Abstract
Introduction
To determine whether trabeculectomy affects postural-induced changes in intraocular pressure (IOP), and whether it is maintained.
Methods
Thirty-six eyes of 36 patients with open-angle glaucoma who were scheduled for their initial trabeculectomy with adjunctive mitomycin C were prospectively examined. The IOP was measured in the sitting and the lateral decubitus position with an ICare rebound tonometer before, and 1, 3, and 12 months after trabeculectomy.
Results
Twenty-nine eyes of 29 patients completed this study. The mean baseline IOP measured with the ICare tonometer was 17.4 ± 4.9 mmHg in the sitting position and 21.3 ± 5.6 mmHg in the lateral decubitus position (p < 0.001). This postural IOP difference, +3.9 mmHg, was reduced to +1.3 ± 1.7 mmHg at 1 month and to +0.8 ± 1.5 mmHg at 3 months after the trabeculectomy (p < 0.001 and p = 0.004 respectively). This decrease in the degree of posture-dependent IOP change was maintained at +1.7 ± 2.2 mmHg at 1 year postoperatively (p < 0.001). In three cases, the postural IOP changes returned to the baseline level, and all three had a failed bleb.
Conclusions
Our results indicate that trabeculectomy not only reduces the IOP but also reduces the degree of posture-induced changes in the IOP. Our findings also speculate that measuring the postural IOP changes after trabeculectomy might provide a clue on the functioning of a filtering bleb.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Glaucoma is a potentially blinding disease and is the second leading cause of visual impairment, as shown in the Tajimi Study conducted in Japan [1]. Although glaucoma is believed to be a multifactorial disease, there is little doubt that intraocular pressure (IOP) plays an important role in the development and progression of glaucomatous optic neuropathy. Short- and long-term variations in the IOP have been shown to occur because of altering physiological conditions, including age, diurnal and seasonal cycles, and postural alterations [2]. Thus, it is difficult to control all of these variables to obtain an exact value of the IOP. In addition, all of these factors make it difficult to determine the effect of the IOP on the progression of glaucomatous optic neuropathy. This is important, because it was recently shown that not only absolute IOP but also its fluctuations might be associated with the progression of glaucomatous optic neuropathy [3, 4]. Of these factors, it was recently shown that postural changes have a close association with functional and morphological impairments in glaucoma [5, 6] and also with the progression of glaucomatous visual field defects [7].
At present, trabeculectomy is recommended to be the first-line surgical therapy for eyes with glaucoma, even if the IOP is within the normal range [8, 9]. In addition to a greater IOP-lowering effect, trabeculectomy has been reported to reduce the degree of IOP elevation after the water drinking test [10]. Trabeculectomy has also been shown to reduce the development of optic disc hemorrhages [11].
There have been studies that investigated the posture-induced changes in IOP after filtering surgery [12–15]. However, the conclusions of these studies are inconsistent, and controversy still exists. Furthermore, there are no data available on the effects of trabeculectomy over time on posture-induced IOP changes. Thus, the purpose of this study was to compare postural IOP changes between before and after trabeculectomy, and to investigate whether the alterations were maintained for at least 1 year.
Materials and methods
Thirty-six eyes of 36 consecutive patients with open-angle glaucoma (OAG), who received their first trabeculectomy with adjunctive mitomycin C (MMC) at the Gifu University Hospital between June 2008 and December 2009, were studied. Preoperatively, the patients were using several ocular hypotensive drugs. The procedures were approved by the Institutional Review Board of Gifu University Graduate School of Medicine. All patients were fully informed on the procedures and gave their written consent before participation.
The preoperative diagnosis of OAG was made based on the following criteria: (1) both eyes had a gonioscopically normal open angle, (2) at least one eye had characteristic visual field defects that corresponded to the location of the glaucomatous disc excavation, and (3) neuroradiologic, rhinologic, and general medical examinations did not disclose any pathological conditions responsible for optic nerve damage. A glaucomatous visual field loss was defined as abnormal when the pattern deviation probability plot showed a cluster of three or more non-edge contiguous points having sensitivity with a probability of less than 5% in the upper or lower hemi-field, and in one of these with a probability of less than 1% on static automated perimetry (Humphrey 30–2, Humphrey Field Analyzer, Humphrey Instruments, San Leandro, CA, USA). A glaucomatous optic nerve appearance was defined as the presence of a focal or a diffuse defect of the optic disc rim to less than 10% of the disc diameter. Patients with any conditions that might induce glaucoma even in one eye were excluded. Also, patients were excluded if they had a history of any intraocular surgery including laser therapy, or had any corneal conditions that prevented reliable IOP measurements. Patients were classified as having normal-tension glaucoma if none of the recorded preoperative IOPs exceeded 21 mmHg in both eyes.
At the baseline, all patients underwent ocular examinations including refraction with an autorefractometer (Topcon KP-8100PA, Tokyo, Japan), best-corrected visual acuity, slit-lamp biomicroscopy, central corneal thickness (CCT) measurement by ultrasonic pachymetry (SP-100 Handy Pachymeter, Tomey, Japan), gonioscopy, direct ophthalmoscopy, and perimetry by Humphrey Field Analyzer with the central 30–2 program.
The IOP measurements were made with a Goldmann applanation tonometer (GAT; Haag–Streit AG, Köniz, Switzerland) at every visit. The IOP measurements with an ICare rebound tonometer (ICare; Tiolat Oy, Helsinki, Finland) were made before and at 1, 3, and 12 monthS after trabeculectomy, in the afternoon by a single examiner throughout the experimental period. To determine posture-induced IOP change, the IOP was first measured in a sitting position with the ICare rebound tonometer. Then the patient was instructed to lie on the bed, and to turn to the lateral decubitus position and the head was placed on a soft pillow. The body was positioned so that the eye scheduled for the surgery was located directly above the fellow eye. The body position was maintained for 5 minutes, and the IOP was measured in this position with the ICare tonometer. The examiner asked the patients to gaze straight ahead to a fixation point, and the IOP measurement was made by touching the transducer to the center of the patients’ cornea. Three consecutive sets of measurements, with six measurements for each set, were made. The average of each set was made automatically, and the averaged values were used for the statistical analyses.
A filtering surgery with antimetabolites was performed when an eye was found to have a progression of the visual field defects and was being treated with the maximum tolerable medications. A modification of Cairns’ technique was used for the trabeculectomy, as described elsewhere [16].
All of the patients were examined approximately 3 times/year, with more frequent visits during the early postoperative period. At each visit, the following ocular examinations were performed: slit-lamp biomicroscopy, funduscopy, and IOP measurements with the GAT. To maintain good control of the IOP, the administration of topical ocular hypotensive drugs and/or laser suture lysis (performed within 1 month after trabeculectomy) were used when necessary. Cases of serious intra- or postoperative complications were removed from the study.
For further analyses, one eye was randomly chosen if both eyes underwent filtering surgery. Paired or unpaired t-tests or repeated measures analysis of variance (ANOVA), followed by multiple comparison Bonferroni tests, were used to evaluate the significance of the IOP changes. The level of significance for each contrast was set at p < 0.05. All statistical analyses were performed using SPSS software version 16.0 (SPSS Japan, Tokyo, Japan.).
Results
Patients
Twenty-nine eyes of 29 patients completed and five patients did not complete our protocol. Of these five, three patients were removed from the study because of severe postoperative complications; one developed a bleb-related endophthalmitis within 3 months after the filtering surgery, and two developed a flat anterior chamber at an early postoperative time. The other two participants had to be referred to other hospitals because their families moved to another prefecture.
The preoperative demographic data of our final 29 participants are shown in Table 1. All of the participants were Japanese. The mean age was 56.8 ± 8.7 years (± SD), and 18 were men. Fourteen had normal-tension glaucoma. The mean baseline IOP with GAT was 16.4 ± 3.7 mmHg. The preoperative mean deviation was −17.04 ± 7.04 decibels, and pattern standard deviation was 13.00 ± 2.42 decibels.
Postoperative intraocular pressures measured with the Goldmann applanation tonometer (GAT)
The time course of the changes in the IOPs measured with GAT are shown in Fig. 1 at the different postoperative times. At one month after the filtering surgery, the average IOP was reduced to 8.4 ± 3.4 mmHg (p < 0.001 versus baseline; Bonferroni test). Thereafter, the average IOP was maintained at 8 to 9 mmHg, although the standard deviation of the IOP was slightly larger (p < 0.001 at 3 and 12 months after trabeculectomy versus the baseline; Bonferroni test). In most cases (26 cases) a filtering bleb appeared to be thin-walled, moist, and avascular or hypovascular at 12 months. However, two patients had thick-walled, hypovascular filtering blebs with relatively low height, and another had a flattened mildly vascularized bleb. These three patients were considered to be failed bleb, and required additional topical ocular hypotensive therapy to maintain the IOP at the 12-month postoperative period.
Time course of intraocular pressures (IOP) changes measured with Goldmann applanation tonometer (GAT) following trabeculectomy. At 1 month after the filtering surgery, the mean IOP was reduced to approximately 8.0 mmHg, and this pressure was maintained up to 3 months. At 12 months after the surgery, the mean IOP was maintained at a level of 9 to 10 mmHg (p < 0.001 at all points versus baseline IOP; Bonferroni test), even though some cases had failed trabeculectomy. Error bars are the standard deviations
Posture-induced changes in intraocular pressures measured with ICare rebound tonometer
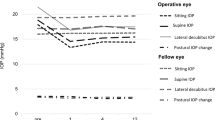
The IOPs measured in the sitting and lying postures with the ICare rebound tonometer are plotted in Fig. 2. The mean baseline IOP with the ICare rebound tonometer was 17.2 ± 4.8 mmHg in the sitting position, and the IOP increased significantly to 21.0 ± 5.5 mmHg in the lateral decubitus position (p < 0.001; paired t-test). One month after trabeculectomy, the IOP in the sitting position was reduced significantly to 9.6 ± 4.0 mmHg, and that in the lateral decubitus position to 10.9 ± 4.5 mmHg. The difference in the IOP in the sitting and lateral decubitus position was still significant (p < 0.001; paired t-test). At 3 months postoperatively, the IOP in the sitting position was 9.1 ± 3.7 mmHg and that in the lateral decubitus position was 10.0 ± 4.0 mmHg (p = 0.004; paired t-test), and at 12 months postoperatively, the IOP in the sitting and the lateral decubitus position was 10.5 ± 4.9 mmHg and 12.2 ± 6.7 mmHg, respectively (p < 0.001; paired t-test). The degree of reduction of the posture-induced IOPs relative to the baseline difference was significant at 1 month, 3 months, and 12 months postoperatively (p < 0.001, p < 0.001, and p = 0.001 respectively; Bonferroni test; Fig. 3). In the three cases that required topical ocular hypotensive agents, the postural IOP changes were larger, at 5 to 9 mmHg, than those in the other cases with no medications at 12 months.
Postural intraocular pressure (IOP) changes measured with an ICare rebound tonometer before and after trabeculectomy over a 12-month period. At the preoperative baseline (n = 29), the mean baseline IOP was 17.2 ± 4.8 mmHg in the sitting position (SP), and increased to 21.0 mmHg by 3.8 ± 2.3 mmHg in the lateral decubitus position (LDP, p < 0.001; paired t-test). One month after the filtering surgery (n = 29), the IOP in the sitting position was 9.6 ± 4.0 mmHg, and was 10.9 ± 4.5 mmHg in the lateral decubitus position (p < 0.001; paired t-test). At 3 months (n = 29), the IOP in the sitting position was 9.1 ± 3.7 mmHg and 10.0 ± 4.0 mmHg in the lateral decubitus position (p = 0.004; paired t-test). At 12 months postoperatively (n = 29), the IOP in the sitting position was 10.5 ± 4.9 mmHg and 12.2 ± 6.7 mmHg in the lateral decubitus position (p < 0.001; paired t-test). The open squares, the upper and lower bars indicate 25–75%, 95%, and 5% percentiles respectively
The postural intraocular pressure (IOP) differences between the sitting position (SP) and the lateral decubitus position (LDP). The differences in the postural IOP decreased statistically significantly from the baseline values at 1 month (n = 29), 3 months (n = 29), and 12 months (n = 29) postoperatively (p < 0.001, p < 0.001, and p = 0.001 respectively; Bonferroni test). However, in three cases with a failed bleb, the postural IOP changes were found to be larger (5 to 9 mmHg) than the remaining cases with successful trabeculectomy at 12 months. The open squares, the upper and lower bars indicate 25–75%, 95%, and 5% percentiles respectively
Discussion
Our results showed that trabeculectomy not only reduced the IOP significantly, but also significantly reduced the degree of posture-induced changes in the IOP. These changes were maintained for at least 1 year after the operation if the filtering bleb was functioning. At present, data on posture-induced changes in the IOP following trabeculectomy are contradictory. Parsley et al. measured IOP in the standing, sitting, and supine position in eyes that had undergone trabeculectomy within 6 years, in the non-operated, medically-treated chronic OAG eyes, and in the control eyes [13]. They found that a change from the sitting to supine position led to a mean IOP increase of 5.49 mmHg in the trabeculectomized eyes and 4.02 mmHg in the non-operated OAG eyes, and 0.9 mmHg in the control eyes [13]. Earlier, Anderson and Grant reported that the rise in the IOP after a postural change was much larger following glaucoma surgery (2.83 mmHg) than in eyes treated with pilocarpine (0.75 mmHg) [12]. Contrary to the above previous reports, Hirooka et al. reported that the averaged IOP difference between the sitting and supine position was reduced by 1.9 mmHg from the baseline 3 months after trabeculectomy [14]. More recently, Weizer et al. found that the posture-induced IOP change was significantly smaller in the trabeculectomized eyes than in the non-operated contralateral eyes (4.6 mmHg versus 6.1 mmHg) [15]. Furthermore, they also demonstrated that decreased bleb height, absence of microcysts, and increased bleb vascularity were associated with larger postural IOP changes [15]. Our findings are compatible with those of Hirooka et al. and Weizer et al.
Most of the earlier studies on posture-induced IOP changes used pneumatic tonometers [2], while we used the ICare rebound tonometer to measure the IOP. The advantages of the ICare tonometer is that the measurements can obtained quickly, it is easy to use, does not require topical anesthesia, and affordable for use on all patients including children. In addition, the ICare IOP values are reproducible [17], although earlier studies [17–20] reported that the ICare IOP values were higher by 0.5 to 2.0 mmHg than the GAT values. Another study showed that the ICare values were acceptable substitute to those obtained by GAT, especially in eyes with low to moderate IOPs [19]. For our study, the ICare tonometer allowed us to measure IOP in the two body positions easily.
At present, there is limited information on IOP in the lateral decubitus position. Most of earlier studies compared the IOP changes from the sitting to the supine positions. The differences in IOP for these two body positions ranged from 1.6 to 8.6 mmHg in eyes with glaucoma [2, 5, 12, 13]. Our ICare IOPs obtained in the lateral decubitus position were significantly higher (mean of 3.8 mmHg) than those in the sitting position. Although there are difficulties in comparing our data with those of earlier studies, our result seemed to be compatible with them. For example, in 20 patients undergoing lung surgery, Hwang et al. reported that the IOP values in the dependent eyes (the lower located eye in the lateral decubitus position) were significantly higher in the anesthetized lateral position than that in the awake prone position 5 minutes after the postural change [21]. These differences were not found in the non-dependent eyes (the upper located eye in the lateral decubitus position) [21]. However, the authors did not compare the IOP in the lateral decubitus position with that in the sitting position, and also found that the IOP in the supine position just after the anesthetic induction was lower by 4 to 5 mmHg than the IOP in the lateral decubitus position after anesthesia, even in the non-dependent eye. The posture-dependent IOP changes seem to occur rapidly within 5 minutes after changing the position, as was shown in normal human eyes [21, 22], and in dog eyes without glaucoma [23]. There were two reasons why the IOP was measured in the lateral decubitus position in the non-dependent eye in our study. The first reason was the ease of measuring the IOP in the non-dependent eye compared to the dependent eye, which might lead to higher reproducibility of our results. The other reason was based on our preliminary data in eyes of normal volunteers, which showed that the IOP in the dependent eye was slightly higher than that in the non-dependent eye. However, the IOP in the lateral decubitus position was significantly increased compared to that in the sitting position, even in the non-dependent eye (data not shown).
Other possible controversial issue is whether the original IOP level would affect the amount of the posture-induced variation in IOP. Armaly and Slamoun found no significant difference in posture-induced IOP rise, irrespective of the original IOP level [24]. On the other hand, Hetland-Eriksen concluded that there was a significant association between the IOP level in the sitting position and the amount of the IOP rise when changing to the supine position [25]. Therefore, it still remains to be elucidated whether decreasing posture-induced IOP elevation, as observed in our present study, is attributable to an additional ability of trabeculectomy or low IOP itself. However, our preliminary study in eyes whose IOPs maintain at a level below 11 mmHg with GAT shows that the posture-induced IOP change in the trabeculectomized eyes was significantly smaller than that in the medically-treated eyes without any surgical interventions (data not shown). Based on our preliminary results, it seems to be unlikely that low IOPs influence the fluctuation due to the body positions.
The mechanism that causes the posture-induced IOP changes has not been determined, although some authors have hypothesized that it is due to choroidal vascular congestion and increased episcleral venous pressure, and might be unrelated to aqueous production [2]. Trabeculectomy yields a newly aqueous pathway through the filtering bleb independent of the episcleral veins, and the use of anti-proliferative agents helps to create an escape area from flourishing vessels. Therefore, it seems reasonable that trabeculectomy will suppress posture-induced IOP alterations. However, there was still a significant IOP difference between the sitting and the lateral decubitus position after filtering surgery, indicating a limitation of trabeculectomy in eliminating postural IOP alterations completely.
There are limitations of our study, which include the relatively small number of patients, dependence on each clinician’s discretion in the choice of the ocular hypotensive agents prescribed, and whether laser suture lyses should be performed to preserve the function of the filtering bleb. However, a report found no significant relationship between the administration of some ocular hypotensive agents and postural IOP changes in NTG patients [26]. A second limitation that our study was hospital-based. And a third limitation was that we did not conduct ultrasound biomicroscopy (UBM) to assess the inner structure of the filtering bleb.
In summary, successful trabeculectomy with adjunctive MMC will reduce not only the absolute IOP but also the degree of posture-induced IOP changes for at least 1 year. Additionally, measuring postural IOP changes might be a method for assessing whether a filtering bleb is functioning. However, further longer follow-up and large-scale investigations would be required to address this issue.
References
Iwase A, Araie M, Tomidokoro A, Yamamoto T, Shimizu H, Kitazawa Y, Tajimi Study Group (2006) Prevalence and causes of low vision and blindness in a Japanese adult population: the Tajimi Study. Ophthalmology 113:1354–1362
Prata TS, De Moraes CG, Kanadani FN, Ritch R, Paranhos A Jr (2010) Posture-induced intraocular pressure changes: considerations regarding body position in glaucoma patients. Surv Ophthalmol 55:445–453
Caprioli J, Coleman AL (2008) Intraocular pressure fluctuation. A risk factor for visual field progression at low intraocular pressures in the advanced glaucoma intervention study. Ophthalmology 115:1123–1129
Nouri-Mahdavi K, Hoffman D, Coleman AL, Liu G, Li G, Gaasterland D, Caprioli J, Advanced Glaucoma Intervention Study (2004) Predictive factors for glaucomatous visual field progression in the Advanced Glaucoma Intervention Study. Ophthalmology 111:1627–1635
Hirooka K, Shiraga F (2003) Relationship between postural change of the intraocular pressure and visual field loss in primary open-angle glaucoma. J Glaucoma 12:379–382
Mizokami J, Yamada Y, Negi A, Nakamura M (2011) Postural changes in intraocular pressure are associated with asymmetrical retinal nerve fiber thinning in treated patients with primary open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol 249:879–885
Kiuchi T, Motoyama Y, Oshika T (2006) Relationship of progression of visual field damage to postural changes in intraocular pressure in patients with normal-tension glaucoma. Ophthalmology 113:2150–2155
Abedin S, Simmons RJ, Grant WM (1982) Progressive low-tension glaucoma: treatment to stop glaucomatous cupping and field loss when these progress despite normal intraocular pressure. Ophthalmology 89:1–6
Collaborative Normal-Tension Glaucoma Study Group (1998) Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol 126:487–497
Mansouri K, Orguel S, Mermoud A, Haefliger I, Flammer J, Ravinet E, Shaarawy T (2008) Quality of diurnal intraocular pressure control in primary open-angle patients treated with latanoprost compared with surgically treated glaucoma patients: a prospective trial. Br J Ophthalmol 92:332–336
Miyake T, Sawada A, Yamamoto T, Miyake K, Sugiyama K, Kitazawa Y (2006) Incidence of disc hemorrhages in open-angle glaucoma before and after trabeculectomy. J Glaucoma 15:164–171
Anderson DR, Grant WM (1973) The influence of position on intraocular pressure. Invest Ophthalmol 12:204–212
Parsley J, Powell RG, Keightley SJ, Elkington AR (1987) Postural response of intraocular pressure in chronic open-angle glaucoma following trabeculectomy. Br J Ophthalmol 71:494–496
Hirooka K, Takenaka H, Baba T, Takagishi M, Mizote M, Shiraga F (2009) Effect of trabeculectomy on intraocular pressure fluctuation with postural change in eyes with open-angle glaucoma. J Glaucoma 18:689–691
Weizer JS, Goyal A, Ple-Plakon P, Trzcinka A, Strong BD, Bruno CA, Junn J, Tseng I, Niziol LM, Musch DC, Moroi SE (2010) Bleb morphology characteristics and effect on positional intraocular pressure variation. Ophthalmic Surg Lasers Imaging 41:532–537
Kitazawa Y, Kawase K, Matsushita H, Minobe M (1991) Trabeculectomy with mitomycin C (a comparative study with fluorouracil). Arch Ophthalmol 109:1693–1698
Martinez-de-la-Casa JM, Garcia-Feijoo J, Castillo A, Garcia-Sanchez J (2005) Reproducibility and clinical evaluation of rebound tonometry. Invest Ophthalmol Vis Sci 46:4578–4580
Jóhannesson G, Hallberg P, Eklund A, Lindén C (2008) Pascal, ICare and Goldmann applanation tonometry—a comparative study. Acta Ophthalmol 86:614–621
Munkwitz S, Elkarmouty A, Hoffmann EM, Pfeiffer N, Thieme H (2008) Comparison of the ICare rebound tonometer and the Goldmann applanation tonometer over a wide IOP range. Graefes Arch Clin Exp Ophthalmol 246:875–879
Davies LN, Bartlett H, Mallen EA, Wolffsohn JS (2006) Clinical evaluation of rebound tonometer. Acta Ophthalmol Scand 84:206–209
Hwang JW, Jeon YT, Kim JH, Oh YS, Park HP (2006) The effect of the lateral decubitus position on the intraocular pressure in anesthetized patients undergoing lung surgery. Acta Anaesthesiol Scand 50:988–992
Loewen NA, Liu JH, Weinreb RN (2010) Increased 24-hour variation of human intraocular pressure with short axial length. Invest Ophthalmol Vis Sci 51:933–937
Broadwater JJ, Schorling JJ, Herring IP, Elvinger F (2008) Effect of body position on intraocular pressure in dogs without glaucoma. Am J Vet Res 69:527–530
Armaly MF, Salamoun SG (1963) Schiotz and applanation tonometry. Arch Ophthalmol 70:603–609
Hetland-Eriksen J (1966) On tonometry. 5. The pressure of glaucomatous eyes measured in the sitting and the lying positions by means of the Goldmann applanation tonometer. Acta Ophthalmol (Copenh) 44:515–521
Kiuchi T, Motoyama Y, Oshika T (2007) Influence of ocular hypotensive eyedrops on intraocular pressure fluctuation with postural change in eyes with normal-tension glaucoma. Am J Ophthalmol 143:693–695
Conflict of interest
The authors have no proprietary or financial interest in any products used in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sawada, A., Yamamoto, T. Effects of trabeculectomy on posture-induced intraocular pressure changes over time. Graefes Arch Clin Exp Ophthalmol 250, 1361–1366 (2012). https://doi.org/10.1007/s00417-012-1942-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-012-1942-7