to reduce the speckle noise. Typical neovascular AMD was diagnosed on the basis of exudative changes corresponding to choroidal neovascularization (CNV) as detected by FA and ICGA. PCV was diagnosed on the basis of the presence of polypoidal structures at the border of a branching vascular network as seen on ICGA [9].
Several studies have reported that the choroidal vascular hyperpermeability (CVH) seen on ICGA was also present in eyes with typical neovascular AMD and PCV. Because CVH can affect choroidal thickness [8, 10,11,12,13,14], we also evaluated the relationship between the choroidal structures and the CVH, which was identified by the presence of multifocal hyperfluorescent regions in the middle and late phases of ICGA [12, 13].
We analyzed the horizontal SS-OCT images by use of the 2 methods described below.
Analyses of the choroidal layer in OCT images
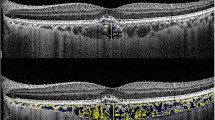
The analyses of the choroidal layer were performed manually according to the method of Branchini and associates [15]. Briefly, the SCT was measured from the hyperreflective line corresponding to the Bruch membrane beneath the RPE and the inner scleral border at the foveal center using the electronic caliper function of the SS-OCT device (Fig. 1). To measure the large choroidal vessel layer (LCVL) thickness, we selected 100 μm as the cutoff size for the large choroidal vessels. The large choroidal vessels horizontally measuring 100 μm or more located closest to the center of the fovea were selected. A horizontal line was then drawn from the innermost point of the large choroidal vessels that intersected the SCT measurement line perpendicularly. The LCVL thickness was measured from the inner scleral border to the intersection point on the SCT measurement line. The distance of the LCVL to the Bruch membrane, which corresponds to the choriocapillaris–medium choroidal vessel layer (CC + MCVL) thickness, was obtained by subtracting the thickness of the LCVL thickness from the SCT. The ratio of the LCVL thickness to the SCT was also calculated.
Analyses of the choroidal layer using swept-source optical coherence tomographic (SS-OCT) image in a healthy eye. The subfoveal choroidal thickness (SCT) was measured at the foveal center using the electronic caliper function of the SS-OCT device. The asterisk represents the large choroidal vessels horizontally measuring 100 µm or more located closest to the center of fovea. A horizontal line (dotted line) was drawn along the inner edge of the large choroidal vessel to intersect the line measuring the SCT. The large choroidal vessel layer (LCVL) thickness was measured from the inner border of the choroidal-scleral junction to the innermost point of the selected large choroidal vessel. The choriocapillaris–medium choroidal vessel layer (CC + MCVL) thickness, which was obtained by subtracting the measurements of the LCVL thickness from the SCT, was the distance from the outer edge of the hyperreflective RPE to the dotted line
Binarization of choroidal OCT images
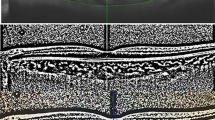
The subfoveal choroidal area of the horizontal OCT image was binarized using a modified version of the Niblack method [16, 17]. Briefly, the OCT image was analyzed using an open-access software, ImageJ (ImageJ V.1.47, National Institutes of Health; available at: http://imagej.nih.gov/ij/). The examined area was selected to be 3 mm wide with the margins 1.5 mm nasal and 1.5 mm temporal to the foveal center (Fig. 2). The region of interest (ROI) was selected and set by the ROI manager in the OCT image. Three choroidal vessels with lumina larger than 100 μm were then randomly selected with the Oval Selection Tool on the ImageJ tool bar, and the average reflective of these areas was determined using the software. The average brightness was set as the minimum value to minimize the noise in the OCT image. The image was then converted to 8 bits and adjusted by the Niblack Auto Local Threshold. The binarized image was converted to an RGB image again, and the luminal area was determined using the Threshold Tool. After adding the data of the distance between pixels, the choroidal, luminal, and stromal areas were automatically calculated. The dark pixels were defined as the luminal areas, and the light pixels, as the stromal areas. The choroidal areas were the sum of the luminal and stromal areas. The protocol details were described previously [16, 17].
Swept-source optical coherence tomographic (SS-OCT) image and converted binarized image in a healthy eye. a The examined area was selected to be 3 mm wide, with margins 1.5 mm nasal and 1.5 mm temporal to the fovea. Three choroidal vessels (asterisks, dark areas) with lumina larger than 100 μm were randomly selected by the Oval Selection Tool. b The OCT image was converted to a binarized image using ImageJ software. The luminal areas and stromal areas are shown. The rectangle surrounded by a white line was excised, and the dark areas were traced by use of the Niblack method. When the binarized image and the margin of the traced areas were merged, the traced areas were shown to correspond with the dark choroidal areas of the OCT image
Statistical analyses
The significance of the differences in the mean values was determined using the Mann–Whitney test. The decimal BCVAs were converted to logarithm of the minimum angle of resolution (logMAR) units for statistical analyses. To investigate the factors determining the difference between typical neovascular AMD and PCV, multivariate logistic regression analysis was performed. Forward and backward stepwise regression was performed using the likelihood-ratio method, in which change in the likelihood-ratio statistic, based on the maximum partial likelihood estimates for the covariate, was used for variable selection. The data were expressed as means ± standard deviations (SDs), and probability values less than .05 were considered significant. All tests were 2-sided, and all statistical analyses were performed using SPSS version 18.0 software (SPSS).
Results
The mean age of the 84 patients was 71.5 ± 9.7 years, with a range of 45–89 years. In 39 eyes (46.4%) of the 84 eyes studied, the diagnosis was typical neovascular AMD, and in the remaining 45 eyes (53.6%), it was PCV. The SCT and intrachoroidal structures could be evaluated in all 84 eyes. The patients’ demographics are shown in Table 1. The age, sex distribution, refractive error (spherical equivalent), and BCVA did not differ significantly between the eyes with typical neovascular AMD and those with PCV.
The results of the choroidal layer analyses and the binarization of the choroidal OCT images for the 2 types of patients are shown in Table 2. The mean SCT and the LCVL thickness were greater in eyes with PCV than in eyes with typical neovascular AMD, but the difference was not significant (P = 0.29 and P = 0.091, respectively). The mean ratio of the LCVL thickness to the SCT was significantly larger in eyes with PCV than in eyes with typical neovascular AMD (P = 0.023). The mean choroidal areas, the mean luminal areas, and the mean stromal areas did not differ significantly between the eyes with typical neovascular AMD and those with PCV (P = 0.47, P = 0.24, and P = 0.73, respectively). In addition, the mean ratio of the luminal areas to the choroidal areas did not differ significantly between the 2 subtypes (P = 0.11). The results of forward and backward stepwise logistic regression analyses showed that the ratio of the LCVL thickness to the SCT was the only factor significantly different between typical neovascular AMD and PCV, after controlling for SCT, LCVL thickness, CC + MCVL thickness, the choroidal areas, the luminal areas, the stromal areas, the ratio of the luminal to the choroidal areas, and CVH (P = 0.017). Representative cases of typical neovascular AMD and PCV are shown in Fig. 3.
Optical coherence tomography (OCT) images of the left eye of an 88-year-old man with typical age-related macular degeneration without choroidal vascular hyperpermeability (CVH). a The subfoveal choroidal thickness (SCT), the large choroidal vessel layer (LCVL) thickness, and the choriocapillaris–medium vessel layer (CC + MCVL) thickness were 337 µm, 267 µm, and 70 µm, respectively. The ratio of the LCVL thickness to the SCT was 0.792. b On the 3-mm-wide binarized OCT image, the choroidal, luminal, and stromal areas were calculated to be 0.876 mm2, 0.567 mm2, and 0.309 mm2, respectively. The ratio of the luminal area to the choroidal area was 0.614. c and d OCT images of the right eye of a 50-year-old man with polypoidal choroidal vasculopathy without CVH. c The SCT, LCVL thickness, and CC + MCVL thickness were 401 µm, 373 µm, and 28 µm, respectively. The ratio of the LCVL thickness to the SCT was 0.930. d On the 3-mm-wide binarized OCT image, the choroidal, luminal, and stromal areas were calculated to be 1.061 mm2, 0.738 mm2, 0.323 mm2, respectively. The ratio of the luminal area to the choroidal area was 0.696
Of the 39 eyes with typical neovascular AMD, 14 eyes (35.9%) had CVH, and 25 eyes (64.1%) had no CVH. The results of the choroidal layer analyses and the binarization method for the eyes with CVH and for those without CVH are shown in Table 3. The mean SCT and the LCVL thickness were significantly greater in eyes with CVH than in those without CVH (P = 0.008 and P = 0.014, respectively). The mean choroidal areas, the mean luminal areas, and the mean stromal areas differed significantly between the eyes with CVH and those without CVH (P = 0.042, P = 0.028, and P = 0.042, respectively). On the other hand, the mean ratio of the LCVL thickness to the SCT and the mean ratio of the luminal areas to the choroidal areas did not differ significantly between the eyes with CVH and those without CVH in typical neovascular AMD (P = 0.70 and P = 0.20, respectively).
Of the 45 eyes with PCV, 20 eyes (44.4%) had CVH and 25 eyes (55.6%) had no CVH. The frequency of CVH in PCV did not differ significantly from that in typical neovascular AMD (P = 0.43). The results of the choroidal layer analyses and the binarization method for the eyes with CVH and for those without CVH are shown in Table 4. No significant differences were found in the choroidal layer analyses and the binarization between the eyes with CVH and those without CVH.
The results of forward and backward stepwise logistic regression analyses showed that the ratio of the LCVL thickness to the SCT was the only factor significantly different between typical neovascular AMD and PCV, after controlling for age, gender, SCT, LCVL thickness, CC + MCVL thickness, the choroidal areas, the luminal areas, the stromal areas, the ratio of the luminal to the choroidal areas, and CVH (odds ratio = 1.71, 95% confidence interval of odds ratio, 1.40–2.03; P = 0.035). Representative cases of typical neovascular AMD and PCV are shown in Fig. 3.
Discussion
In this study, we used choroidal layer analyses and a binarization method to compare the intrachoroidal structures with the horizontal SS-OCT images in eyes with typical neovascular AMD and in eyes with PCV. The results showed that the mean ratio of the LCVL thickness to the SCT was significantly larger in eyes with PCV than in eyes with typical neovascular AMD. Multivariate logistic regression analyses showed that the ratio of the LCVL thickness to the SCT was the only factor that can differentiate typical neovascular AMD from PCV. This suggests that the large choroidal vessels were expanded in eyes with PCV, and this observation is consistent with previously reported histopathologic findings for PCV eyes [18, 19]. To the best of our knowledge, this is the first study to quantitatively demonstrate the similarities and differences in the intrachoroidal structures of eyes with typical neovascular AMD and those with PCV.
In the histopathologic specimens of PCV eyes, dilated choroidal vessels were seen beneath the Bruch membrane, and the vascular wall of the vessels seemed to be thin [19]. Yang and associates [20] showed that the SCT and the largest diameter of the hyporeflective lumen in EDI-OCT were greater in eyes with PCV than in healthy control eyes. The findings of those earlier studies support our findings that the ratio of LCVL thickness to the SCT was significantly larger in eyes with PCV. On the other hand, no difference was found in the intrachoroidal structures of PCV eyes with and without CVH. In eyes with PCV, it might be difficult to distinguish the intrachoroidal structures of eyes with CVH and of those without CVH only by OCT images, since eyes with PCV have variably dilated vessels in the choroid and large variations regardless of the presence or absence of CVH.
With the binarization method, the luminal areas, the choroidal areas, and the ratio of the luminal to the choroidal areas tended to be greater in eyes with PCV than in those with typical neovascular AMD, but the differences were not significant. The reason the binarization method did not find significant differences in the current study might be due to the differences in methodology between the choroidal layer analyses and the binarization method.
On the other hand, the current study revealed that the intrachoroidal structures in eyes with typical neovascular AMD with CVH were different from those without CVH. In typical neovascular AMD, the mean SCT, LCVL thickness, choroidal areas, luminal areas, and stromal areas in the eyes with CVH were significantly greater than in those without CVH. CVH was originally described as a characteristic finding in central serous chorioretinopathy (CSC) [21, 22]. In terms of the relationship between CVH and neovascular AMD, Sasahara and associates originally reported that the frequency with CVH in eyes with typical neovascular AMD was 1.9% [13]. Other recent studies reported higher frequencies of 12.4–37.5% [8, 10] in typical neovascular AMD and of 26.9–9.3% in PCV [8, 10, 12, 14]. These findings are comparable to this study’s findings. In previous studies, the eyes with CVH had greater SCT than did those without CVH both in eyes with typical neovascular AMD and in eyes with PCV [8, 12, 14]. Our results suggest that the difference in the choroidal layer compositions between the eyes with CVH and those without CVH was especially significant in typical neovascular AMD. In the eyes with typical neovascular AMD accompanied by CVH, not only the luminal areas but also the stromal areas were significantly expanded. These results suggest that CVH might cause leakage of fluid from the choroidal vascular plexus into the interstitial tissues. Recently, it was reported that pachychoroid neovasculopathy had more frequent CVH and greater SCT than did neovascular AMD [23]. Eyes with a pachychoroid are characterized by dilated outer choroidal vessels with type 1 CNV located under the RPE. Further studies will elucidate the differences in intrachoroidal structures in eyes with and without a pachychoroid.
The current study had several limitations. First, the number of eyes studied was small. The SCT in eyes with PCV did not differ significantly from that in eyes with typical neovascular AMD. The results of earlier studies showed that the mean SCT was greater in eyes with PCV than in eyes with typical neovascular AMD [6,7,8]. The reason for this discrepancy between the current study and those earlier studies might be due to the relatively small sample size or to the large variations in the SCT. Second, the choroidal structural analyses were performed only at the foveal area. Third, in the Niblack method, the black and white pixels may not always correspond with the luminal and stromal areas of the choroid. Fourth, although the choroidal thickness and intrachoroidal structure may be affected by the axial length [24, 25], this fact was not considered in this study. Finally, this was a retrospective study, and especially, the choroidal layer analyses were manually performed. More objective methods using automated software are needed.
In conclusion, we demonstrated that the choroidal layer composition differed significantly between typical neovascular AMD and PCV, but the difference in the intrachoroidal structure was not apparent from the results of the binarization method. The differences in intrachoroidal structure between eyes with CVH and eyes without CVH were more evident in typical neovascular AMD than in PCV. The clinical significance needs to be confirmed in future prospective studies.
References
Wong TY, Chakravarthy U, Klein R, Mitchell P, Zlateva G, Buggage R, et al. The natural history and prognosis of neovascular age-related macular degeneration: a systematic review of the literature and meta-analysis. Ophthalmology. 2008;115:116–26.
Laude A, Cackett PD, Vithana EN, Yeo IY, Wong D, Koh AH, et al. Polypoidal choroidal vasculopathy and neovascular age-related macular degeneration: same or different disease? Prog Retin Eye Res. 2010;29:19–29.
Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina. 1990;10:1–8.
Spaide RF, Koizumi H, Pozonni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146:496–500.
Yun S, Tearney G, de Boer J, Ifttimia N, Bouma B. High-speed optical frequency-domain imaging. Opt Express. 2003;11:2953–63.
Koizumi H, Yamagishi T, Yamazaki T, Kawasaki R, Kinoshita S. Subfoveal choroidal thickness in typical age-related macular degeneration and polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. 2011;249:1123–8.
Chung SE, Kang SW, Lee JH, Kim YT. Choroidal thickness in polypoidal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology. 2011;118:840–5.
Jirarattanasopa P, Ooto S, Nakata I, Tsujikawa A, Yamashiro K, Oishi A, et al. Choroidal thickness, vascular hyperpermeability, and complement factor H in age-related macular degeneration and polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2012;53:3663–72.
Spaide RF, Yannuzzi LA, Slakter JS, Sorenson J, Orlach DA. Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina. 1995;15:100–10.
Kim JH, Chang YS, Lee TG, Kim CG. Choroidal vascular hyperpermeability and punctate hyperfluorescent spot in choroidal neovascularization. Invest Ophthalmol Vis Sci. 2014;56:1909–15.
Miyake M, Tsujikawa A, Yamashiro K, Ooto S, Oishi A, Tamura H, et al. Choroidal neovascularization in eyes with choroidal vascular hyperpermeability. Invest Ophthalmol Vis Sci. 2014;55:3223–30.
Koizumi H, Yamagishi T, Yamazaki T, Kinoshita S. Relationship between clinical characteristics of polypoidal choroidal vasculopathy and choroidal vascular hyperpermeability. Am J Ophthalmol. 2013;155:305–13.
Sasahara M, Tsujikawa A, Musashi K, Gotoh N, Otani A, Mandai M, et al. Polypoidal choroidal vasculopathy with choroidal vascular hyperpermeability. Am J Ophthalmol. 2006;142:601–7.
Maruko I, Iida T, Sugano Y, Saito M, Sekiryu T. Subfoveal retinal and choroidal thickness after verteporfin photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2011;151:594–603.
Branchini LA, Adhi M, Regatieri CV, Nandakumar N, Liu JJ, Laver N, et al. Analysis of choroidal morphologic features and vasculature in healthy eyes using spectral-domain optical coherence tomography. Ophthalmology. 2013;120:1901–8.
Sonoda S, Sakamoto T, Yamashita T, Shirasawa M, Uchino E, Terasaki H, et al. Choroidal structure in normal eyes and after photodynamic therapy determined by binarization of optical coherence tomographic images. Invest Ophthalmol Vis Sci. 2014;55:3893–9.
Izumi T, Koizumi H, Maruko I, Takahashi Y, Sonoda S, Sakamoto T, et al. Structural analyses of choroid after half-dose verteporfin photodynamic therapy for central serous chorioretinopathy. Br J Ophthalmol. 2017;101:433–7.
Rosa RH Jr, Davis JL, Eifrig CW. Clinicopathologic reports, case reports, and small case series: clinicopathologic correlation of idiopathic polypoidal choroidal vasculopathy. Arch Ophthalmol. 2002;120:502–8.
Nakajima M, Yuzawa M, Shimada H, Mori R. Correlation between indocyanine green angiographic findings and histopathology of polypoidal choroidal vasculopathy. Jpn J Ophthalmol. 2004;48:249–55.
Yang LH, Jonas JB, Wei WB. Optical coherence tomographic enhanced depth imaging of polypoidal choroidal vasculopathy. Retina. 2013;33:1584–9.
Hayashi K, Hasegawa Y, Tokoro T. Indocyanine green angiography of central serous chorioretinopathy. Int Ophthalmol. 1986;9:37–41.
Guyer DR, Yannuzzui LA, Slakter JS, Sorenson JA, Ho A, Orlock D. Digital indocyanine green videoangiography of central serous chorioretinopathy. Arch Ophthalmol. 1994;112:1057–62.
Miyake M, Ooto S, Yamashiro K, Takahashi A, Yoshikawa M, Akagi-Kurashige Y, et al. Pachychoroid neovasculopathy and age-related macular degeneration. Sci Rep. 2015;5:16204.
Wei WB, Xu L, Jonas JB, Shao L, Du KF, Wang S, et al. Subfoveal choroidal thickness: the Beijing Eye Study. Ophthalmology. 2013;120:175–80.
Sonoda S, Sakamoto T, Yamashita T, Uchino E, Kawano H, Yoshihara N, et al. Luminal and stromal areas of choroid determined by binarization method of optical coherence tomographic images. Am J Ophthalmol. 2015;159:1123–31.
Acknowledgement
The authors thank Yuji Yamamoto, Department of Ophthalmology, Kyoto Prefectural University of Medicine, for statistical expertise. This study was supported in part by grant no. 25670739 from the Ministry of Education, Culture, Sports, Science and Technology-Japan (Dr. Koizumi).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Y. Takahashi, None; H. Koizumi, Grant (Novartis), Moderator fees (Alcon, Bausch + Lomb, Bayer, Canon, HOYA, Kowa, NIDEK, Novartis, Santen, Senju, Topcon, Wakamoto); T. Hasegawa, Moderator fees (Alcon, Bayer, Kowa, Novartis, Santen, Senju); T. Izumi, None; I. Maruko, Moderator fees (Alcon, Bayer, NIDEK, Novartis, Santen, Senju, Topcon); S. Sonoda, None; T. Sakamoto, Grant (HOYA, Kowa, Novartis, Senju, Wakamoto), Consultant fees (Bausch + Lomb, Bayer, Novartis, Wakamoto), Moderator fees (Alcon, Kowa, Novartis, Pfizer, Santen, Senju); T. Iida, Grant (NIDEK, Novartis, Santen), Moderator fees (Bayer, Novartis, Santen).
Additional information
Corresponding author: Hideki Koizumi
About this article
Cite this article
Takahashi, Y., Koizumi, H., Hasegawa, T. et al. Comparison of subfoveal choroidal structures in typical neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Jpn J Ophthalmol 62, 576–583 (2018). https://doi.org/10.1007/s10384-018-0615-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-018-0615-4