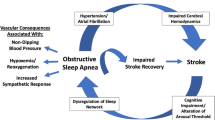
Abstract
Background
Low arousal threshold plays a part in the pathogenesis of obstructive sleep apnea (OSA) and may be improved by sedatives. Sedative antidepressants are frequently prescribed for stroke patients due to their high prevalence of insomnia and depression. However, the effect of sedative antidepressants on the severity of OSA in stroke patients has not been studied well.
Methods
In a double-blinded randomized crossover pilot study, 22 post-acute ischemic stroke patients (mean age, 61.7 ± 10.6 y) with OSA received 100 mg of trazodone or a placebo just before polysomnography, with approximately 1 week between measures. The study also measured baseline heart rate variability and 24-h ambulatory blood pressure.
Results
Administration of trazodone significantly increased the percentage time of slow-wave sleep (31.5 ± 13.2 vs. 18.4 ± 8.7%; P < 0.001) and improved almost all the parameters of OSA severity, including the apnea–hypopnea index (AHI, 25.4 ± 15.4 vs. 39.1 ± 18.4 events/h; P < 0.001), the respiratory arousal index (9.8 (5.8–11.95) vs. 14.1 (11.3–18.7) events/h; P < 0.001), and the minimum oxygen saturation (80.2 ± 9.1 vs. 77.1 ± 9.6%; P = 0.016). Responders to therapy (AHI reduced by > 50%; n = 7/22) had predominant OSA during rapid-eye-movement sleep and decreased sympathetic tone, as reflected in significantly lower mean blood pressure, diastolic blood pressure, and normalized low-frequency power.
Conclusions
Obstructive sleep apnea with comorbid ischemic stroke may be a distinctive phenotype which responds quite well to trazodone, decreasing OSA severity without increasing nocturnal hypoxia.
Trial Registration
Clinicaltrials.gov: NCT04162743, 2019/11/10.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple factors contribute to obstructive sleep apnea (OSA), an illness that is amenable to precision medicine. Eckert et al. proposed a PALM scale (Passive critical closing pressure of the upper airway, Arousal threshold, Loop gain, and Muscle responsiveness) as a tool for developing novel targeted treatments for OSA based on pathophysiologic characteristics [1]. Among the four proposed endotypes, arousal includes both cortical arousal associated with electroencephalographic changes and subcortical arousal associated with autonomic reactions such as blood pressure (BP) and heart rate (HR) [2]. Arousals fragment sleep, causing insomnia and excessive daytime sleepiness; they usually arise at the end of respiratory events [3, 4] and contribute to the pathogenesis of OSA [4, 5]. Low respiratory arousal threshold, which is found in up to one-third of moderate-to-severe OSA patients, is defined as the occurrence of arousal from sleep with a small rise in ventilatory drive [1, 6]. While arousals occurring at a high arousal threshold prevent asphyxia, premature arousals at lower arousal thresholds may worsen OSA severity [7, 8]. A low arousal threshold phenotype has been frequently observed in non-obese OSA patients and may predict poor tolerance of continuous positive airway pressure (CPAP), because the noise, facial mask, and airway pressure of CPAP may further aggravate sleep disruption [9]. The low arousal threshold phenotype can be predicted by allocating a score of 1 to each clinically available polysomnography (PSG) parameters, including the severity of OSA (apnea–hypopnea index, AHI < 30 events/h), the minimum SpO2 (minimum oxygen saturation measured by pulse oximetry SpO2 > 82.5%) and the predominance of apneas vs. hypopneas (Fhypopneas > 58.3%) [10]. A summed score of two or above predicted low arousal thresholds in middle-aged and unselected participants, with a sensitivity of 80.4% and a specificity of 88.0% [10].
Based on the above, numerous sedating agents have been tested on OSA patients with low arousal thresholds. Sedatives with muscle-relaxant effects generally prolonged respiratory events and did not decrease OSA severity [11, 12]. Recently, sedative antidepressants and non-myorelaxant sedatives have been found to have mixed effects on the severity of OSA [6, 13,14,15]. Eckert et al. reported that 100 mg of trazodone, a sedative antidepressant, increased NREM (non-rapid eye movement) respiratory arousal threshold without obvious change in level of oxygen saturation, AHI, arousal index, genioglossus muscle activity, and upper airway collapsibility in seven middle-aged and obese OSA patients with low respiratory arousal threshold [16]. On the contrary, Smales et al. found that 100 mg of trazodone caused a significant reduction in AHI without obvious change in oxygen saturation, level of arousal index, and NREM arousal threshold in 15 unselected OSA patients [17]. The reasons for these two contradictory findings may be related to samples studied and the lack of evaluation of REM-related OSA.
Research regarding the effect of sedative antidepressants on stroke patients with OSA has additional importance, because insomnia and depression have been reported in more than 50% of stroke patients [18, 19], among whom OSA is also highly prevalent [20]. In one study, about half of stroke patients with insomnia fulfilled the diagnostic criteria for depression [18]. Therefore, sedative antidepressants are frequently prescribed for insomnia in stroke patients with undiagnosed OSA, especially in the context of depression. In another study, mirtazapine, a noradrenergic and specific serotonergic antidepressant, did not universally improve OSA severity in 10 ischemic or hemorrhagic stroke patients. The authors recommended closely monitoring OSA severity at least by overnight pulse oximetry before and during the first month of prescribing of mirtazapine [15]. Trazodone, a serotonin modulator with non-myorelaxant sedative properties, may be a third-line treatment for adult chronic insomnia [21]. Whether the same precautions taken when prescribing mirtazapine to stroke patients should be taken when prescribing trazodone is currently unknown.
To the best of our knowledge, the effect of sedative antidepressants on the severity of OSA in ischemic stroke patients has not been well studied. We hypothesized that trazodone does not worsen nocturnal oxygen saturation in ischemic stroke patients with OSA and might have beneficial effects on stroke patients with OSA and a low arousal threshold. We selected trazodone because of its anti-depressant and non-myorelaxant sedative effect.
Methods
This study consecutively recruited post-acute ischemic stroke patients who were admitted to the rehabilitation ward in a teaching hospital. The exclusion criteria were lack of clear consciousness or unstable neurologic signs, unstable medical conditions such as delirium, active infection, evidence of overt congestive cardiac failure, chronic obstructive pulmonary disease, end-stage-renal-disease receiving dialysis, allergy to trazodone, and taking monoamine oxidase inhibitors or any medication that may affect respiration. After the baseline PSG study, patients were excluded if their level of AHI was less than five events per hour or if they had sleep-related breathing disorders other than OSA (e.g., Cheyne-Stroke respiration, central sleep apnea and central hypoventilation syndrome). The study used a randomized double-blinded and placebo-controlled crossover pilot design in which participants were assigned to groups according to the allocation order (placebo first or treatment first). One of the authors who was not involved in patient recruitment and outcome evaluation generated the allocation order using a random number generator. Qualified participants would be studied overnight by PSG on two separate occasions with approximately a 1-week washout period. Patients were administered either 100 mg of trazodone (Trazone Tab, Taoyuan, Taiwan) or an indistinguishable placebo orally just before PSG based on their allocation order. Trazodone dose was based on previous studies [16, 17]. Administration of other medications was kept as constant as possible during the washout period.
The study was conducted in accordance with the Declaration of Helsinki, approved by the Institutional Review Board of Chang Gung Medical Foundation and registered with clinicaltrials.gov (NCT04162743). Informed consent was obtained from all participants and/or their legal guardians.
Clinical evaluation
A comprehensive history of all participants was taken, including demographic data, current medications and risk factors for stroke. Cardiac arrhythmia was assessed by 12-lead electrocardiogram (ECG) or 24-h Holter ECG if the stroke mechanism was unexplained after the first-line investigation. On the date of PSG examination, the Barthel index [22], Epworth sleepiness scale [23], and Patient Health Questionnaire-9 [24] were used to evaluate current stroke severity, the propensity to sleep, and depression, along with the following physical parameters including height and weight. Given that both insomnia and OSA share several common daytime symptoms [25], a patient was deemed positive for insomnia if they gave a positive answer (i.e., more than 16 nights per month) to any of these questions: Do you (1) have trouble initiating sleep? (2) Wake up during the night without being able to get back to sleep? (3) Have early morning awakenings and find it difficult to get back to sleep? (4) Take sleeping medications or other sleep aids to improve your sleep?
Polysomnography
Polysomnography examination was performed at the sleep laboratory using the Compumedics Profusion System (Australia) from 10:00 PM to 7:00 AM. Six electroencephalographic channels, an electrocardiogram, an electrooculogram, a submentalis and bilateral anterior tibial surface electromyogram, airflow sensors (nasal pressure cannula and oronasal thermistor), thoracic and abdominal inductance plethysmography belts and finger pulse oximetry were included. A recording time of at least six hours was required for the PSG study to be valid. We used the American Academy of Sleep Medicine (AASM) scoring manual version 2.0.3 to diagnose sleep apnea [26]. Apnea was defined as the cessation of airflow for at least 10 s; hypopnea was defined as a reduction of > 30% in airflow for > 10 s with either an arousal or oxygen desaturation ≥ 3%. The AHIREM was computed as the sum of apnea and hypopnea events during REM (rapid eye movement) sleep divided by the REM sleep duration (hours). Additionally, CSA was diagnosed when ≥ 50% of respiratory events were the central type.
Twenty-four-hour ambulatory BP monitoring
The 24-h ambulatory BP monitoring was recorded by validated oscillometric devices (Watch BP O3, Microlife AG, Widnau, Switzerland) over the non-paretic arm one day after participants underwent their first PSG study. All participants were instructed to avoid caffeine intake on the day of the investigations. Measurements were taken at 30 min intervals for 24 h with an appropriate cuff size and the result was arbitrarily divided into two parts: a daytime period from 7 AM to 10 PM and a nighttime period from 10 PM to 7 AM. Patients were allowed routine rehabilitation and daily activities and were instructed to stay still with their arm relaxed and extended during each BP measurement. Prespecified quality criteria including > 15 daytime and > 8 nighttime measurements were required for a successful 24-h ambulatory BP monitoring.
Heart rate variability
Heart rate variability was assessed in a well-controlled room using a QHRV device with HW6 hardware (Medeia Ltd., Sofia, Bulgaria) between 7:30 AM and 08:00 AM before breakfast in the supine position on the same day of the 24-h ambulatory BP monitoring. Participants with arrhythmia were excluded from the analysis. The absolute power of low-frequency (LF), high-frequency (HF) and very-low-frequency bands were calculated from spectral analyses using a five-minute segment of ECG signals. Normalized HF and LF power were defined as the power of the HF and LF divided by the sum of HF, LF and very-low-frequency absolute power and represented indexes of modulation of the parasympathetic and sympathetic branches of the autonomic nervous system [27], respectively.
Statistics
The normality of the data distribution was assessed using the Shapiro–Wilk test and data are reported as mean ± standard deviation or median and inter-quartile range. Either paired Student t tests or Wilcoxon signed rank tests were used to evaluate the effects of trazodone versus placebo on the sleep indices, according to whether they were normally distributed. Statistical power analyses used G-Power version 3.1.9.5 software (Heinrich-Heine-University, Düsseldorf, Germany) with a two-tailed α of 0.05, a power of 0.8, and an effect size of 0.6 according to the reported improvement in AHI of 10 events/h by the administration of trazodone [17] and the standard deviation of AHI (17 events/h) from our previous results [28], resulting in 24 subjects.
Whether patients had low arousal threshold was calculated according to the equation above [10] using baseline PSG data. The effect size of the improvement in AHI was calculated using Cohen’s d. To calculate the effect size we used standard deviation of AHI while taking placebo. Participants were classified as responders if the level of AHI when taking trazodone decreased by more than 50% of the level of AHI when taking the placebo. In order to assess the difference between responders and nonresponders, we compared categorical variables using a Fisher’s exact test and compared continuous variables using the unpaired t test or the Mann–Whitney U test. Statistical analyses were performed using SPSS software (IBM SPSS Statistics 25, SPSS Inc., Chicago, IL) and a P value of < 0.05 was regarded as statistically significant.
Results
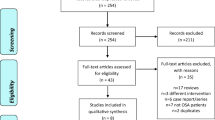
Twenty-six patients were enrolled and 22 of them (12 women and 10 men) completed the study (Fig. 1). The baseline characteristics of patients who completed the study are presented in Table 1. Almost all the parameters of OSA severity, except mean SpO2, were significantly improved in the trazodone group as compared to those in placebo group (Table 2). After administering trazodone, the mean improvement in AHI was 13.7 ± 12.1 events/h and the effect size was 0.74. Only one patient showed a mild increase in AHI from 64.1 events/h while taking the placebo to 65.6 events/h while taking trazodone (Fig. 2). Previous studies suggested that a 3% difference in oxygen saturation level by pulse oximetry was the minimum detectable and clinically relevant change in stroke patients [29]. None of the participants was found to have more than a 3% decrease in mean and minimum SpO2 after administration of trazodone (Fig. 2). In addition, the percentage time of slow-wave sleep (SWS) was also significantly improved in the trazodone group (Table 2). No obvious adverse effects of trazodone were noted.
Individual effects of trazodone on obstructive sleep apnea (OSA) severity and nocturnal oxygen saturation. Black line represents those patients considered nonresponders to therapy, whereas gray line represents responders (see text for definitions of responders/nonresponders). a Trazodone significantly reduced the mean apnea–hypopnea index (trazodone, 25.4 ± 15.4 vs. placebo, 39.1 ± 18.4; P < 0.001). Only one patient’s AHI increased from 64.1 events/h while taking placebo to 65.6 events/h while taking trazodone. b Mean level of mean oxygen saturation measured by pulse oximetry (SpO2) was not significantly different (trazodone, 94.2 ± 1.3 vs. placebo, 93.7 ± 1.3; P = 0.099) between two conditions. c Trazodone significantly increased the mean level of minimum SpO2 (trazodone, 80.2 ± 9.1 vs. placebo, 77.1 ± 9.6; P = 0.016). None of the participants was found to have more than 3% decrease in mean and minimum SpO2 after administration of trazodone
Seven out of 22 participants had a more than 50% improvement in AHI level when taking trazodone and were classified as responders. The baseline characteristics of responders and nonresponders are presented in Table 3. No statistically significant differences in age, body mass index, presence of insomnia or depression, stroke severity, arousal index, nocturnal SpO2, and OSA severity was found between responders and nonresponders. The percentage of patients predicted to have the low arousal threshold phenotype was not different between two groups either. Mean BP and diastolic BP levels were significantly lower in the responder group. The heart rate variability analysis was not performed on two nonresponders due to arrhythmia and the normalized LF power of the responders was significantly lower than that of the nonresponders. Moreover, responders tended to be predominantly female, had higher percentage time of SWS, higher levels of AHIREM and mean SpO2, and slower HR. The absence of statistically significant differences in sex, AHIREM and percentage time of SWS between the two groups may be a type 2 error due to the small sample size. When comparing the post-treatment OSA parameters of responders against nonresponders (Table 4), responders significantly reduced AHIREM on trazodone versus placebo (40.5 ± 21.3 vs. 61.5 ± 17.6 events/h, p = 0.013) and nonresponders significantly reduced spontaneous arousal index on trazodone versus placebo (4.6 ± 2.9 vs. 9.2 ± 7.1 events/h, p = 0.006).
Discussion
Trazodone not only raised minimum oxygen saturation in ischemic stroke patients with OSA, but also decreased the severity of OSA as expressed by AHI and oxygen desaturation index. Decreased sympathetic tone and predominant OSA during REM sleep instead of presence of low respiratory arousal threshold predicts good response to trazodone therapy.
Trazodone modestly decreases OSA severity and improves minimum oxygen saturation in ischemic stroke patients with OSA. Typically, the prescription of a sedative in people with severe OSA or nocturnal hypoxia should be contraindicated or closely monitored. This study recruited patients with a wide range of OSA severity and nocturnal hypoxia and found that the modest improvement in AHI (13.7 ± 12.1 events/h) was similar to the improvement of 13.6 and 10.2 events/h by the oral appliance [30] and trazodone, [17] respectively. Only a few participants showed mild worsening of AHI or nocturnal hypoxia after administration of trazodone, but it did not reach the level of clinical relevance, which suggests that diagnosis and close monitoring of OSA severity is not initially needed when prescribing trazodone for depression or insomnia in ischemic stroke patients. If the baseline arousal threshold is high, the administration of trazodone might result in a certain increase in respiratory event duration and consequent worsening nocturnal hypoxia. Significant improvement in overall minimum oxygen saturation at night implies that the baseline arousal threshold is not high and trazodone did not suppress the upper airway dilator muscle among ischemic stroke patients with OSA. Prior findings that a low arousal threshold phenotype is frequently found in non-obese OSA patients [9] offer further support for this speculation, since the patients in this study were non-obese. In meta-analyses of randomized clinical trials, CPAP therapy for OSA does not reduce stroke risk; nevertheless, patients with good CPAP adherence (> 4 h per day) may benefit [31]. Recent review studies have reported that adherence to CPAP in stroke patients is very low (12–25%) and insomnia may promote CPAP intolerance [32]. Although trazodone is not as effective as CPAP in alleviating OSA, it may be combined with CPAP to reduce CPAP intolerance in stroke patients with OSA. Indeed, the combination of trazodone and CPAP might be clinically relevant to stroke patients with comorbid OSA and insomnia, as a way of improving CPAP compliance in this patient subgroup. Further research is suggested to make any definite claims regarding the effect of combination therapy of trazodone and CPAP on CPAP adherence and stroke-related outcomes.
Trazodone decreased OSA severity. The mechanism that accounts for its therapeutic effect may be multifactorial. Our findings differ from the two previously mentioned studies of trazodone [16, 17]. The current study found a significant decrease of respiratory arousal index and increased percentage time of SWS, level of minimum SpO2, and percentage of sleep time spent with oxygen saturation below 90 and 80%. Differences in study populations might account for the discordant results. The participants in the previous two studies were typical OSA patients who were middle-aged and obese without other comorbidities. Old age, non-obesity and stroke per se in this study might be the reasons for their good response to trazodone. Increased spontaneous arousals and decreased SWS due to normal aging [33] might make elderly patients more susceptible to the administration of trazodone. Studies have found that the medullary serotonergic neurons play an important role in central respiratory chemoreception and carbon dioxide-induced arousal [34]. The significant improvement in sleep fragmentation, as expressed by the reduction of respiratory arousal index, may be associated with the effect of trazodone on the medullary serotonergic neurons and could be the main reason for the resulting decrease in OSA severity. The findings that trazodone significantly increased the percentage time of SWS are consistent with results of previous insomnia studies [35]. As OSA severity is reduced significantly during SWS [36], the significantly increased percentage time of SWS due to trazodone may also lead to decreased OSA severity. In contrast, the findings that mirtazapine did not significantly increase SWS in stroke patients with OSA [15] might be the reason why it failed to decrease or even exacerbated OSA severity. The dissimilar response to trazodone and mirtazapine in terms of OSA severity may be attributed to the different pharmacology of these two sedative antidepressants. Other possible mechanisms to explain the therapeutic effect of trazodone may relate to the modulating effect of serotonin on the upper airway motorneurons [37].
Decreased sympathetic tone and predominant OSA during REM sleep predicted good response to trazodone for reducing OSA severity. Although this study was underpowered to perform subgroup analysis, some inferences can still be made. Given the respiratory arousal threshold during REM sleep is lower than that of NREM sleep [38, 39], the effect of trazodone on respiratory arousal might be more pronounced in the REM sleep. Therefore, the marginally higher baseline AHIREM and the significant decrease in AHIREM after administration of trazodone might be the main reason for the better response of the responders. The findings that the female-predominant responder group tended to have higher baseline AHIREM and percentage time of SWS are in agreement with previous studies examining sex differences in OSA [40]. Previous studies also showed that females have a greater difference in AHI between SWS and REM sleep as compared to males [36]. Therefore, the beneficial effect of increasing the percentage time of SWS to reduce OSA severity may be more obvious in females, which could be the reason why females are likely to respond better to trazodone for reducing OSA severity. The elevated normalized LF power, mean and diastolic BP, and marginal increased HR in the nonresponders suggest that the sympathetic system is more activated in the nonresponders as compared to the responders. The central respiratory chemoreception and carbon dioxide-induced arousal is not only regulated by the medullary serotonergic neurons but also the locus coeruleus noradrenergic neurons [34]. Besides, the locus coeruleus noradrenergic neurons also act as a major autonomic nucleus and play a crucial role in the modulation of autonomic function [41]. Therefore, it is biologically plausible that sympathetic tone may reflect the activities of locus coeruleus and be a predictor of response to trazodone in regulating arousal.
Traditional parameters of OSA, including arousal index, failed to predict the response to trazodone among the stroke patients in this study. As the baseline OSA parameters did not differ between responders and nonresponders, it is not surprising that the low arousal threshold calculated using the equation of Edwards et al. [10] failed to predict the response to trazodone in this study. Participants’ characteristics may be the reason for discrepant results, as the participants in the study by Edwards et al. [10] were mainly middle-aged and obese adults without obvious major comorbidities. Therefore, further studies are needed to validate the equation presented by Edwards et al. in stroke patients. Moreover, whether the respiratory arousal threshold measured by the nadir esophageal or epiglottic pressure preceding cortical arousal [10] can be a predictor of the response to trazodone for reducing OSA severity in ischemic stroke patients deserves further study.
This study has several limitations. Firstly, detailed pathophysiologic characteristics of OSA as mentioned above were not measured, which means the mechanisms that account for the therapeutic effect are speculative. Secondly, the small sample size meant the subgroup analysis of responders and nonresponders was exploratory and underpowered. Moreover, this study lacks other clinically relevant outcome measures in addition to the severity of OSA and oxygen saturation. Furthermore, this crossover study evaluated the effect of trazodone on one night only. Therefore, longitudinal studies to see whether the treatment effects persist or decrease over time due to drug tolerance are indicated. Lastly, this study recruited ischemic stroke patients with moderate-to-severe disability who were admitted to the hospital for inpatient rehabilitation. Thus, our results cannot be generalized to patients with mild stroke or severe stroke and impaired consciousness.
Conclusion
Obstructive sleep apnea with comorbid ischemic stroke may be a special phenotype which responds quite well to trazodone for decreasing OSA severity. The findings that trazadone improved the minimum SpO2 level should ease concerns that trazodone may worsen nocturnal hypoxia in patients with ischemic stroke. Future longitudinal studies with larger sample size and clinically relevant outcome measures are suggested.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A (2013) Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med 188(8):996–1004. https://doi.org/10.1164/rccm.201303-0448OC
Thomas RJ (2003) Arousals in sleep-disordered breathing: patterns and implications. Sleep 26(8):1042–1047
Rees K, Spence DP, Earis JE, Calverley PM (1995) Arousal responses from apneic events during non-rapid-eye-movement sleep. Am J Respir Crit Care Med 152(3):1016–1021. https://doi.org/10.1164/ajrccm.152.3.7663777
Younes M (2004) Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med 169(5):623–633. https://doi.org/10.1164/rccm.200307-1023OC
Eckert DJ (1985) Younes MK (2014) Arousal from sleep: implications for obstructive sleep apnea pathogenesis and treatment. J Appl Physiol 116(3):302–313. https://doi.org/10.1152/japplphysiol.00649.2013
Eckert DJ, Owens RL, Kehlmann GB, Wellman A, Rahangdale S, Yim-Yeh S, White DP, Malhotra A (2011) Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci 120(12):505–514. https://doi.org/10.1042/cs20100588
Ratnavadivel R, Stadler D, Windler S, Bradley J, Paul D, McEvoy RD, Catcheside PG (2010) Upper airway function and arousability to ventilatory challenge in slow wave versus stage 2 sleep in obstructive sleep apnoea. Thorax 65(2):107–112. https://doi.org/10.1136/thx.2008.112953
Wains SA, El-Chami M, Lin H-S, Mateika JH (2017) Impact of arousal threshold and respiratory effort on the duration of breathing events across sleep stage and time of night. Respir Physiol Neurobiol 237:35–41. https://doi.org/10.1016/j.resp.2016.12.009
Gray EL, McKenzie DK, Eckert DJ (2017) Obstructive sleep apnea without obesity is common and difficult to treat: evidence for a distinct pathophysiological phenotype. J Clin Sleep Med 13(1):81–88. https://doi.org/10.5664/jcsm.6394
Edwards BA, Eckert DJ, McSharry DG, Sands SA, Desai A, Kehlmann G, Bakker JP, Genta PR, Owens RL, White DP, Wellman A, Malhotra A (2014) Clinical predictors of the respiratory arousal threshold in patients with obstructive sleep apnea. Am J Respir Crit Care Med 190(11):1293–1300. https://doi.org/10.1164/rccm.201404-0718OC
Berry RB, Kouchi K, Bower J, Prosise G, Light RW (1995) Triazolam in patients with obstructive sleep apnea. Am J Respir Crit Care Med 151(2):450–454
Hoijer U, Hedner J, Ejnell H, Grunstein R, Odelberg E, Elam M (1994) Nitrazepam in patients with sleep apnoea: a double-blind placebo-controlled study. Eur Respir J 7(11):2011–2015
Carter SG, Berger MS, Carberry JC, Bilston LE, Butler JE, Tong BKY, Martins RT, Fisher LP, McKenzie DK, Grunstein RR, Eckert DJ (2016) Zopiclone increases the arousal threshold without impairing genioglossus activity in obstructive sleep apnea. Sleep 39(4):757–766. https://doi.org/10.5665/sleep.5622
Carberry JC, Grunstein RR, Eckert DJ (2019) The effects of zolpidem in obstructive sleep apnea—an open-label pilot study. J Sleep Res 28(6):e12853. https://doi.org/10.1111/jsr.12853
Brunner H (2008) Success and failure of mirtazapine as alternative treatment in elderly stroke patients with sleep apnea-a preliminary open trial. Sleep Breath 12(3):281–285
Eckert DJ, Malhotra A, Wellman A, White DP (2014) Trazodone increases the respiratory arousal threshold in patients with obstructive sleep apnea and a low arousal threshold. Sleep 37(4):811–819. https://doi.org/10.5665/sleep.3596
Smales ET, Edwards BA, Deyoung PN, McSharry DG, Wellman A, Velasquez A, Owens R, Orr JE, Malhotra A (2015) Trazodone effects on obstructive sleep apnea and non-REM arousal threshold. Ann Am Thorac Soc 12(5):758–764. https://doi.org/10.1513/AnnalsATS.201408-399OC
Leppävuori A, Pohjasvaara T, Vataja R, Kaste M, Erkinjuntti T (2002) Insomnia in ischemic stroke patients. Cerebrovasc Dis 14(2):90–97
Farner L, Wagle J, Engedal K, Flekkøy KM, Wyller TB, Fure B (2010) Depressive symptoms in stroke patients: a 13 month follow-up study of patients referred to a rehabilitation unit. J Affect Disord 127(1–3):211–218. https://doi.org/10.1016/j.jad.2010.05.025
Lyons OD, Ryan CM (2015) Sleep apnea and stroke. Can J Cardiol 31(7):918–927
Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M (2008) Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med 4(5):487–504
Shah S, Vanclay F, Cooper B (1989) Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol 42(8):703–709. https://doi.org/10.1016/0895-4356(89)90065-6
Johns MW (1991) A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14(6):540–545
de Man-van GJ, Hafsteinsdottir TRNPD, Lindeman EMDPD, Burger HMDPD, Grobbee DMDPD, Schuurmans MRNPD (2012) An efficient way to detect poststroke depression by subsequent administration of a 9-item and a 2-item patient health questionnaire. Stroke 43(3):854–856
Wallace DM, Wohlgemuth WK (2019) Predictors of insomnia severity index profiles in United States veterans with obstructive sleep apnea. J Clin Sleep Med 15(12):1827–1837. https://doi.org/10.5664/jcsm.8094
Berry R, Brooks R, Gamaldo C (2014) The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, Version 2.0.3. American Academy of Sleep Medicine, Darien, IL
Burr RL (2007) Interpretation of normalized spectral heart rate variability indices in sleep research: a critical review. Sleep 30(7):913
Yu C-C, Huang C-Y, Kuo W-K, Chen C-Y (2019) Continuous positive airway pressure improves nocturnal polyuria in ischemic stroke patients with obstructive sleep apnea. Clin Interv Aging 14:241–247. https://doi.org/10.2147/CIA.S193448
Rowat AM, Wardlaw JM, Dennis MS, Warlow CP (2001) Patient positioning influences oxygen saturation in the acute phase of stroke. Cerebrovasc Dis 12(1):66–72. https://doi.org/10.1159/000047683
Ramar K, Dort LC, Katz SG, Lettieri CJ, Harrod CG, Thomas SM, Chervin RD (2015) Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015. J Clin Sleep Med 11(7):773–827. https://doi.org/10.5664/jcsm.4858
Bassetti CLA, Randerath W, Vignatelli L, Ferini-Strambi L, Brill AK, Bonsignore MR, Grote L, Jennum P, Leys D, Minnerup J, Nobili L, Tonia T, Morgan R, Kerry J, Riha R, McNicholas WT, Papavasileiou V (2020) EAN/ERS/ESO/ESRS statement on the impact of sleep disorders on risk and outcome of stroke. Eur Respir J 55(4):1901104. https://doi.org/10.1183/13993003.01104-2019
Mello-Fujita L, Kim LJ, Palombini LdO, Rizzi C, Tufik S, Andersen ML, Coelho FM (2015) Treatment of obstructive sleep apnea syndrome associated with stroke. Sleep Med 16(6):691–696. https://doi.org/10.1016/j.sleep.2014.12.017
Edwards BA, O’Driscoll DM, Ali A, Jordan AS, Trinder J, Malhotra A (2010) Aging and sleep: physiology and pathophysiology. Semin Respir Crit Care Med 31(5):618–633. https://doi.org/10.1055/s-0030-1265902
Guyenet PG, Abbott SBG (2013) Chemoreception and asphyxia-induced arousal. Respir Physiol Neurobiol 188(3):333–343. https://doi.org/10.1016/j.resp.2013.04.011
Mendelson WB (2005) A review of the evidence for the efficacy and safety of trazodone in insomnia. J Clin Psychiatry 66(4):469–476
Subramanian S, Hesselbacher S, Mattewal A, Surani S (2013) Gender and age influence the effects of slow-wave sleep on respiration in patients with obstructive sleep apnea. Sleep Breath 17(1):51–56. https://doi.org/10.1007/s11325-011-0644-4
Veasey SC (2003) Serotonin agonists and antagonists in obstructive sleep apnea. Am J Respir Med 2(1):21–29. https://doi.org/10.1007/BF03256636
Messineo L, Eckert DJ, Lim R, Chiang A, Azarbarzin A, Carter SG, Carberry JC (2020) Zolpidem increases sleep efficiency and the respiratory arousal threshold without changing sleep apnoea severity and pharyngeal muscle activity. J Physiol 598(20):4681–4692. https://doi.org/10.1113/JP280173
Berry RB, Gleeson K (1997) Respiratory arousal from sleep: mechanisms and significance. Sleep 20(8):654–675
Basoglu OK, Tasbakan MS (2018) Gender differences in clinical and polysomnographic features of obstructive sleep apnea: a clinical study of 2827 patients. Sleep Breath 22(1):241–249
Samuels ER, Szabadi E (2008) Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part II: physiological and pharmacological manipulations and pathological alterations of locus coeruleus activity in humans. Curr Neuropharmacol 6(3):254–285. https://doi.org/10.2174/157015908785777193
Funding
Study funded by the Chang Gung Medical Research Council under Contract No. CMRPG2I0021.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Chang Gung Medical Foundation.
Informed consent to participate
Informed consent was obtained from all participants and/or their legal guardians.
Informed consent for publication
Patients signed informed consent regarding publishing their data and photographs.
Rights and permissions
About this article
Cite this article
Chen, CY., Chen, CL. & Yu, CC. Trazodone improves obstructive sleep apnea after ischemic stroke: a randomized, double-blind, placebo-controlled, crossover pilot study. J Neurol 268, 2951–2960 (2021). https://doi.org/10.1007/s00415-021-10480-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-021-10480-2