Abstract
Background
The aim of this study was to compare the intraocular pressure (IOP) results measured by the iCare rebound tonometer with those obtained by the Goldmann applanation tonometer (GAT) over a wide range of IOP values. Furthermore, the comfort level of the iCare measurement was evaluated.
Method
The study included 75 eyes of 75 patients. The patients were divided into three groups (7–15 mmHg n = 25, 16–22 mmHg n = 25, 23–60 mmHg n = 25). The measurements were taken by two independent observers in a masked fashion. All patients were asked about discomfort during the iCare measurement. To establish the agreement between the two devices, a Bland-Altman analysis was performed.
Results
Overall, the 95% confidence interval of the differences between the two devices was −8.67 to 10.25 mmHg and in 62.7%, the iCare measurement was within ±3 mmHg of the GAT measurements. The distribution of the differences in IOP was similar, from 7–22 mmHg. In the higher IOP range (23–60 mmHg), however, the deviation was almost twice as large. The measurement with the iCare tonometer was well tolerated; 100% of the patients denied any discomfort.
Conclusions
The iCare tonometer is a mobile alternative to GAT in a low to moderate IOP range, but our findings show a greater deviation than previously reported. In high IOP values, measurements with the iCare tonometer do not correlate well with GAT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The measurement of intraocular pressure (IOP) is a fundamental basic examination in daily ophthalmologic routine. Especially in the management of glaucoma the assessment of IOP is the most important factor for diagnosis and follow-up. It is well known that IOP is a risk factor for glaucoma and its reduction until now the only proven therapy to slow down disease progression [1, 11]. The most accurate measurement of IOP would be an invasive manometric measurement, so far this is only possible in an experimental setting [13]. In clinical practice, the worldwide accepted gold standard for IOP measurement is Goldmann applanation tonometry (GAT) [22]. Its principle is well known [10]. A new and also mobile alternative to the GAT is the iCare rebound tonometer (Tiolat Oy, Helsinki, Finland). It has recently been available on the German market (CE approval 2003), introduced by Kontiola in the year 2000. In this study, we compared the iCare rebound tonometer with measurements obtained by GAT. The technique of the rebound tonometry (dynamic or impact tonometry) [8, 15, 17, 19] can be explained briefly as follows: A fairly light moving probe (24.0 mg) is fired off repeatedly onto the eye (six times per measurement). The deceleration of the probe after bouncing back from the cornea is determined and calculated into the IOP in mmHg, displayed on the iCare device. Detailed physical explanation is found in the literature [16]. The hand-held iCare tonometer measures without exerting pressure and is faster than the corneal reflex, so it does not require local anaesthesia.
The purpose of this study was to compare the IOP values measured by the iCare with those of the GAT over a wide range of IOP. Furthermore, the comfort level of the iCare measurement was evaluated.
Materials and methods
Seventy-five eyes of 75 patients were measured; 30 male and 45 female patients were included. The mean age (standard deviation) of the patients was 57.75 (SD ± 17.26) years. All patients were recruited from the Department of Ophthalmology, University of Mainz, Germany.
The patients were divided into three groups: Group A with an IOP within the 7–15 mmHg range, group B with an IOP within the 16–22 mmHg range, and group C with an IOP within the 23–60 mmHg range. These ranges were based on the International Standard for human eye tonometers ISO 8612 [2].
All patients underwent a slit-lamp examination to exclude patients with corneal pathologies such as previous keratoplasty surgery or refractive surgery, scarring, edema, epithelial lesions and higher astigmatism of >3 diopters. The classification into the three different IOP groups was based on the GAT readings. All patients were informed about the study and gave informed consent prior to inclusion in the study.
The perception of comfort versus discomfort was performed by using a visual analog scale (VAS). The VAS is one of the most frequently used scales for the measurement of pain/discomfort [6]. A 10-mm line is marked at one side with discomfort and on the other side with comfort. The patient has to mark his perception on the 10-mm line to show the comfort level.
The measurements were performed by two experienced investigators (SM-GAT), (AE-Icare) in a masked fashion. This means that both investigators did not know the IOP result of the other tonometer. To avoid any aqueous massage by GAT measurements [3, 18] only one GAT measurement was taken and the iCare measurement was performed first. The iCare software is adjusted for six consecutive measurements, the highest and the lowest measurement is automatically discarded and the IOP is calculated from the remaining four IOP values. If a faulty measurement is obtained, the software does not accept the measurement.
The GAT (Haag-Streit, Switzerland) measurement followed the iCare measurement by using one eye drop of commercially available local anaesthetic (Oxybuprocain + Fluorescein).
To avoid any statistical bias, one study eye was randomly chosen, except for patients in whom only one eye could be measured due to exclusion criteria.
Statistical analysis
Statistical analysis was performed using the SPSS statistical software program, version 14 (SPSS Inc, Chicago, IL). Descriptive statistics was performed for demographic characteristics of the study population. Descriptive analysis including mean values and standard deviations IOP measurements using both instruments was performed. The calculated Pearson correlation coefficient r indicates a high correlation with r = 0.7–0.99, a moderate correlation with r = 0.4–0.69 and no correlation with r < 0.4. A Bland-Altman analysis was used to assess agreement between the instruments [4]. The Bland-Altman analysis is used to compare two different measurement methods. The difference between each IOP measurement was plotted against the mean. The y-axis represents the difference in IOP (iCare-GAT), the x-axis the mean of both measurements (iCare+GAT)/2). Furthermore, the mean difference of the measurements, the standard deviation (SD) and the 95% confidence interval (CI) of the differences were calculated.
Results
The correlation coefficient for the iCare and Goldmann Tonometer was 0.87 and the r2 value was 0.76 (Fig. 1). The mean IOP reading with the GAT was 20.80 ± 9.38 mmHg (range 8–60 mmHg). The mean IOP reading with the iCare was 21.59 ± 9.17 mmHg (range 9–51 mmHg).
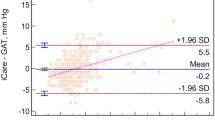
The Bland-Altman plot (Fig. 2) showed the following results: On the y-axis the difference between the methods is plotted against the mean of the two measurements on the x-axis.
The mean difference (Δ) between the iCare and GAT measurement was 0.79 mmHg, with a standard deviation (SD) of 4.73 mmHg and a 95% confidence interval (Mean Δ ± 2 × SD) of −8.67 to 10.25 mmHg (Table 1).
In almost two-thirds of the study population the iCare measurements were within ±3 mmHg of the GAT measurements and 48.1% of all iCare measurements were within ±2 mmHg, 25.4% within ±1 mmHg of GAT measurements. However, 17.1% of all measurements by iCare tonometry showed ±5 mm Hg deviation from GAT measurements.
In the 3 different IOP groups the standard deviations for the difference between the iCare and GAT measurement were 3.25 (IOP 7–15 mmHg), 3.96 (IOP16–22 mmHg) and 6.13 (IOP 23–60 mmHg). Both measurements were within ±3 mmHg in 64% (IOP 7–15 mmHg and IOP16–22 mmHg, respectively) and 60% for the high pressure group (IOP 23–60 mmHg) (Table 2).
The iCare measurement was very well tolerated; 100% of the patients did not feel any discomfort, when they were asked using the visual analog scale after the examination.
Discussion
A new technical device for the measurement of IOP always has to prove its accuracy in comparison to a current gold standard. In tonometry this gold standard is Goldmann applanation tonometry [22, 25].
Therefore, this study compared the IOP readings of the iCare rebound tonometer with those by GAT by measuring outpatients in a daily clinical setting. Furthermore, the comfort level of the iCare measurement was evaluated.
A new approach in this study was the division into three IOP groups, covering an IOP range from 7–60 mmHg, providing new information about the capability of the iCare tonometer to measure within a wide range of IOP.
For GAT a intrasubject variation of more than 3 mmHg between two GAT measurements has been reported in 30% of the measured eyes [21] and calibration errors over ±2.5 mmHg [25]. Therefore, some authors suggest an error ≤ ± 2.5 mmHg for an acceptable balance between tolerance and clinical accuracy.
In this study we defined a “tolerable” difference range from −3 to 3 mmHg. Group A (range 7–15 mmHg) and B (range 16–22 mmHg) showed similar results with 64% of all measurements within ±3 mmHg and group C (range 23–60 mmHg) slightly worse results with 60% of all measurements within ±3 mmHg.
In group C 28% of the differences between the two devices were greater than 5 mmHg. The standard deviation of group C with a confidence interval of −12.56–11.98 mmHg difference was almost twice as large as the standard deviation for group A and B.
This demonstrates that iCare measurements in a high IOP range are not accurate enough for clinical decision taking. Several recent studies have been published to determine the accuracy of the IOP measurement with the iCare rebound tonometer compared to GAT [5, 7, 9, 12]:
Iliev et al. [12] report a 95% confidence interval of −3,2 mmHg to 5,2 mmHg and 84.1% of the IOP readings within ±3 mmHg difference. Compared to our study they found higher correlations between both devices; however, fewer patients with high IOP readings were included. Brusini et al. found a similar 95% confidence interval compared to our group 1 and 2 of −7 to 6.6 mmHg, but a better ±3 mmHg difference interval of 74.1% [5].
In comparison with other hand-held devices such as the Keeler Pulsair 3000 a 95% CI to GAT from 1.75 to −2.72 mmHg with an IOP range from 10 mmHg to 44 mmHg has been shown [20].
For the digital tonometer TGDc-01 PRA a 95% CI to GAT from −17 to 10 mmHg and for the Tonopen a 95% CI to GAT from −6 to 7 mmHg has been reported [24].
The comfort level of the iCare in our study corresponds to that of other studies, which report a very high patients’ comfort level [14, 24].
The present study might be limited by disregarding the influence of the central corneal thickness (CCT). It has been demonstrated that the iCare is affected by CCT and adjusting the IOP to the individual corneal thickness should be considered [5, 12]. However, some authors suggest that the GAT and the iCare are equally affected and this would neutralise this effect on our results [12].
Another source of bias could be the defined order in which the iCare and GAT measurement was performed. It was assumed that the tonography effect of the iCare tonometer due to aqueous massage is very small and was almost negligible. This was the reason for performing the iCare measurements first. The probe used on the cornea weighs only 26.5 mg and has a 1.7-mm diameter plastic end-tip. Schreiber et al. noted a tonography effect of only 0.6 mmHg after a series of 18 successive measurements [23]. We performed six successive measurements, consequently a much smaller IOP reduction can be considered.
In summary, this study demonstrates a moderate agreement between the new iCare and GAT in normal to moderate elevated IOP, and a poor agreement in the higher IOP range. Compared to previously reported papers our findings show a greater deviation between the iCare and GAT measurement in all three IOP groups.
The iCare might be useful in disabled patients, e.g., in old peoples homes, in rural areas and an alternative to GAT, when a mobile tonometer is needed. It is easy to use and very well tolerated by the patients.
It can also be helpful for patients who are difficult to measure with GAT, due to blepharospasm during the GAT measurement, or for patients who should be measured without the need to apply any eye drops.
In a low to moderate IOP range the iCare might be an acceptable substitute to GAT, but in patients with high IOP the standard deviation + confidence interval of the differences is very large, which leads us to the conclusion that the iCare measurement on its own is not accurate enough as a substitute for GAT measurement.
References
The Advanced Glaucoma Intervention Study (AGIS) : 7 (2000) The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol 130(4):429–440
Internationaler Standard für Augentonometer ISO 8612 (2001) Beuth-Verlag GmbH, Berlin
Bechrakis E (1966) On spontaneous decrease of pressure in applanation tonometry. Ophthalmologica 151(5):604–614
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1(8476):307–310
Brusini P, Salvetat ML, Zeppieri M, Tosoni C, Parisi L (2006) Comparison of ICare tonometer with Goldmann applanation tonometer in glaucoma patients. J Glaucoma 15(3):213–217
Chapman CR, Casey KL, Dubner R, Foley KM, Gracely RH, Reading AE (1985) Pain measurement: an overview. Pain 22(1):1–31
Davies LN, Bartlett H, Mallen EA, Wolffsohn JS (2006) Clinical evaluation of rebound tonometer. Acta Ophthalmol Scand 84(2):206–209
Dekking HM, Coster HD (1967) Dynamic tonometry. Ophthalmologica 154(1):59–74
Fernandes P, Diaz-Rey JA, Queiros A, Gonzalez-Meijome JM, Jorge J (2005) Comparison of the ICare rebound tonometer with the Goldmann tonometer in a normal population. Ophthalmic Physiol Opt 25(5):436–440
Goldmann H, Schmidt T (1957) About applanation tonometry. Ophthalmologica 134(4):221–242
Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M (2002) Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol 120(10):1268–1279
Iliev ME, Goldblum D, Katsoulis K, Amstutz C, Frueh B (2006) Comparison of rebound tonometry with Goldmann applanation tonometry and correlation with central corneal thickness. Br J Ophthalmol 90(7):833–835
Kohlhaas M, Boehm AG, Spoerl E, Pursten A, Grein HJ, Pillunat LE (2006) Effect of central corneal thickness, corneal curvature, and axial length on applanation tonometry. Arch Ophthalmol 124(4):471–476
Kontiola A, Puska P (2004) Measuring intraocular pressure with the Pulsair 3000 and Rebound tonometers in elderly patients without an anesthetic. Graefes Arch Clin Exp Ophthalmol 242(1):3–7
Kontiola AI (2000) A new induction-based impact method for measuring intraocular pressure. Acta Ophthalmol Scand 78(2):142–145
Kontiola AI (2003) Developing impact tonometers for clinical use and glaucoma research. Finland: Department of Ophthalmology, University of Helsinki
Kontiola AI, Goldblum D, Mittag T, Danias J (2001) The induction/impact tonometer: a new instrument to measure intraocular pressure in the rat. Exp Eye Res 73(6):781–785
Krakau CE, Wilke K (1971) On repeated tonometry. Acta Ophthalmol (Copenh) 49(4):611–614
Obbink J (1931) Onderzoek naar het verband tusschen inwendigen oogdruk en ballistische reacties. Thesis, Utrecht, The Netherlands
Parker VA, Herrtage J, Sarkies NJ (2001) Clinical comparison of the Keeler Pulsair 3000 with Goldmann applanation tonometry. Br J Ophthalmol 85(11):1303–1304
Phelps CD, Phelps GK (1976) Measurement of intraocular pressure: a study of its reproducibility. Albrecht Von Graefes Arch Klin Exp Ophthalmol 198(1):39–43
Sandhu SS, Chattopadhyay S, Birch MK, Ray-Chaudhuri N (2005) Frequency of goldmann applanation tonometer calibration error checks. J Glaucoma 14(3):215–218
Schreiber W, Vorwerk CK, Langenbucher A, Behrens-Baumann W, Viestenz A (2007) A comparison of rebound tonometry (ICare) with TonoPenXL and Goldmann applanation tonometry. Ophthalmologe 104:299–304
van der Jagt LH, Jansonius NM (2005) Three portable tonometers, the TGDc-01, the ICARE and the Tonopen XL, compared with each other and with Goldmann applanation tonometry*. Ophthalmic Physiol Opt 25(5):429–435
Wessels IF, Oh Y (1990) Tonometer utilization, accuracy, and calibration under field conditions. Arch Ophthalmol 108(12):1709–1712
Acknowledgements
For the study the iCare rebound tonometer was provided by PESCHKE GmbH, Nürnberg, Germany. This was the only source of funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Munkwitz, S., Elkarmouty, A., Hoffmann, E.M. et al. Comparison of the iCare rebound tonometer and the Goldmann applanation tonometer over a wide IOP range. Graefes Arch Clin Exp Ophthalmol 246, 875–879 (2008). https://doi.org/10.1007/s00417-007-0758-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-007-0758-3