Abstract
Purpose
To evaluate the results of a 3-year follow-up of intravitreal pegaptanib sodium injection as maintenance therapy for the treatment of neovascular age-related macular degeneration (AMD) in Japanese patients.
Methods
In this prospective, uncontrolled interventional study, 20 eyes of 19 patients with treatment-naïve AMD who had received 3 consecutive monthly injections of 0.5 mg/0.05 mL ranibizumab as the induction treatment and had shown clinical/anatomical improvement were enrolled. An intravitreal injection of 0.3 mg/0.09 mL pegaptanib sodium was administered as the maintenance therapy every 6 weeks. Booster treatments using ranibizumab were allowed if clinical deterioration was judged to be present. The primary outcome measures were the best-corrected visual acuity (BCVA) and the central foveal thickness (CFT) as evaluated using spectral-domain optical coherence tomography.
Results
Sixteen of the 20 eyes (80 %) were assessed at the 3-year follow-up. The mean logMAR BCVA improved significantly from 0.56 ± 0.31 before the induction treatment to 0.24 ± 0.25 at baseline (P < 0.001) and was well maintained at 156 weeks (0.25 ± 0.28, P = 0.938). Moreover, the mean CFT also decreased significantly from 346 ± 111 μm before the induction treatment to 232 ± 54 μm at baseline (P < 0.001) and was well preserved at 156 weeks (210 ± 59 μm, P = 0.278). Thirteen eyes (81.3 %) received an unscheduled booster treatment, and no severe systemic or ocular side effects occurred during follow-up.
Conclusion
Intravitreal pegaptanib sodium injection as the maintenance therapy was effective in stabilizing the vision of patients with AMD in whom induction treatment led to improved BCVA, as evaluated at the 3-year follow-up.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Age-related macular degeneration (AMD) is the leading cause of severe, irreversible vision loss in older adults [1, 2]. Because of a rapidly aging population, the incidence of AMD is expected to continue to increase in the future [3]. Severe vision loss in patients with AMD is caused by the development of choroidal neovascularization (CNV) [4].
Several agents that inhibit the action of vascular endothelial growth factor (VEGF) have recently been used for the management of CNV in patients with AMD. Ranibizumab (Lucentis; Novartis Pharma, Basel, Switzerland and Genentech, South San Francisco, CA, USA) is a recombinant humanized anti-VEGF antibody fragment targeting all isoforms of VEGF-A [5]. Two years of results obtained in both the MARINA and ANCHOR studies have shown that patients with all types of CNV who were treated with monthly intravitreal injections of ranibizumab (IVR) exhibited an improvement in their final mean best-corrected visual acuity (BCVA) [6, 7]. However, patients who received IVR treatments on an as-needed basis tended to exhibit visual decline after they had received the monthly ranibizumab treatments for 2 years [8]. Therefore, attempts to maintain the efficacy results obtained during the induction phase of IVR with less frequent dosing were expected.
In contrast, pegaptanib sodium (Macugen; Valeant Pharmaceuticals/Pfizer, New York, NY, USA) has also been reported to be effective against CNV related to AMD [9, 10]. Recently, an initial inductive dose of a nonspecific VEGF inhibitor, followed by maintenance therapy with pegaptanib sodium and as-required booster doses of ranibizumab, was prospectively studied in a LEVEL study [11]. Similarly, encouraging results were reported in Japan in a study named the LEVEL-J study [12]. However, the long-term outcomes of intravitreal pegaptanib sodium (IVP) as the maintenance therapy have not been reported.
The purpose of this study was to evaluate the results of a 3-year follow-up after IVP as the maintenance therapy for the treatment of neovascular AMD in Japanese patients.
Patients and methods
We performed a prospective, uncontrolled interventional study examining 20 eyes in 19 patients aged 50 years or older who had been diagnosed with subfoveal neovascular AMD. These 20 eyes were enrolled from the 77 eyes enrolled in the LEVEL-J study [12]. After they had completed the 54-week study period, the patients were followed for at least 156 weeks. The first IVP administration was defined as study entry or baseline. All patients had BCVA that was better than 20/400, underwent 3 consecutive induction treatments with ranibizumab before study entry, and showed clinical/anatomical improvement, as determined by the investigator. Clinical improvement was defined as an improvement of at least 0.2 logMAR at the time of study entry. All patients were treated at the Yokohama City University Medical Center between November 2009 and February 2011. The study was approved by our institute’s ethics committee. Informed consent was obtained from all eligible patients before the procedures.
Patients with other causes of CNV secondary to high myopia, angioid streaks, hereditary disorders, uveitis, or any other secondary diseases were not enrolled in the study. In addition, patients who had previously received treatment for AMD, such as laser photocoagulation, submacular surgery, photodynamic therapy, or intravitreal injection of any other anti-VEGF agent, were also excluded. Furthermore, patients with eye disease such as glaucoma, macular hole, diabetic retinopathy, or rhegmatogenous retinal detachment that could potentially influence the visual acuity of the studied eye were also excluded.
At baseline, all patients received 0.3 mg/0.09 mL pegaptanib sodium via an intravitreal injection through the pars plana by use of a 30-gauge needle. The injection was administered every 6 weeks as the maintenance therapy. Because this study was performed during routine clinical practice, the dosing interval for pegaptanib sodium could be changed up to a maximum of 12 weeks. Unscheduled IVR treatments for AMD (booster therapy) were allowed if clinical deterioration was judged to be present by the evaluating physician. Booster injections were performed if any of the following changes were observed and judged to have worsened: (1) visual acuity loss of ≥0.2 logMAR due to exudative changes; (2) new macular hemorrhage; or (3) evidence of persistent or recurrent subretinal fluid accumulation, intraretinal edema, or enlargement of a pigment epithelial detachment (PED) as diagnosed using spectral-domain optical coherence tomography (SD-OCT; Cirrus high-definition OCT; Carl Zeiss, Dublin, California, USA) during the study period.
The main outcome measures were BCVA and central foveal thickness (CFT) as determined using SD-OCT. BCVA was converted to logMAR equivalents for the statistical analysis. The Wilcoxon signed-rank test was used to compare the data. All analyses were performed using SPSS version 17 software (IBM, Armonk, NY, USA). Probability values less than 0.05 were considered significant.
Results
Of the 19 patients enrolled in this study, two (2 eyes) withdrew because they stopped visiting the hospital at weeks 105 and 122, respectively. One patient (2 eyes) was switched to monthly ranibizumab treatment during the maintenance phase because of frequent recurrences. Finally, a total of 16 eyes in 16 patients (80 %) were assessed at the 3-year follow-up. The baseline characteristics and clinical data before the induction treatment, at baseline, and after the maintenance therapy are shown in Table 1. Of the 16 patients included in the series, eight were men. The patients’ age ranged from 65 to 85 years (mean age 74.8 ± 6.1 years).
Among the 16 eyes, five (31.3 %) were diagnosed as having classic CNV, eight (50.0 %) as having occult CNV, one (6.3 %) as having polypoidal choroidal vasculopathy (PCV), and two (12.5 %) as having retinal angiomatous proliferation (RAP).
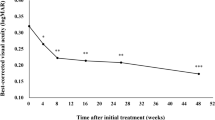
The mean logMAR BCVA improved significantly from 0.56 ± 0.31 before the induction treatment to 0.24 ± 0.25 at baseline (P < 0.001) and was well maintained at 156 weeks (0.25 ± 0.28, P = 0.938; Fig. 1).
Changes in the mean logMAR BCVA between the initial visit and the 3-year follow-up after the maintenance therapy. The mean logMAR BCVA improved significantly from 0.56 ± 0.31 before the induction treatment to 0.24 ± 0.25 at baseline (P < 0.001) and was well maintained at 156 weeks (0.25 ± 0.28, P = 0.938)
In contrast, the mean CFT also decreased significantly from 346 ± 111 μm before the induction treatment to 232 ± 54 μm at baseline (P < 0.001) and was well preserved at 156 weeks (210 ± 59 μm, P = 0.278; Fig. 2).
The mean number of injections of both pegaptanib sodium and booster therapy administered during the 156 weeks was 24.4 ± 4.6. The number of pegaptanib injections was 17.1 ± 3.5, while the mean number of booster injections of ranibizumab was 7.3 ± 5.3. Thirteen eyes (81.3 %) received unscheduled booster treatments. Among the patients who received booster treatments, the mean time between the onset of the maintenance therapy and the first booster was 23.8 ± 28.6 weeks. No systemic side effects, such as severe cardiac disease or cerebrovascular events, occurred during the follow-up period. One patient (6.3 %) experienced ocular side effects, i.e., elevated intraocular pressure (>21 mmHg), but the pressure was successfully reduced using medication.
Figure 3 shows a case report.
Patient 12 in Table 1, a 67-year-old woman, presented with diminished visual acuity in her right eye. a Color fundus photograph of the right eye shows a serous retinal detachment (SRD) with a hard exudate. SD-OCT before the induction treatment revealed serous retinal detachment with occult CNV showing a double-layer sign. Her visual acuity was 0.5 in the right eye. b OCT after 3 consecutive monthly ranibizumab injections showed a reduction in SRD. Her visual acuity had improved to 0.8. She started receiving maintenance therapy using pegaptanib sodium. c However, at 29 weeks after the initiation of the maintenance therapy, SRD was observed, although her visual acuity was maintained at 0.9. A booster treatment of ranibizumab was administered. d 4 weeks after the booster treatment, the SRD had disappeared, and the maintenance therapy with pegaptanib sodium was reinitiated. e At the 3-year follow-up, the patient’s visual acuity had stabilized at 0.8 without exudative findings
Discussion
Our results demonstrated that IVP as the maintenance therapy was effective in stabilizing vision in Japanese patients with AMD in whom the induction treatment led to improved BCVA, as evaluated at the 3-year follow-up. Although a recent report showed that switching therapy to pegaptanib was effective [13], to our knowledge, no other published reports have shown the safety and efficacy outcomes of IVP administration as the maintenance therapy for patients with AMD during a long-term follow-up.
According to studies examining the effects of anti-VEGF agents on CNV, patients often receive multiple injections on a monthly basis for a long period of time to maintain the improved BCVA [6, 7]. Although the visual acuity significantly improved in those previous studies, whether monthly dosing was the optimal dosing interval remained unclear. The CATT study showed that patients who were treated as needed received an average of 12.6 injections over 2 years. However, the as-needed treatment resulted in a smaller gain in visual acuity than that achieved with monthly injections [14]. Recently, administration every 2 months of aflibercept, a fusion protein that binds to the members of the VEGF family, produced improvements similar to those achieved using monthly ranibizumab [15]. However, these 2 agents are associated with some systemic safety-related complications, such as cerebrovascular risk [16, 17]. Therefore, it is important to determine an appropriate protocol for improving and maintaining visual acuity in patients with AMD while minimizing exposure to nonselective inhibition. To resolve these problems, in the present study, maintenance therapy using pegaptanib sodium, which is a specific VEGF165 inhibitor, was investigated.
Regarding the postinjection BCVA, the mean logMAR BCVA was well maintained when compared with that at baseline, throughout the 3-year observation period. Although 13 of the 16 patients (83.1 %) required unscheduled booster treatments, the mean number of ranibizumab injections was only 7.3 at 156 weeks. This result suggests that ongoing injections of pegaptanib sodium may suppress recurrence and may be effective in minimizing the need for booster injections, thereby enabling an improved visual acuity to be maintained for at least 3 years. Furthermore, a previous report indicated that vision loss after 2 years of monthly ranibizumab injections was related to retinal pigment epithelium (RPE) abnormalities, suggesting that treatments targeting photoreceptor and RPE survival, such as those with neuroprotective and antiinflammatory effects, may be necessary for maintaining visual gain [18]. Nishijima et al. [19] reported that VEGF120 (equivalent to human VEGF121) may be the most important factor for neuroprotection in rats and that no decrease in retinal ganglion cell viability was observed when VEGF-A was blocked with pegaptanib. Therefore, maintenance therapy with pegaptanib sodium, which does not bind to VEGF121, may minimize photoreceptor and RPE damage and enable the maintenance of the improved visual acuity for as long as 3 years. However, among the 20 eyes enrolled in this study, 2 eyes of 1 patient were switched to monthly ranibizumab injections because the pegaptanib sodium injections were ineffective. Therefore, we have to bear in mind that it is still difficult to maintain the BCVA of some patients with AMD.
The main limitation of the present study was the relatively small sample size. A larger sample size is required to clarify the results more precisely in the near future. This study also examined patients who had experienced a gain in visual acuity after the induction therapy. Maintenance therapy with pegaptanib should also be studied in patients with visual acuity loss or no change in visual acuity during the induction phase. Furthermore, there may be important underlying differences among exudative AMD, PCV, and RAP patients. However, similar results were archived for any type of lesion subtypes in this study.
In conclusion, IVP as the maintenance therapy was effective in stabilizing vision in patients with AMD in whom induction treatment led to improved BCVA, as evaluated at the 3-year follow-up.
References
Bressler NM, Bressler SB, Fine SL. Age-related macular degeneration. Surv Ophthalmol. 1988;32:375–413.
Bressler NM, Bressler SB, Congdon NG, Ferris FL 3rd, Friedman DS, Klein R, et al. Potential public health impact of age-related eye disease study results: Areds report No. 11. Arch Ophthalmol. 2003;121:1621–4.
Bressler NM. Age-related macular degeneration is the leading cause of blindness. JAMA Ophthalmol. 2004;291:1900–1.
Ferris FL 3rd, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984;102:1640–2.
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31.
Kaiser PK, Blodi BA, Shapiro H, Acharya NR. Angiographic and optical coherence tomographic results of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2007;114:1868–75.
Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology. 2009;116(57–65):e55.
Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K, Group S-US. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology. 2013;120:2292–9.
Gragoudas ES, Adamis AP, Cunningham ET Jr, Feinsod M, Guyer DR, Group VISiONCT. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351:2805–16.
Gonzales CR, Group VISiONCT. Enhanced efficacy associated with early treatment of neovascular age-related macular degeneration with pegaptanib sodium: an exploratory analysis. Retina. 2005;25:815–27.
Friberg TR, Tolentino M. Pegaptanib sodium as maintenance therapy in neovascular age-related macular degeneration: the LEVEL study. Br J Ophthalmol. 2010;94:1611–7.
Ishibashi T, Group L-JS. Maintenance therapy with pegaptanib sodium for neovascular age-related macular degeneration: an exploratory study in Japanese patients (LEVEL-J study). Jpn J Ophthalmol. 2013;57:417–23.
Shiragami C, Ono A, Kobayashi M, Manabe S, Yamashita A, Shiraga F. Effect of switching therapy to pegaptanib in eyes with the persistent cases of exudative age-related macular degeneration. Medicine. 2014;93:e116.
Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119:1388–98.
Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–48.
Ueta T, Yanagi Y, Tamaki Y, Yamaguchi T. Cerebrovascular accidents in ranibizumab. Ophthalmology. 2009;116:362.
Ueta T. Safety in aflibercept versus ranibizumab. Ophthalmology. 2014;121:e5.
Rosenfeld PJ, Shapiro H, Tuomi L, Webster M, Elledge J, Blodi B, et al. Characteristics of patients losing vision after 2 years of monthly dosing in the phase III ranibizumab clinical trials. Ophthalmology. 2011;118:523–30.
Nishijima K, Ng YS, Zhong L, Bradley J, Schubert W, Jo N, et al. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Pathol. 2007;171:53–67.
Conflicts of interest
M. Inoue, Grant (Pfizer); K. Kadonosono, Grant (Pfizer); A. Arakawa, None; S. Yamane, None; T. Ishibashi, Grant (Pfizer).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Inoue, M., Kadonosono, K., Arakawa, A. et al. Long-term outcome of intravitreal pegaptanib sodium as maintenance therapy in Japanese patients with neovascular age-related macular degeneration. Jpn J Ophthalmol 59, 173–178 (2015). https://doi.org/10.1007/s10384-015-0374-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-015-0374-4