Abstract
The aim of this paper was to investigate whether both neurocognitive and social cognitive performances were different between remitted first-episode schizophrenia patients, non-remitters and healthy controls (HC). We assessed social cognition (Degraded Facial Affect Recognition Task—DFAR and Emotional Mentalizing Task—EMT) and neurocognition (Wechsler Adult Intelligence Scale and Word Learning Test—WLT) in 174 remitted first-episode schizophrenia patients, 110 non-remitted first-episode schizophrenia patients and 320 HC. Multivariate analyses of variance with age, gender and IQ as covariates (MANCOVA) were performed to compare mean cognitive test scores between the three groups. Remitted first-episode schizophrenia patients performed significantly worse than HC only in one verbal memory task (WLT immediate recall; p = 0.004); in the same test, they were significantly better than non-remitters (p = 0.027). Non-remitted first-episode schizophrenia patients, differently from remitters, performed significantly worse than HC in terms of social cognition (EMT—p < 0.05 and DFAR—p < 0.05). Remitted first-episode schizophrenia patients presented worse cognitive performance than HC in verbal memory tasks, but not in facial affect recognition and in ToM, while non-remitters did; these results suggest that neurocognitive deficits are the core hallmark of schizophrenia and that social cognition is relatively unaffected in remitted patients after their first episode.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Poor daily life activities, low social and occupational functioning levels and cognitive impairment are core features of schizophrenia [1, 2], most of them persisting outside of acute exacerbations of the disorder. This leads schizophrenia patients to experience low ability to live independently and to have successful social interactions.
Cognitive impairment has been detected in high-risk and ultra-high-risk populations for psychosis and in nearly remitted first-episode schizophrenia patients, with different degrees of improvement after a follow-up period of 8 months [3–5]; it is widely accepted that deficits in cognitive domains such as working memory, attention and executive functions do not improve when symptoms disappear [1, 6]. Being specific and enduring features of schizophrenia, neurocognitive deficits are considered trait markers of the disorder, together with brain changes, genetic polymorphisms and immunological abnormalities [7].
Whether remitted schizophrenia patients reach complete functional recovery is rather debated. It has been demonstrated that symptomatic remission is associated with better daily functioning than non-remitters [8]; Boden et al. [9] reported that remitted first-episode schizophrenia patients presented more functional behaviours and life satisfaction than non-remitters, while Bobes et al. [10] found that only about 10 % of patients reached relevant functioning levels during remission.
Social cognition, defined as the cognitive processes subtending interactions in the social world [11, 12], is impaired in schizophrenia patients as well as neurocognition, but it is not so clear whether social cognitive deficits persist despite the remission of the acute symptomatology. Of note, they were also present in nearly remitted first-episode schizophrenia patients [4] and a recent meta-analysis demonstrated that social cognitive performance is also abnormal during the prodromal phases of the disorder in ultra-high-risk population of young individuals with respect to healthy controls (HC) [13]. Taken together, these findings suggest that abnormalities in social cognitive tasks, starting before the onset of the disorder, are a core feature of schizophrenia.
Furthermore, some authors demonstrated that stable schizophrenia outpatients presented social cognitive impairment particularly in the theory of mind (ToM—the ability to recognize emotions and intentions of others) [14–16]. These findings have been confirmed by a number of studies despite the use of different criteria to define remission: Mehta et al. [17] demonstrated that ToM and other social cognitive domains were impaired in both remitted patients and HC, and Rodríguez-Sosa et al. [18] reported social cognitive deficits in discharged patients, but they excluded first-episode patients; meta-analyses by Sprong [19] and Bora et al. [20, 21] supported the hypothesis that social cognitive deficits, particularly ToM impairment, are trait markers of schizophrenia. Of note, these meta-analyses compared studies involving patients with acute symptoms and authors suggested in their conclusions to study first-episode schizophrenia patients in full remission [21]. In particular, Bora et al. [20] analysed papers with non-homogeneous samples, involving also chronic schizophrenia patients and only few remitted patients. In contrast, some studies have demonstrated that ToM impairment is transient, and it is associated with the acute exacerbations of the disorder, particularly with delusional symptoms [22, 23]. Particularly, Pousa et al. [23] involved remitted schizophrenia patients, not only after the first episode. Also Balogh et al. [24] sustained the hypothesis that social cognitive deficits are state markers of the disorder. One study reported that ToM and emotion processing improved, but impairment persisted after clinical remission, thus supporting both the state and trait hypotheses [25].
Some variables such as the definition of “remission” which is different among studies, selection of tools to assess cognition, dimensions of cognitive functions assessed could potentially influence the results. For example, in some studies, social cognition, particularly ToM, has been assessed using subjective scales. To our knowledge, this is the first study using Emotional Mentalizing Task [26] to compare remitted first-episode schizophrenia patients and HC in terms of ToM abilities. The test investigates four subdomains and provides a specific and reliable measure of the different ToM skills (inference of second-order false beliefs, first-order true beliefs, first- and second-order emotions). Furthermore, differently from previous studies, our paper included patients who presented both features (clinical remission and only one schizophrenia episode), being the sample unbiased and powerful. To date, whether social cognitive deficits are considered a trait or state marker of the disorder is rather debated. Evidence, based on mixed samples and non-specific tools, is not sufficient to settle the issue.
Purpose of the present study is to investigate whether neurocognitive and social cognitive deficits are present in first-episode schizophrenia even though patients are in remission. We hypothesized that social cognitive deficits may be more pronounced in non-remitted first-episode schizophrenia patients compared to remitters and HC, also in the light of their lower insight. [27], and that social cognition is preserved when symptoms disappear after the first schizophrenia episode.
Methods
A total of 284 first-episode schizophrenia patients (110 non-remitters and 174 remitters) and 320 HC were recruited as part of the ongoing multicentre “Genetic Risk and Outcome in Psychosis” (GROUP) study in the Netherlands. The procedure of recruitment, informed consent and approval by the accredited Medical Ethics Review Committee (METC) has been described in a previous report on the GROUP study [28].
Inclusion criteria included: age between 16 and 60; fluency in Dutch language; ability and willing to give informed consent; a diagnosis of schizophrenia according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition [29], as assessed by the Comprehensive Assessment of Symptoms and History interview (CASH) [30]; monotherapy with antipsychotic treatment; and lifetime first psychotic episode occurred at least 2 years before. Remission of psychiatric symptoms was defined according with the remission criteria for schizophrenia defined by Andreasen et al. [31].
Exclusion criteria were as follows: history of head trauma and the presence of a medical or neurological illness associated with psychiatric symptoms or affecting cognition.
Demographic and clinical variables such as age, sex, age at onset, duration of observation, cannabis misuse, urine cannabis, urine cocaine, urine amphetamines and pharmacological treatment were collected. Haloperidol dose equivalent of antipsychotics was calculated according to the method defined by Andreasen et al. [32]. Symptoms severity was assessed by the Positive and Negative Syndrome Scale (PANSS) [33].
HC did not have: a current or lifetime psychiatric disorder, as assessed by the CASH [30]; clinical conditions affecting cognitive performance (e.g. dementia, hypothyroidism); and a first-degree family member with a lifetime psychotic or mood disorder for the influence on cognitive performances [34].
All the measures used in the GROUP project were selected on the basis of established reliability and validity as well as on their feasibility for use in large multisite studies [28].
Neurocognitive assessment
Estimated IQ: Wechsler Adult Intelligence Scale (WAIS III) [35, 36]. The arithmetic (working memory), digit symbol-coding (processing speed), block design (reasoning and problem-solving) and information subtests (verbal comprehension) of the WAIS III were administered to estimate IQ. The sum of the four subtests yields a measure of estimated IQ.
Verbal memory: Word learning test (WLT) [37]. The test consists of 15 words presented three times on a computer display. After each presentation, patients had to write down as many words as they could remember. The sum of the words correctly recalled during the three trials was a measure of immediate recall. Twenty minutes after immediate recall, delayed recall was recorded. Patients had to write down in 1 min all words they remembered, which was the delayed recall score. Finally, the original list along with 15 distracter words was presented. Patients had to indicate by button press whether a presented word was a member of the original list or not.
Social cognitive assessment
Emotion perception: Degraded Facial Affect Recognition Task (DFAR) [38]. The task uses black and white photographs of four different actors (two males and two females) depicting four emotions: angry, happy, fearful and neutral. The task comprises 64 trials consisting of 16 face presentations in each emotion category. The emotions are shown with 75 % intensity in order to increase the difficulty of the task. Subjects are asked to indicate the emotional expression of each face with a button press and to respond as accurately as possible. Outcome is the proportion of faces correctly recognized as neutral, happy, fearful and angry emotions (range 0–100 %).
ToM: Conflicting beliefs and Emotions Task–Emotional Mentalizing Test (EMT) [26, 39]. The task comprises eight vignettes dealing with a social situation of either exclusion or threat and featuring two protagonists A and B. In the vignette, A holds a true first-order belief and B holds a false second-order belief. Each belief is associated with an emotional state, one characterized by positive and one by negative valence. Participants are asked six questions after each vignette, designed to assess their understanding of the two conflicting beliefs and conflicting emotional states. Two first-order, two second-order and two control questions are included. The first-order questions tested participants’ ability to deduce from the story the belief and emotional state of actor A. The second-order questions tested participants’ understanding of the false belief of actor B on the thoughts of actor A as well as the associated emotional state of actor A perceived by actor B. The score 0 is assigned for a wrong response, 1 for a partially correct response (partial mental state) and 2 for a correct response (full mental state). Range was 0–8 for each question.
Statistical analyses
Descriptive analyses of the total sample were performed. Groups of patients divided according to the current antipsychotic treatment were compared in terms of haloperidol equivalent doses using a univariate analysis of variance (one-way ANOVA).
Demographic and clinical variables were compared between groups (remitted schizophrenia patients at first episode, non-remitters and HC) using Chi-square test with Bonferroni’s corrections for dichotomous variables or multivariate analyses of variance (MANOVAs) for continuous variables.
Multivariate analyses of variance considering age, gender and IQ as covariates (MANCOVAs) were performed to compare mean cognitive test scores between the three groups.
Statistical Package for Social Sciences (SPSS) for Windows (version 21.0) was used as statistical programme.
Results
The total sample (N = 604) consisted of 110 non-remitted schizophrenia patients at first episode, 174 remitted first-episode schizophrenia patients and 320 HC. The mean age of the total sample was 31.56 (± 9.21) years; 385 subjects (63.7 %) were males and 219 (36.3 %) females. Patients presented a mean duration of observation of 6.87 (± 4.17) years. Other demographic and clinical variables are summarized in Table 1.
Groups of patients treated with different antipsychotics did not differ in terms of haloperidol equivalents (F = 0.819; p = 0.515) (Table 2).
No statistically significant differences were found between the three groups (remitters/non-remitters/HC) in terms of urine cocaine (χ2 = 4.114; df = 2; p = 0.089) and urine amphetamines (χ2 = 4.498; df = 2; p = 0.181).
The three groups were statistically different for age (F = 16.533; p < 0.001), gender (χ2 = 59.443; df = 2; p < 0.001), urine cannabis (χ2 = 22.154; df = 2; p < 0.001), cannabis misuse (χ2 = 31.103; df = 2; p < 0.001) and WAIS estimation total IQ (F = 58.077; p < 0.001). In particular, HC were older than schizophrenia first-episode patients (both remitters and non-remitters); both patients’ groups had significantly more males than females as compared to HC; non-remitters were more frequently positive at the cannabis urine test than remitted first-episode schizophrenia patients and HC, while both patients’ groups presented cannabis misuse more frequently than HC; finally, non-remitted first-episode schizophrenia patients had statistically significant lower IQ than remitters and HC, while HC presented the highest IQ of the total sample.
Descriptive statistics (mean and standard deviation) and post hocs of neurocognition and social cognition of the three groups are summarized in Table 3.
The three groups were not significantly different for WLT retention rate (F = 0.012; p = 0.988), DFAR neutral faces (F = 1.918; p = 0.148), DFAR happy faces (F = 1.958; p = 0.142), DFAR fearful faces (F = 2.175; p = 0.115), EMT second-order emotion (F = 1.605; p = 0.202), EMT first-order belief (F = 1.453; p = 0.235) and EMT control questions (F = 1.981; p = 0.139).
Statistically significant differences were found in terms of WLT immediate recall (F = 14.489; p < 0.001), WLT delayed recall (F = 4.287; p = 0.014), DFAR angry faces (F = 4.108; p = 0.017), DFAR percentage of total correct (F = 5.979; p = 0.003), EMT second-order belief (F = 6.520; p = 0.002) and EMT first-order emotion (F = 3.412; p = 0.034).
Post hocs revealed that HC performed better than both remitters and non-remitters in one of the memory tests, the immediate recall (p < 0.005), while they had higher scores than non-remitters only in delayed recall (p = 0.015). Furthermore, non-remitted first-episode schizophrenia patients performed worse than remitters in WLT immediate recall (p = 0.027) (Fig. 1).
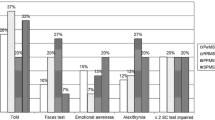
In all the social cognitive tests with a statistically significant difference among groups, non-remitters performed worse than HC (p < 0.05), while the difference between remitted first-episode schizophrenia patients and HC was not significant (Fig. 2).
Discussion
This study investigated whether social cognition (facial emotion recognition and emotional mentalizing) and verbal memory performances were different between first-episode schizophrenia patients in remission, first-episode schizophrenia patients not in remission and healthy controls.
The main finding of the study is that while non-remitted first-episode schizophrenia patients presented both neurocognitive and social cognitive impairment as compared to HC, remitted first-episode schizophrenia patients presented only one verbal memory deficit (in the subtest of immediate recall) as compared to HC, while they were not different in terms of facial affect recognition or ToM (emotional mentalizing).
Interestingly, our study shows that even the best-outcome patients appear to have neurocognitive deficits in the verbal memory domain, while their social cognition seems to be preserved, differently from worse-outcome patients (non-remitters after a first schizophrenia episode). Furthermore, remitted first-episode schizophrenia patients did also have lower IQ as compared to healthy controls, as well as non-remitters. Controlling for IQ, the results remained the same (i.e. verbal memory deficits and intact social cognition in remission).
The results of the present study are in line with previous evidence that cognitive disabilities, such as memory deficits, are a trait marker of schizophrenia [40, 41] and support the hypothesis that impairment in social cognition may primarily be a state-dependent characteristic of the illness [23].
In our sample, one of the verbal memory tests was impaired even during symptomatic remission, differently from facial affect recognition and ToM measures. Some authors stated that social cognition is related to cognitive performance: Sachs and colleagues [42] found a correlation between emotion discrimination and some cognitive domains, such as verbal memory or language processing; Bora et al. [43] demonstrated that remitted schizophrenia patients presented ToM disabilities that may be related to the general cognitive impairment; Fernandez-Gonzalo et al. [44, 45] showed that neuropsychological variables differently influenced first- and second-order ToM tasks and that in first-episode schizophrenia patients, executive functions performance was related to ToM. On the other hand, some authors investigated specificity and severity of social cognitive impairment in schizophrenia and argued that ToM is not influenced by cognitive performance [46–48], thus supporting the hypothesis that social cognition and neurocognition may be underpinned by different pathogenetic mechanisms. According to our findings, it is not possible to affirm whether social cognition is influenced by neurocognitive variables or not, but our results lean towards the hypothesis that they are independent.
Finally, in our study, DFAR total score is significantly worse between non-remitters and HC, specifically angry face recognition is altered in first-episode schizophrenia patients not in remission, compared to HC. Our results are consistent with previous findings showing that schizophrenia is associated with impaired ability to recognize negative emotions, such as anger and fear with respect to healthy controls or other psychiatric disorders [49–52]. This could be explained by the presence of residual PANSS paranoid/delusional symptoms, which are present in these patients who did not gain remission after a first schizophrenia episode. Other explanations should be considered while interpreting these findings, such as the effects on cognition caused by antipsychotic medication and/or cannabis misuse.
The data from this paper can help to answer this question: after a first episode of schizophrenia do patients in remission have cognitive deficits? Our results answer Yes for few neurocognitive abilities, but No for social cognition. For this reason, cognitive remediation programmes are warranted in remitted schizophrenia patients and social cognition should be considered a target for early rehabilitation programmes.
The study has some limits. One of the most important is the limited cognitive assessment: only one neurocognitive domain (verbal memory) has been assessed in our sample, ToM abilities were evaluated using only one test (i.e. Emotional Mentalizing Task), and attributional style was not examined. Further studies using a complete neurocognitive battery are needed to confirm our results. Other limits of the study are the different antipsychotic treatment among patients and the poor matching of the three groups. For example, antipsychotic doses in terms of haloperidol equivalents were different between remitters and non-remitted schizophrenia patients, but it was expected that patients in remission take lower antipsychotic doses compared with patients still presenting psychotic symptoms. Furthermore, it has been demonstrated that second-generation antipsychotic did not influence cognitive performance in schizophrenia [53]. Finally, the cross-sectional design may have limited the interpretation of the results: it would be possible that remitters were a better prognosis group of patients originally with higher IQ and better social cognitive performance and that the duration of remission had influenced their performance.
References
Cornblatt BA, Keilp JG (1994) Impaired attention, genetics, and the pathophysiology of schizophrenia. Schizophr Bull 20:31–46
San L, Ciudad A, Alvarez E, Bobes J, Gilaberte I (2007) Symptomatic remission and social/vocational functioning in outpatients with schizophrenia: prevalence and associations in a cross-sectional study. Eur Psychiatr 22:490–498
Kelleher I, Murtagh A, Clarke MC, Murphy J, Rawdon C, Cannon M (2013) Neurocognitive performance of a community-based sample of young people at putative ultra high risk for psychosis: support for the processing speed hypothesis. Cogn Neuropsychiatr 18:9–25
Lee SY, Bang M, Kim KR (2015) Impaired facial emotion recognition in individuals at ultra-high risk for psychosis and with first-episode schizophrenia, and their associations with neurocognitive deficits and self-reported schizotypy. Schizophr Res 165:60–65
Niendam TA, Bearden CE, Zinberg J, Johnson JK, O’Brien M, Cannon TD (2007) The course of neurocognition and social functioning in individuals at ultra-high risk for psychosis. Schizophr Bull 33:772–781
Hofer A, Bodner T, Kaufmann A (2011) Symptomatic remission and neurocognitive functioning in patients with schizophrenia. Psychol Med 41:2131–2139
Chen Y, Bidwell LC, Norton D (2006) Trait vs. state markers for schizophrenia: identification and characterization through visual processes. Curr Psychiatr Rev 2:431–438
Karow A, Moritz S, Lambert M, Schöttle D, Naber D, EGOFORS Initiative (2012) Remitted but still impaired? Symptomatic versus functional remission in patients with schizophrenia. Eur Psychiatr 27:401–405
Boden R, Sundstrom J, Lindstrom E, Lindstrom L (2009) Association between symptomatic remission and functional outcome in first-episode schizophrenia. Schizophr Res 107:232–237
Bobes J, Ciudad A, Alvarez E, San L, Polavieja P, Gilaberte I (2009) Recovery from schizophrenia: results from a 1-year follow-up observational study of patients in symptomatic remission. Schizophr Res 115:58–66
Green MF, Olivier B, Crawleym JN, Penn DL, Silverstein S (2005) Social cognition in schizophrenia: recommendations from the measurement and treatment research to improve cognition in schizophrenia new approaches conference. Schizophr Bull 31:882–887
Penn DL, Sanna LJ, Roberts DL (2008) Social cognition in schizophrenia: an overview. Schizophr Bull 34:408–411
Lee TY, Hong SB, Shin NY, Kwon JS (2015) Social cognitive functioning in prodromal psychosis: a meta-analysis. Schizophr Res 164:28–34
Herold R, Tényi T, Lénárd K, Trixler M (2002) Theory of mind deficit in people with schizophrenia during remission. Psychol Med 32:1125–1129
Inoue Y, Yamada K, Hirano M (2006) Impairment of theory of mind in patients in remission following first episode of schizophrenia. Eur Arch Psychiatr Clin Neurosci 256:326–328
Pinkham AE (2014) Social cognition in schizophrenia. J Clin Psychiatr 75:14–19
Mehta UM, Thirthalli J, Naveen Kumar C, Keshav Kumar J, Keshavan MS, Gangadhar BN (2013) Schizophrenia patients experience substantial social cognition deficits across multiple domains in remission. Asian J Psychiatr 6:324–329
Rodríguez Sosa JT, Gil Santiago H, Trujillo Cubas A et al (2013) Social cognition in patients with schizophrenia, their unaffected first degree relatives and healthy controls. Comparison between groups and analysis of associated clinical and sociodemographic variables. Rev Psiquiatr Salud Ment 6:160–167
Sprong M, Schothorst P, Vos E, Hox J, Van Engeland H (2007) Theory of mind in schizophrenia: meta-analysis. Br J Psychiatr 191:5–13
Bora E, Yucel M, Pantelis C (2009) Theory of mind impairment in schizophrenia: meta-analysis. Schizophr Res 109:1–9
Bora E, Pantelis C (2013) Theory of mind impairments in first-episode psychosis, individuals at ultra-high risk for psychosis and in first-degree relatives of schizophrenia: systematic review and meta-analysis. Schizophr Res 144:31–36
Drury VM, Robinson EJ, Birchwood M (1998) ‘Theory of mind’ skills during an acute episode of psychosis and following recovery. Psychol Med 28:1101–1112
Pousa E, Duñó R, Brébion G, David AS, Ruiz AI, Obiols JE (2008) Theory of mind deficits in chronic schizophrenia: evidence for state dependence. Psychiatr Res 158:1–10
Balogh N, Egerházi A, Berecz R, Csukly G (2014) Investigating the state-like and trait-like characters of social cognition in schizophrenia: a short-term follow-up study. Schizophr Res 159:499–505
Maat A, van Montfort SJ, de Nijs J, GROUP Investigators et al (2015) Emotion processing in schizophrenia is state and trait dependent. Schizophr Res 161:392–398
Shaw P, Lawrence EJ, Radbourne C, Bramham J, Polkey CE, David AS (2004) The impact of early and late damage to the human amygdala on ‘theory of mind’ reasoning. Brain 127:1535–1548
Vohs JL, Lysaker PH, Liffick E et al (2015) Metacognitive capacity as a predictor of insight in first-episode psychosis. J Nerv Ment Dis 203:372–378
Korver N, Quee PJ, Boos HB, Simons CJ, De Haan L, Investigators GROUP (2012) Genetic risk and outcome of psychosis (GROUP), a multi-site longitudinal cohort study focused on gene-environment interaction: objectives, sample characteristics, recruitment and assessment methods. Int J Method Psychiatr Res 21:205–221
American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders. 4th ed., text revision. American Psychiatric Association, Washington
Andreasen NC, Flaum M, Arndt S (1992) The comprehensive assessment of symptoms and history (CASH). An instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatr 49:615–623
Andreasen NC, Carpenter WT Jr, Kane JM, Lasser RA, Marder SR, Weinberger DR (2005) Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatr 162:441–449
Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC (2010) Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatr 67:255–262
Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276
Buoli M, Caldiroli A (2015) IQ as a cognitive marker of genetic liability in relatives of schizophrenia patients. Am J Psychiatr 172:793–794
Blyler CR, Gold JM, Iannone VN, Buchanan RW (2000) Short form of the WAIS-III for use with patients with schizophrenia. Schizophr Res 46:209–215
Wechsler D (1997) WAIS-III: Wechsler adult intelligence scale, administration and scoring manual, 3rd edn. Psychol Corp, San Antonio
Saan R, Deelman B (1986) De 15-woordentest A en B (een voorlopige handleiding). Afdeling Neuropsychologie, AZG, Groningen
van’t Wout M, Aleman A, Kessels RP, Laroi F, Kahn RS (2004) Emotional processing in a non-clinical psychosis-prone sample. Schizophr Res 68:271–281
Swart M, Kortekaas R, Aleman A (2009) Dealing with feelings: characterization of trait alexithymia on emotion regulation strategies and cognitive-emotional processing. PLoS ONE 4:e5751
Faraone SV, Seidman LJ, Kremen WS, Toomey R, Pepple JR, Tsuang MT (2000) Neuropsychologic functioning among the nonpsychotic relatives of schizophrenic patients: the effect of genetic loading. Biol Psychiatr 48:120–126
Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ (2009) Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology 23:315–336
Sachs G, Steger-Wuchse D, Kryspin-Exner I, Gur RC, Katschnig H (2004) Facial recognition deficits and cognition in schizophrenia. Schizophr Res 68:27–35
Bora E, Gökçen S, Kayahan B, Veznedaroglu B (2008) Deficits of social-cognitive and social-perceptual aspects of theory of mind in remitted patients with schizophrenia: effect of residual symptoms. J Nerv Ment Dis 196:95–99
Fernandez-Gonzalo S, Pousa E, Jodar M, Turon M, Duño R, Palao D (2013) Influence of the neuropsychological functions in theory of mind in schizophrenia: the false-belief/deception paradigm. J Nerv Ment Dis 201:609–613
Fernandez-Gonzalo S, Jodar M, Pousa E et al (2014) Selective effect of neurocognition on different theory of mind domains in first-episode psychosis. J Nerv Ment Dis 202:576–582
Bozikas VP, Giannakou M, Kosmidis MH et al (2011) Insights into theory of mind in schizophrenia: the impact of cognitive impairment. Schizophr Res 130:130–136
Mehta UM, Thirthalli J, Subbakrishna DK, Gangadhar BN, Eack SM, Keshavan MS (2013) Social and neuro-cognition as distinct cognitive factors in schizophrenia: a systematic review. Schizophr Res 148:3–11
Pickup GJ, Frith CD (2001) Theory of mind impairments in schizophrenia: symptomatology, severity and specificity. Psychol Med 31:207–220
Leppänen JM, Niehaus DJ, Koen L, Du Toit E, Schoeman R, Emsley R (2006) Emotional face processing deficit in schizophrenia: a replication study in a South African Xhosa population. Schizophr Res 84:323–330
van’t Wout M, Aleman A, Kessels RP, Cahn W, De Haan EH, Kahn RS (2007) Exploring the nature of facial affect processing deficits in schizophrenia. Psychiatr Res 150:227–235
Goghari VM, Sponheim SR (2013) More pronounced deficits in facial emotion recognition for schizophrenia than bipolar disorder. Compr Psychiatr 54:388–397
Allott KA, Rice S, Bartholomeusz CF et al (2015) Emotion recognition in unaffected first-degree relatives of individuals with first-episode schizophrenia. Schizophr Res 161:322–328
Nielsen RE, Levander S, Kjaersdam Telléus G, Jensen SO, Østergaard Christensen T, Leucht S (2015) Second-generation antipsychotic effect on cognition in patients with schizophrenia–a meta-analysis of randomized clinical trials. Acta Psychiatr Scand 131:185–196
Acknowledgments
The infrastructure for the GROUP study is funded by the Geestkracht programme of the Dutch Health Research Council (ZON-MW, Grant Number 10-000-1002) and matching funds from participating universities and mental healthcare organizations (Site Amsterdam: Academic Psychiatric Centre AMC, Ingeest, Arkin, Dijk en Duin, Rivierduinen, Erasmus MC, GGZ Noord Holland Noord; Site Utrecht: University Medical Centre Utrecht, Altrecht, Symfora, Meerkanten, RIAGG Amersfoort, Delta; Site Groningen: University Medical Center Groningen, Lentis, GGZ Friesland, GGZ Drenthe, Dimence, Mediant, GGZ De Grote Rivieren and Parnassia Bavo Groep; Site Maastricht: Maastricht University Medical Center, GGZ Eindhoven en de Kempen, GGZ Midden-Brabant, GGZ Oost-Brabant, GGZ Noord-en Midden-Limburg, Mondriaan Zorggroep, Prins Clauscentrum Sittard, RIAGG Roermond, Universitair Centrum Sint-Jozef Kortenberg, CAPRI University of Antwerp, PC Ziekeren Sint-Truiden, PZ Sancta Maria Sint-Truiden, GGZ Overpelt, OPZ Rekem). The research leading to these results has received funding from the European Community’s Seventh Framework Program under Grant Agreement No. HEALTH-F2-2009-241909 (Project EU-GEI).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr Buoli, Dr Serati and Dr Caldiroli do not have any affiliation with or financial interest in any organization that might pose a conflict of interest with the present article. Prof. Altamura has served as a consultant or on Advisory Boards for Roche, Merck, AstraZeneca, Bristol Myers Squibb, Janssen/Cilag and Lundbeck. Dr Cahn has been an unrestricted research grant holder with Eli Lilly, BMS, Lundbeck, Sanofi-Aventis, Janssen-Cilag, AstraZeneca and Schering-Plough.
Rights and permissions
About this article
Cite this article
Caldiroli, A., Buoli, M., Serati, M. et al. General and social cognition in remitted first-episode schizophrenia patients: a comparative study. Eur Arch Psychiatry Clin Neurosci 266, 639–647 (2016). https://doi.org/10.1007/s00406-016-0701-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-016-0701-x