Abstract
Background
To determine if asymmetrical postural change-induced elevations in intraocular pressure (IOP) in eyes of patients with primary open-angle glaucoma (POAG) were associated with asymmetries in functional and structural damage.
Methods
Enrolled were 132 eyes of 66 patients with POAG (male/female = 22/44, age = 55.2 ± 13.4 years). IOP was first measured in a seated position and then in a supine position at 10-minute intervals over a 60-minute period using a TonoPen XL. The Humphrey visual field 30-2 program and a fast retinal nerve fiber layer (RNFL) thickness acquisition protocol of Stratus optical coherence tomography (OCT) were performed. Mean deviation (MD) and average total RNFL thickness were compared between eyes with the greater magnitude of postural change-induced IOP elevation (ΔIOP) and those with the smaller magnitude of ΔIOP.
Results
The MDs of the eyes with larger ΔIOP (6.21 ± 3.18 mmHg) and smaller ΔIOP (3.02 ± 0.37 mmHg) were −12.31 ± 7.63 dB and −9.67 ± 6.80 dB respectively (p = 0.0176). The average total RNFL thickness was 64.33 ± 17.83 μm in the former and 68.56 ± 15.10 μm in the latter (p = 0.049). The MDs and RNFL thickness were not significantly different between the eyes that had higher and lower IOP values measured in the seated position.
Conclusions
In patients with asymmetrical POAG, the magnitude of IOP elevation induced by postural changes may be related to differences in the severity of both functional and structural damage between the eyes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Elevated intraocular pressure (IOP) is the only established, treatable risk factor for the development and progression of glaucomatous optic neuropathy [1]. Thus, IOP assessment is of particular importance for the management of glaucoma patients. However, it is well-known that IOP has short-term and long-term fluctuations. Whether or not IOP fluctuation is a risk factor for the progression of glaucomatous optic neuropathy has been a matter of controversy [2]. With regard to short-term IOP fluctuations, previous studies [3–11] have indicated that IOP increases following a postural change from a seated to a supine position, and that the magnitude of the IOP elevation was higher in glaucoma patients than in controls. More recently, several reports [12–16] have suggested that the magnitude of the increase in IOP that occurs with this postural change may be associated with the development and progression of glaucomatous visual field defects.
It is well-known that the diagnosis and monitoring of glaucomatous optic neuropathy should include a combination of structural and functional tests, because these two types of test are not completely correlated and are complementary to each other [17]. Thus, whether or not there is a positive association between postural change-induced IOP increases and structural damage in the eyes of patients with primary open-angle glaucoma (POAG) was not predictable, even if the eyes exhibited the correlation between the postural IOP fluctuation and functional damage. In addition, the structure–function relationship in eyes with early- to moderate-stage glaucoma is known to be poor compared with those with advanced-stage glaucoma [18, 19]. This is either because the standard deviations for both the visual field testing and structural analyses are large for eyes with early-stage glaucoma [19], or because the structural damage may precede functional damage during the pathogenesis of glaucoma [20]. However, no previous studies have examined the effect that postural IOP changes have on the structural damage due to glaucomatous optic neuropathy, including retinal nerve fiber layer (RNFL) thinning.
In this study, we sought to determine whether or not an asymmetry in postural change-induced IOP increases in individuals with POAG correlated with differences in the degree of thinning of the RNFL between their eyes. An additional aim of this study was to examine the relationship between visual field defects and RNFL thinning separately, by comparing these parameters in the eyes that had a larger rise in IOP and in the fellow eyes that had a smaller rise in IOP, in order to examine the impact of the degree of structural damage on the structure–function relationship in the same population.
Methods
All investigations were conducted in accordance with the ethical standards stated in the 1964 Declaration of Helsinki and with the approval of the Institutional Review Board of Kobe University Graduate School of Medicine. Written informed consent was obtained from all participants.
Subjects
We recruited 66 patients with bilateral POAG [22 male and 44 female, 25 to 81 years of age (median 55, mean 55.2 ± 13.4)] who regularly visited the Glaucoma Clinic at Kobe University Hospital and who underwent supine position IOP measurement between January 2008 and June 2009. They were enrolled irrespective of their IOP values and their use of ocular hypotensive agents. In order to carry a diagnosis of POAG, patients had to have: 1) a normal anterior chamber angle, 2) a glaucomatous optic disc appearance (i.e., a vertical cup-to-disc ratio of 0.6 or more, a vertical cup to disc asymmetry between the eyes of 0.2 or more, and neuroretinal rim thinning, notching, or nerve fiber layer defects), and 3) corresponding visual field defects that were defined by consecutive, repeated abnormal results on standard automated perimetry (SAP) [i.e., a glaucoma hemifield test that was outside of the normal limits, a pattern standard deviation with p < 0.05, or three or more contiguous points with p < 0.05 (at least one of which had a p-value of <0.01) in the pattern deviation plot].
SAP was performed with the Humphrey Field Analyzer (HFA) 750 (Carl-Zeiss Meditec, Dublin, CA, USA) using the Swedish Interactive Threshold Algorithm standard strategy and the central 30-2 program. The visual field reliability criteria included fixation losses and false-positive and false-negative rates of less than 15%.
Central corneal thickness (CCT) was measured with a specular microscope (NONCON ROBO; Konan Medical, Nishinomiya, Japan).
The exclusion criteria were: 1) best-corrected visual acuity less than 10/20, 2) previous intraocular surgery, 3) intra- and/or extraocular diseases that could potentially affect the visual field (i.e., optic disc anomalies, ischemic optic neuropathy, retinal diseases, intraorbital and intracranial space-occupying lesions, and diabetes), 4) myopia greater than −6 D, 5) astigmatism greater than ±3.0 D, and 6) media opacity that was associated with a lens nuclear sclerosis grade of ≥3.
Optical coherence tomography (OCT)
The RNFL thickness was measured using the fast RNFL thickness acquisition protocol of the Stratus OCT (Carl Zeiss Meditec, Inc, Dublin, CA USA) after pupillary dilation as has been described elsewhere [18, 21]. Briefly, a total of three peripapillary circular scans, each composed of 256 A scans, were acquired consecutively using a circle with a diameter of 3.46 mm centered on the optic disc. An automated computer algorithm delineated the anterior and posterior margins of the RNFL. The average total RNFL thickness measurement that was obtained from the 360-degree circular scans was then used for further analyses. Images that were unfocused, poorly centered, had a signal strength of <6, or that failed the RNFL segmentation algorithm were excluded. OCT recording and analysis were performed by one of the authors (YY), who was masked to the supine position IOP measurements of the included patients.
Supine position IOP measurement
Patients first underwent routine ophthalmic examination and Goldmann applanation tonometry in a dimly lit room. After a 15-minute rest period, the patient had his/her IOP measured in a seated position with a TonoPen XL (Mentor, Norwell, MA, USA). Ten minutes later, the patient was instructed to lie down on an examination bed in another dimly lit room. A pillow was used to facilitate patient head positioning whenever it was required. IOP was measured immediately after lying down and every 10 minutes thereafter over a 60-minute period. Topical anesthetics were instilled in patients’ eyes before each IOP measurement. TonoPen XL IOP measurements were performed as follows [22]. Immediately before the initial measurement at the sitting position, the instrument was calibrated. Next, six consecutive measurements were made, from which the average was automatically calculated and displayed. Values with p > 0.05 were discarded and another recording was made. The procedures were performed three times and the mean value was used as the IOP for each time point. The same examiner (JM) performed all of the measurements, and was blinded to the OCT data and HFA data.
The difference between the initial IOP measurement that was taken in the seated position and the maximal IOP measurement that was obtained during the supine position was defined as ΔIOP. For each patient, the eye that had a larger ΔIOP was categorized into the larger ΔIOP eye group, while the fellow eye that had a smaller ΔIOP was categorized into the smaller ΔIOP eye group.
All patients underwent HFA, OCT, and supine position IOP measurements within a 3-month period.
Statistical analyses
All statistical evaluations were performed using StatView version 5.0 software (SAS Institute, Cary, NC, USA) and MedCalc (version 10.4.0, MedCalc Software, Mariakerte, Belgium). The postural change from the seated to supine position and time course of changes during the supine position in IOP measurements for larger and smaller ΔIOP eye groups were compared independently using the repeated-measure analysis of variance (ANOVA) and the Dunnett test as a post-hoc test. The IOPs of the larger and smaller ΔIOP eye groups at each time point were compared using paired t-tests. The number of eyes that exhibited the maximum IOP measurement at a specific time point during the supine position was plotted as a histogram. The mean values for the seated IOP, maximum supine IOP, ΔIOP, CCT, the number of eye drops that patients used, the HFA mean deviation (MD), and the average total RNFL thickness were compared between larger ΔIOP eyes and smaller ΔIOP eye groups using paired t-tests. The mean MD and RNFL thickness of eyes with higher seated-position IOP values and lower seated-position IOP values were also compared with paired t-tests. The sample size was calculated by setting the mean = −12.3 dB, the null hypothesis value = −9.67 dB, and the standard deviation = 7.63, the type I error = 0.05, and the type II error = 0.20 using MedCalc (for single means). A linear regression analysis was also performed to examine the correlation between MD and the average total RNFL thickness for the larger and smaller ΔIOP eye groups independently. The obtained correlation coefficient was compared between the larger and smaller ΔIOP eye groups. All p-values <0.05 were considered to be statistically significant.
Results
Table 1 shows mean ± standard deviation and ranges for the seated and maximum supine IOP, ΔIOP, and CCT values, as well as the number of eye drops that were used for larger and smaller ΔIOP eye groups. Figure 1 demonstrates the postural and time course changes that occurred in IOP for larger and smaller ΔIOP eye groups. On average, the IOP became significantly elevated upon lying down from the seated position, remained elevated for up to 10 min after lying down, and then stabilized at 20 min and thereafter both for larger and smaller ΔIOP eye groups. The difference in IOPs between the larger and smaller ΔIOP eye groups became statistically significant at 10 minutes after lying down and remained significant for the remainder of the 60-minute testing period. The IOPs of both eye groups returned to the basal levels immediately after the postural change from the supine to the seated position. The CCT and the number of eye drops that were used did not differ between the larger and smaller ΔIOP eye groups (Table 1).
Intraocular pressure (IOP) fluctuations following postural changes. ΔIOP indicates the difference in IOP between the initial IOP measured in the seated position and the maximum IOP obtained while patients were in the supine position. Closed circles represent larger ΔIOP eyes, whereas open circles represent smaller ΔIOP eyes. Bars indicate the standard deviation. The time courses of intra-eye IOP changes were compared with repeated-measure of analysis of variance and the Dunnett test. * and ** indicate p < 0.05 compared to the initial IOPs measured in seated position for larger ΔIOP eyes and for smaller ΔIOP eyes respectively. Inter-eye differences of IOPs at each time point were compared with the paired t-test, for which the p-values are described on the top of the figure
Interestingly, the time point at which IOPs reached their maximum value while patients were in the supine position was highly variable among the eyes (Fig. 2). Although the frequency was highest at 10 minutes after becoming supine, a very similar frequency distribution was observed at the other time points, implying that the time point at which an eye exhibited its maximal IOP was not predictable, at least during this time frame.
A frequency distribution of the number of eyes that reached their maximal intraocular pressure (IOP) measurement at each time point while patients were in the supine position. ΔIOP is designated as in Fig. 1. Black columns represent larger ΔIOP eyes, whereas white columns represent smaller ΔIOP eyes
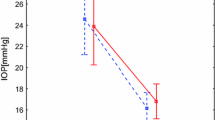
When larger and smaller ΔIOP eye groups were compared, the former had significantly worse MD (−12.31 ± 7.63, ranging from −34.17 to 0.98, dB) and a thinner average total RNFL thickness (64.33 ± 17.83 μm, range 25.84 to 107.07) than the latter (MD: −9.67 ± 6.80 dB, range −29.67 to 1.82; RNFL thickness: 68.56 ± 15.10 μm, range 37.40 to 105.31) (Fig. 3). In comparison, there were no differences in the MD or the average total RNFL thickness between eyes that had a higher seated-position IOP measurement and those that had a lower seated-position IOP measurement (Fig. 4).
The difference in average total retinal nerve fiber layer (RNFL) thickness and mean deviation (MD) between larger ΔIOP eyes and smaller ΔIOP eyes. ΔIOP is designated as in Fig. 1. RNFL thickness was measured with the Stratus OCT. Bars indicate the standard error of mean. Comparisons were made with paired t-tests
The difference in average total retinal nerve fiber layer (RNFL) thickness and mean deviation (MD) between eyes with higher and lower sitting intraocular pressure (IOP) measurements while in the seated position. RNFL thickness was measured with the Stratus OCT. Bars indicate the standard error of the mean. Comparisons were made with paired t-tests
Linear regression analyses demonstrated a positive correlation between the MD and the average total RNFL thickness both for the larger and smaller ΔIOP eye groups (Fig. 5). The correlation coefficient (=0.655) in the former group was statistically higher than that (=0.409) in the latter (p < 0.05).
Scatter plots and regression analyses of the relationship between average total retinal nerve fiber layer (RNFL) thickness and mean deviation (MD). Top: larger ΔIOP eyes: r = 0.655, p < 0.0001. Bottom: smaller ΔIOP eyes: r = 0.409, p = 0.0005. ΔIOP is designated as in Fig. 1. RNFL thickness was measured with the Stratus OCT
Discussion
Many previous reports [3–16] have indicated that IOP increases with a postural change from a seated to a supine position, which was further confirmed in this study. However, the magnitude of the IOP increase has varied among these reports, although eyes with glaucoma or ocular hypertension consistently exhibited a more marked postural change-induced IOP increase than normal eyes; previously reported measurements ranged from 2.5 to 5.6 mmHg in healthy eyes [3–6, 10, 13] and from 3.5 to 8.6 mmHg in glaucomatous or ocular hypertensive eyes [3, 4, 16]. In this study, the average increase in IOP was 6.21 mmHg in the larger ΔIOP eye group and 3.02 mmHg in the smaller ΔIOP eye group. The inter-study differences in previously reported postural change-induced increases in IOP arise from many factors, including differences in subject inclusion and exclusion criteria and differences in the methods that were used to obtain IOP measurements. Some investigators used a pneumatonometer [5, 6, 10, 13], while others used the Perkins hand-held applanation tonometer [3, 14–16]. In this study, we used the TonoPen XL. A previous paper [23] demonstrated better within-visit and across-visit reproducibility of postural IOP changes measured with the TonoPen XL compared to those measured with the Pulsair tonometer. In addition, previous studies [3–16] have measured a supine IOP at a fixed time point. However, we found that the time point at which IOPs reached their maximal values while patients were in the supine position varied greatly between the eyes, and was thus not predictable. Thus, the assessment of postural changes in IOP using a fixed time for IOP measurements while patients are in the supine position may miss the truly maximal IOP value, and therefore underestimate the ΔIOP and decrease the reproducibility of the results. On the other hand, repeatedly measuring IOP with short intervals as we did in the present study may lead to a cumulative applanation effect that could result in the underestimation of IOP. However, a previous study [24] showed that a rest period of 5 minutes was long enough to eliminate the previous applanation effect. We think that the 10-minute interval used in this study exerted only a negligible unfavorable effect on the serial IOP measurement and the calculation of ΔIOP if it exerted any negative impact at all.
Kiuchi et al. [14, 15] have demonstrated that the IOP fluctuation induced by a postural change was a risk factor for the development and progression of normal-tension glaucoma (NTG), and that ocular hypotensive eye drops had no effect on the postural change-induced IOP increases that occurred [16]. According to their studies, an MD slope was correlated both with the absolute values of the supine position IOP and the magnitude of IOP elevation 30 minutes after entering the supine position in 60 eyes of 33 patients with NTG [14]. In another paper, [15] they reported that more advanced visual field defects were observed in eyes with a greater magnitude of postural change-induced IOP elevation in 20 untreated patients with NTG. Hirooka et al. [13] also demonstrated that the magnitude of IOP elevation upon moving from the seated to the supine position was significantly greater in the eyes that had more advanced visual field damage in patients with asymmetrical POAG. The present study confirmed these earlier observations regarding the negative impact of the postural fluctuation of IOP on glaucomatous visual field loss. However, these previous reports did not evaluate the presence of structural damage or disease progression related to the postural IOP changes that they reported.
The present study demonstrated that the eyes that had a greater magnitude of postural change-induced IOP elevation had smaller average RNFL thicknesses in patients with POAG; this proved for the first time that there is a positive association between postural change-induced IOP increases and structural damage in the eyes of patients with POAG. A somewhat related and intriguing recent study by Gunns et al. [25] examined the relationship between the diurnal variation in IOP and RNFL thickness using a scanning laser polarimetry (SLP) in eyes with exfoliation syndrome and in normal eyes. They reported that eyes with exfoliation syndrome that exhibited a higher diurnal IOP variation had thinner RNFL thickness compared with the normal controls [25]. The present study and the study by Gunns et al. [25] suggest that the occurrence of short-term fluctuations in IOP may itself be a risk factor for RNFL thinning, which is a structural marker of glaucomatous optic neuropathy.
This study also demonstrated a positive structure–function relationship even when eyes were stratified by ΔIOP magnitude, further expanding upon the previous reports by us [18] and by others [19]. On the other hand, the correlation between RNFL thickness reduction and visual field damage in eyes with smaller ΔIOPs was weaker than that observed in eyes with larger ΔIOPs. One possible reason for this may be that the group of eyes with smaller ΔIOPs consisted of eyes that were in a relatively early stage of glaucoma, because the structure–function relationship in eyes with early- to moderate-stage glaucoma is poorer than that in those with advanced-stage glaucoma, as mentioned earlier [18, 19].
The mechanisms underlying the IOP increase that is induced by postural change are not fully understood. There are several hypotheses for this, including an episcleral venous pressure rise [3, 8], choroidal vessel congestion [3, 8], and an increased aqueous humor production or outflow resistance [9] while patients are in the supine position. However, a central hypervolemia during the supine position may activate volume receptors that cause an increased fluid excretion from the kidney and the subsequently increased plasma colloid osmotic pressure [11]. This condition creates a blood–ocular colloid pressure gradient that could dehydrate the vitreous body through the retina and uvea, and may eventually exert a negative force with regard to IOP [11]. Additionally, the prolonged IOP elevation that occurs while patients are in the supine position as well as the asymmetrical postural change-induced IOP elevation that occurs in the eyes of the same individual, which was demonstrated in the present study as well as in previous studies [13–16], imply that complex mechanisms may regulate the postural changes in IOP, and suggest that additional factors that have not yet been identified may be involved.
It should also be emphasized that the magnitude of postural changes in IOP may not be the sole cause for the asymmetrical visual field damage that was present in our patients. Postural changes also affect blood pressure and, subsequently, ocular perfusion pressure [26, 27]. Compromised autoregulatory control of ocular hemodynamics may therefore be a contributing factor to the pathogenesis of glaucoma [28]. Choi et al. [27] demonstrated that larger circadian fluctuations of mean ocular perfusion pressures were significantly associated not only with visual functional damage but also with RNFL thickness reduction as measured by SLP. Kiuchi et al. [14] reported that the MD slope was also correlated with systolic blood pressure in the supine position.
Other limitations of our study include the relatively small sample size as well as possible selection bias with regard to subject enrollment. Almost all subjects used ocular hypotensive eye drops, which may have affected the postural change-induced IOP increases that we observed. However, Kiuchi et al. [16] recently demonstrated that the use of timolol, latanoprost, or brinzolamide eye drops, which were the most common eye drops that patients used in the present study, did not alter the magnitude of postural change-induced IOP elevation.
Recent interest has been generated in the possibility of measuring IOP without postural change during sleep at night, in order to evaluate the actual diurnal IOP fluctuations, as several researchers have demonstrated nocturnal elevation of IOP when patients were sleeping in the supine position [29, 30]. However, not all practicing physicians can perform 24-h IOP measurements for all of their glaucoma patients. If the current protocol for measuring IOP for 1 h in the supine position could be used as a substitute for the 24-h IOP measurement, or if it can be shown to reflect (at least in part) the 24-h IOP fluctuations, the data obtained from this protocol may be valuable for glaucoma patient care and management in the future.
In conclusion, the magnitude of IOP elevation induced by postural change from a seated to supine position differed between the eyes of individual patients with POAG. Not only the visual field loss but also the RNFL thickness reduction was more advanced in the eyes with a greater magnitude of postural change-induced IOP elevation. Further studies are needed to determine whether or not the assessment of postural change-induced IOP fluctuations could potentially be used as a substitute for 24-h IOP measurements in the care of glaucoma patients.
References
Weinreb RN, Khaw PT (2004) Primary open-angle glaucoma. Lancet 363:1711–1720
Caprioli J, Coleman AL (2008) Intraocular pressure fluctuation. A risk factor for visual field progression at low intraocular pressures in the Advanced Glaucoma Intervention Study. Ophthalmology 115:1123–1129
Anderson DR, Grant MW (1973) The influence of position on intraocular pressure. Invest Ophthalmol 12:204–212
Krieglstein GK, Langham ME (1975) Influence of body position on the intraocular pressure of normal and glaucomatous eyes. Ophthalmologica 171:132–145
Leonard TJK, Kerr Muir MG, Kirkby GR, Hitchings RA (1983) Ocular hypertension and posture. Br J Ophthalmol 67:362–366
Tsukahara S, Sasaki T (1984) Postural change of IOP in normal persons and in patients with primary wide open-angle glaucoma and low-tension glaucoma. Br J Ophthalmol 68:389–392
Smith TJ, Lewis J (1985) Effect of inverted body position intraocular pressure. Am J Ophthalmol 99:617–618
Friberg TR, Sanborn G, Weinreb RN (1987) Intraocular and episcleral venous pressure increase during inverted posture. Am J Ophthalmol 103:523–526
Carlson KH, McLaren JW, Topper JE, Brubaker RF (1987) Effect of body position on intraocular pressure and aqueous flow. Investig Ophthalmol Vis Sci 28:1346–1352
Yamabayashi S, Aguilar RN, Hosoda M, Tsukahara S (1991) Postural change of intraocular and blood pressures in ocular hypertension and low tension glaucoma. Br J Ophthalmol 75:652–655
Chiquet C, Custaud M-A, Le Tran AP, Millet C, Gharib C, Denis P (2003) Changes in intraocular pressure during prolonged (7-day) head-down tilt bedrest. J Glaucoma 12:204–208
Lietz A, Kaiser HJ, Stumpfig D, Flammer J (1995) Influence of posture on the visual field in glaucoma patients and controls. Ophthalmologica 209:129–131
Hirooka K, Shiraga F (2003) Relationship between postural change of the intraocular pressure and visual field loss in primary open-angle glaucoma. J Glaucoma 12:379–382
Kiuchi T, Motoyama Y, Oshika T (2006) Relationship of progression of visual field damage to postural changes in intraocular pressure in patients with normal-tension glaucoma. Ophthalmology 113:2150–2155
Kiuchi T, Motoyama Y, Oshika T (2010) Postural response of intraocular pressure and visual field damage in patients with untreated normal-tension glaucoma. J Glaucoma 19:191–193
Kiuchi T, Motoyama Y, Oshika T (2007) Influence of ocular hypotensive eyedrops on intraocular pressure fluctuation with postural change in eyes with normal-tension glaucoma. Am J Ophthalmol 143:693–695
Wollstein G, Schuman JS, Price LL, Aydin A, Strak PC, Hertzmark E, Lai E, Ishikawa H, Mattox C, Fujimoto JG, Paunescu LA (2005) Optical coherence tomography longitudinal evaluation of retinal nerve fiber layer thickness in glaucoma. Arch Ophthalmol 123:464–470
Naka M, Kanamori A, Tatsumi Y, Fujioka M, Nagai-Kusuhara A, Nakamura M, Negi A (2009) Comparison of mean deviation with AGIS and CIGTS scores in association with structural parameters in glaucomatous eyes. J Glaucoma 18:379–384
Hood DC, Kardon RH (2007) A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res 26:688–710
Harwerth RS, Quigley HA (2006) Visual field defects and retinal ganglion cell losses in patients with glaucoma. Arch Ophthalmol 124:853–859
Kanamori A, Naka M, Nagai-Kusuhara A, Yamada Y, Nakamura M, Negi A (2008) Regional relationship between retinal nerve fiber layer thickness and corresponding visual field sensitivity in glaucomatous eyes. Arch Ophthalmol 126:1500–1506
Kanamori A, Nakamura M, Nakanishi Y, Yamada Y, Negi A (2005) Long-term glial reactivity in rat retinas ipsilateral and contralateral to experimental glaucoma. Exp Eye Res 81:48–56
Wilson MR, Baker RS, Mohammadi P, Wheeler NC, Lee DA, Scott C (1993) Reproducibility of postural changes in intraocular pressure with the Tono-pen and Pulsair tonometers. Am J Ophthalmol 116:479–483
Wilke K (1972) Effect of repeated tonometry: genuine and sham measurements. Acta Ophthalmol 50:574–582
Gumus K, Bozkurt B, Sonmez B, Irkec M, Orhan M, Saracbasi O (2006) Diurnal variation of intraocular pressure and its correlation with retinal nerve fiber analysis in Turkish patients with exfoliation syndrome. Graefes Arch Clin Exp Ophthalmol 244:170–176
Singleton CD, Robertson D, Byrne DW, Joos KM (2003) Effect of posture on blood and intraocular pressures in multiple system atrophy, pure autonomic failure, and baroreflex failure. Circulation 108:2349–2354
Choi J, Kim KH, Jeong J, Cho H, Lee CH, Kook MS (2007) Circadian fluctuation of mean ocular perfusion pressure is a consistent risk factor for normal-tension glaucoma. Investig Ophthalmol Vis Sci 48:104–111
Galambos P, Vafiadis J, Vilchez SE, Wagenfeld L, Matthiessen ET, Richard G, Klemm M, Zeits O (2006) Compromised autoregulatory control of ocular hemodynamics in glaucoma patients after postural change. Ophthalmology 113:1832–1836
Liu JH, Zhang X, Kripke DF, Weinreb RN (2003) Twenty-four-hour intraocular pressure pattern associated with early glaucomatous changes. Investig Ophthalmol Vis Sci 44:1586–1590
Hara T, Hara T, Tsuru T (2006) Increase of peak intraocular pressure during sleep in reproduced diurnal changes by posture. Arch Ophthalmol 124:165–168
Acknowledgement
This study was supported in part by Grants-in-Aid No. 22390324 (AN, MN) and No. 20592043 (MN, AN) from the Ministry of Education, Culture Sports, Science and Technology of the Japanese Government.
Author information
Authors and Affiliations
Corresponding author
Additional information
Financial disclosure
No financial interest with any groups.
Rights and permissions
About this article
Cite this article
Mizokami, J., Yamada, Y., Negi, A. et al. Postural changes in intraocular pressure are associated with asymmetrical retinal nerve fiber thinning in treated patients with primary open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol 249, 879–885 (2011). https://doi.org/10.1007/s00417-010-1565-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-010-1565-9