Abstract
Purpose
Recently, guidelines on the annotation of dynamic human embryo monitoring recommended screening for the presence of planar blastomere arrangement at the 4-cell stage. This observational study was set up in order to analyze whether developmental kinetics of planar human embryos are different from tetrahedral ones.
Methods
Therefore, embryos of 115 consecutive ICSI patients (showing 32 planar and 554 tetrahedral embryos) were cultured in a new time-lapse system (Miri TL) and their embryos were annotated for morphokinetic development and screened for irregular cleavages and morphological dysmorphisms.
Results
Significantly less planar embryos reached blastocyst stage and showed worse quality as compared to regular tetrahedral embryos. The rate of bi- and/or multinucleation was also significantly higher in the affected group. Irregular cleavages, particularly embryo rolling, were more often seen in planar embryos. Morphokinetics between planar and tetrahedral were distinguishable up to 4-cell stage (t2–t4), thereafter the observed delay in planar embryos (t8) was more likely the result of a higher rate of arrested embryos in the planar group.
Conclusions
Planar embryos are associated with both a significant increase in irregular cleavage as well as a delay in preimplantation development. This indicates that planar embryos are rather abnormal and should only be considered for transfer if no other embryos are available.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although a considerable proportion of embryo transfers is performed at blastocyst stage [1], number and quality of embryos, patient decision, or working load in the IVF laboratory force embryologist and clinicians to transfer at the cleavage stage (which is practically the period between days 2 and 4).
Cleavage is considered to be the period after fertilization when the zygote starts to develop into a multicellular organism. Due to mitotic cleavages the cytoplasmic volume of the fertilized ovum is divided into numerous smaller nucleated cells, called blastomeres [2]. From the beginning of assisted reproduction these cleavage stage embryos have been scored and chosen for intrauterine transfer according to their cell number and the percentage of cytoplasmic fragmentation [3, 4]. Through the years additional cleavage stage features were also thought to be of predictive value in terms of implantation, such as multinucleation [5], uneven blastomere cleavage [6], pattern of fragmentation [7], shape of the embryo [8], or cytoplasmic pitting [9], to name only a few. Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology [10] finally found a consensus what should be the minimum standard of reporting cleavage stage embryo quality. The parameters chosen were stage-specific cell number and size, fragmentation, and presence of multinucleation and, interestingly, all three of them have independent prognostic power to predict live birth [11].
Other authors more focused on dynamic events than relying on simple static embryo morphology. Lundin et al. [12] found that early mitosis to the two-cell stage is a strong biological indicator of embryo potential. Two groups [13, 14] used the visibility of blastomere nuclei, or the synchronicity of blastomere cell cycles to put it differently, to estimate implantation capacity.
Taking into account dynamic processes and combine this information with conventional embryo morphology [15] is the principle of “morphokinetics” which is about to take over the leading role in embryo scoring since time-lapse imaging is an increasingly upcoming and promising tool. Its common use led an expert time-lapse user group [16] to publish a consensus on how to annotate dynamic human embryo development. They also defined how to annotate exact cleavage timings, duration of cell cycles, and synchronicity of these events.
One important additional aspect of this pivotal paper is the characterization of irregular cleavages, all of which are thought to be negatively correlated with further outcome. While rapid cleavage [17], trichotomous mitosis [18], cell fusion [19], and embryo rolling [20] happen quite frequently, other phenomena, such as the planar arrangement of cells on day 2, are less often documented.
The latter cleavage pattern seems to be associated with a reduced implantation potential [21,22,23] and a causal relationship with aneuploidy was hypothesized [21]. So far no details on morphokinetics of such planar embryos were available. This lack of information led us to annotate morphokinetics of embryos showing planar arrangement of their four cells and to additionally screen them for morphological anomalies.
Methods
According to our internal standard operation procedure embryos or blastocysts which have been derived from planar embryos are not considered for transfer [21] and, consequently, no implantation data are available. Since time-lapse technology in our lab is routinely applied, we refrained from applying for IRB approval for the present observational study. Patients gave informed consent and were not charged for time-lapse imaging. Since this was an observational study and a rate of less than 5% of planar embryos was expected (based on Ebner et al. [21]) no power analysis was done; however, the rather low number of observations was considered during statistical evaluation.
In the study phase a total of 115 ICSI cases (which represented ca. 35% of all cycles treated in this 5-month period) were incubated in a rather new time-lapse incubator [24] characterized by separate incubation chambers (Miri TL®, Esco Medical, Egaa, Denmark). Decision which patients to incubate to the Miri TL was based on patients wish, availability of empty chambers and patient history. It is important to note that the patients were not randomized and tendentially more good prognosis patients (in terms of oocyte number only) were allocated to the time-lapse incubator in order to get more morphokinetical information up to blastocyst stage. All annotations were done by the same embryologist (TE). However, presence of planar and tetrahedral embryos (Fig. 1) was not only recorded in Miri TL patients, but also in those whose embryos were cultured in a conventional bench top incubator (MINC™, Cook Medical, Vienna, Austria). Although not part of this study, it should be highlighted that percentage of planar embryos was comparable in both types of incubators indicating that culturing in the Miri TL® did not induce planar cleavage.
Blastomere constellation on day 2 of preimplantation development. a Regular tetrahedral appearance. b Planar arrangement of blastomeres showing incomplete cleavage (study object). c Planar array of cells as the result of an ovoid-shaped zona pellucida. d Planar embryo with unsuspicious cleavage rotated by 90°. It should be noted that embryos depicted in c and d were not considered as study objects
The mean age of the 115 female partners was 32.8 ± 4.9 years. Approximately one-third of the patients suffered from secondary infertility and medical indication was as expected in average IVF clients (12% PCOS and 8% endometriosis). All but 5 partners (95.6%) suffered from male subfertility, but no cases of TESE were included.
Controlled ovarian hyperstimulation was performed using an antagonist protocol in the majority of cases (86.1%). Therefore, either recombinant FSH (Elonva® or Puregon®, MSD, Vienna, Austria) or a urinary product (Menopur®, Ferring, Vienna, Austria) was administered. In the long agonist scheme down regulation was done with Buserelin (Suprecur®, Sanofi-Aventis, Vienna, Austria), whereas the gonadotrophins were the same. On average, stimulation took 10.6 ± 1.8 days and the estradiol level on the day of ovulation induction was found to be 1954 ± 1232 pg/ml.
No later than 40 h after ovulation induction ICSI was performed. Immediately after ICSI all injected oocytes were loaded in a CultureCoin® (Esco Medical) which is the culture dish specially designed for the Miri TL®. Irrespective of the fact how many injected gametes had to be loaded all 14 wells of the CultureCoin® were filled with 30 µl of EmbryoAssist medium (Origio, Berlin, Germany) under mineral oil (Gynemed, Lensahn, Germany) in order to allow for proper preincubation (a minimum of 2 h was required as assessed during process validation) in a milieu of 5% oxygen and 6.8% CO2. As recommended by an experienced time-lapse user group [16], t0 (start of ICSI) was set as the mid-point from when injection begins and ends. This time point is used as the start of the video sequence and all annotated morphokinetic parameters refer to this event (measured in hours post-injection).
Images were taken by a built-in Zeiss objective (× 20) with a numerical aperture of 0.35 specialized for 635 nm illumination using red light. Video sequences consisted of more than 10,000 images (120 h, 7 focal planes, image frequency 5 min). All videos were analyzed with the Miri® TL Viewer software (Supplementary Videos 1 and 2). Variables were annotated according to previously published guidelines [16] and included t2PB, tPNa, tPnf, t2-t8, tSC, tSB, and tB. Special care was taken to identify irregular cleavage events such as embryo rolling, trichotomous mitotic divisions, rapid cleavage, and cell fusion. In addition, it was screened for morphological abnormalities with known correlation to aneuploidy or developmental arrest/delay (pronuclear arrangement, bi/multinucleation, uneven cleavage).
Fertilization check was carried out in the morning of day 1 (16–20 h post-injection). Incorrect PN arrangement was noted once PN were located in the periphery or did not show contact. Further cleavage up to blastocyst stage was scored according to Alpha and ESHRE guidelines [10]. All scoring was done using the video sequences. It is noteworthy to mention that on day 3 of in vitro culture embryos were transferred to fresh preincubated CultureCoins® with 30 µl droplets of BlastAssist medium (Origio).
Although the vast majority of cases resulted in blastocyst transfers (n = 109), six patients had all their embryos vitrified on day 5 due to OHSS or endometrial deficiencies, however, this scenario did not affect our data set, since all planar and tetrahedral embryos could be followed up to 120 h post-ICSI, thus, no data were lost. It is important to note that no embryo transfer was canceled due to an impaired morphokinetic development.
Statistics
A Fisher’s exact test was performed for the analysis of contingency tables. It is noteworthy, that a logarithmic transformation of the time data was done in order to ensure homogeneity of error variance (Levene’s test) as well as normal distribution of the residuals (Kolmogorov–Smirnov test). We performed an analysis of variance (ANOVA) on the transformed data (factors: type, arrest, and the interaction factor type*arrest as well as subject and subject*type to account for individual patients). A Bonferroni correction for post hoc analysis was applied. As usual the level of significance was P < 0.05. All analyses were done using MatLab 2014b and SPSS 23.0.
Results
Within the study period in 24 patients a total of 32 embryos were found to be planar which represented 5.5% of all embryos (n = 586) cultured in the time-lapse incubator. As indicated in Table 1, approximately every fourth embryo (32/134) was found to have planar arrangement of blastomeres in affected patients (showing at least one planar embryo).
Tetrahedral embryos significantly more often survived until blastocyst stage and showed better quality blastocysts as compared to planar embryos (P < 0.05). This could be correlated to higher rates of bi- or multinucleation (P < 0.001) in planar embryos. Tendentially, uneven cleavage (P = 0.06) and incorrect PN arrangement (P = 0.09) were also more frequently observed in planar embryos.
Apart from these morphological parameters, morphokinetics in planar embryos were also negatively affected. In particular, embryo rolling (indicating cleavage problems) was observed more frequently (P < 0.001). All in all, Table 1 highlights that significantly less planar embryos were characterized by an inconspicuous development neither showing morphological nor morphokinetical anomalies compared to the tetrahedral group in which close to 80% of the embryos showed normal cleavage behavior (P < 0.001). Developmental stop in embryos showing “embryo rolling” was not related to blastomere arrangement on day 2 since planar (9/13) and tetrahedral (4/6) embryos showed similar rates of arrest.
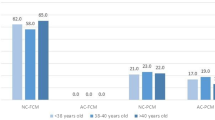
Post hoc analysis revealed that in the case of embryo arrest times of t3 (P = 0.049), t7 (P = 0.022), and t8 (P = 0.01) were significantly delayed in planar embryos as compared to normal tetrahedral ones. As indicated in Fig. 2, the difference between the two subtypes of cleaved embryos was significant for the times t2 (P = 0.046), t3 (P = 0.015), t4 (P = 0.001), and t8 (P = 0.017).
Conclusions
With the use of a new time-lapse imaging system, the present study investigated in detail the prevalence and dynamic changes of planar embryos. Normal day-2 embryos show a tetrahedral arrangement of four cells with three blastomeres lying side by side (Fig. 1a). The said constellation is the result of two sequential cleavages, a meridional one followed by an equatorial cleavage or vice versa [2].
Cleavage anomalies include embryos that are characterized by a particular planar constellation of four blastomeres with presumed incomplete cleavage (Fig. 1b). Incomplete cleavage is not only supported by the presence of a consistent contact area (resulting in the loss of the spherical shape of blastomeres), but also by fluorescence studies [25]. Gardner [25] highlighted that an injected dye in 4-cell planar embryos spread to all three sister blastomeres (indicating a persistent bridge between all four cells), whereas in tetrahedral embryos spreading of the fluorescent label was restricted to the immediate daughter cell.
A sperm centriole defect has been suggested to cause this particular type of 4-cell arrangement [21], which can be characterized by the sequence of two consecutive meridional or equatorial cleavages [2, 26].
In mouse, such a series of two aligned cleavages may induce that progeny of the blastomeres populate different regions of the blastocyst which in turn may result in lower implantation and live-birth rates [27, 28]. The same negative correlation has been documented for human embryos [21, 22]. The reason for this negative impact of the planar blastomere constellation on implantation is unclear; however, there is evidence that aneuploidy plays a major role [21]. This scenario led Ciray et al. [16] to recommend the annotation of a planar arrangement of cells on observation (tPA).
Theoretically, the altered orientation of the cleavage planes in planar embryos may cause aberrant distributions of animal and vegetal ooplasm, including cell organelles and maternally inherited transcripts/proteins, between the blastomeres [26, 29, 30]. This would in part explain the impaired cleavage to blastocyst stage in non-tetrahedral embryos [21, 26] if one is an advocate of the “pre-patterning model” [25, 27, 28]. For the sake of completeness it should be clarified that others [31, 32] do not support the idea that the axes of a mouse blastocyst are anticipated at oocyte stage. Also in human, the pre-patterning model was questioned because at 4-cell stage all individual blastomeres had the potential to develop into both cell lineages at blastocyst stage [33, for review see 34]. Typically, those planar embryos that make it to the blastocyst stage are characterized by a circular arrangement of blastomeres on day 3 [21].
Alternatively, any decline or delay in blastulation can be the manifestation of a reduction in cell–cell contacts which are a prerequisite for timed compaction and blastulation [8]. Liu et al. [23] elegantly highlighted the clinical significance of intracellular contact points at four-cell stage using time-lapse imaging. Once again they highlighted that fewer than six cell–cell contacts at the end of the four-cell stage (corresponding to non-tetrahedral formation of blastomeres as shown in Fig. 1c, d) are associated with a compromised subsequent development and reduced implantation potential.
However, they [23] also pointed out the main problem that goes with all studies on planar embryos which is the inconsistency in defining planar arrangement of blastomeres on day 2 [30]. Indeed, Cauffman et al. [26] overgeneralized the morphological dysmorphism of non-tetrahedral arrangement of blastomeres since they collectively analyzed “regular” planar embryos (Fig. 1c, 1d) with four one-layered blastomeres lying side by side [8] and planar embryos with “incomplete cleavage” (Fig. 1b) having the shape of a “tetrafoliate clover” [21]. The obvious misinterpretation of planarity within embryologists may explain differences in incidence and outcome [21, 22, 26, 35].
Interestingly, in a recent time-lapse study it could be demonstrated that two out of three planar embryos corrected their suboptimal planar structure by establishing a tetrahedral arrangement (with six intracellular contacts) by the end of the four-cell stage [23]. In detail, video sequences revealed that in such a case blastomeres gradually established contact by migrating towards each other.
It is important to note that the present work exclusively deals with planar embryos showing incomplete cleavage and the shape of a tetrafoliate clover. The fact that none of the studied planar 4-cell embryos corrected (or could not correct) its constellation of blastomeres once more emphasizes the existence of two different entities of planar embryos that should not be mixed up.
Since embryo development is a dynamic process embryo appearance can change with time. Thus, it is very likely that the actual spatial configuration of cells can rarely be detected via static snapshot observations. Time-lapse imaging here offers a powerful tool in order to accurately follow dynamic events. Particularly, bi- and multinucleated blastomeres are often missed if embryos are scored only once a day. Goodman et al. [36], e.g., observed a fivefold increase in the prevalence of multinucleation when they retrospectively reviewed their embryos using time-lapse technique. Ergin et al. [37] would have missed approximately 70% of all multinucleated embryos by simply relying on static observations once a day. These papers nicely explain why our previous (static) results on the rate of multinucleation [21] could not be confirmed in the present data set working with a highly dynamic technique such as time-lapse imaging.
To our best knowledge, this is the first morphokinetic study exclusively focusing on a particular planar embryo type with bad prognosis [21]. In the present study, the presence of significantly more irregular cleavages and morphological dysmorphisms related to genetical constitution suggests deselecting clover-shaped planar embryos because there could be the theoretical risk of aneuploidy. They should only be considered for transfer if no other embryos are available.
Although using time-lapse technique, the frequency of multinucleation was comparable between euploid and aneuploid embryos [38], it was found to be increased in embryos that showed rapid cleavage [19], a phenomenon which has previously been reported to have an adverse effect on subsequent implantation [17]. Above that, morphokinetics between tPNf and t5 were significantly delayed by 1–2.5 h [38], a finding which further confirms our results. Another explanation for the developmental delay and subsequent arrest in the present manuscript comes from Joergensen et al. [39] who published that 3PN ICSI zygotes more often show early cleavage to three cells, but then are characterized by a longer stay at the 3-cell stage which precedes embryonic delay or arrest. This scenario strongly resembles the one seen in embryos with trichotomous mitosis which in the present study was observed twice as often in planar than in tetrahedral embryos. Whichever phenomenon may gain influence on preimplantation development, present results indicate that in planar embryos developmental problems emerge rather early, e.g., between t2 and t4 while the later differences in timing are the result of a higher rate of embryo arrest in planar embryos. Aberrant fertilization (e.g., the sporadic presence of a single pronucleus) further contributes to developmental delay/arrest since it takes some time to rearrange for 2PNa and t2.
The causal relationship between aneuploidy and morphokinetics discussed is highly controversial and so far optimistic publications [40, 41] and reluctant ones [42, 43] balance each other. The most prominent work indicating such a correlation was published by Campbell et al. [44] identifying early initiation of blastulation (tSB) and time to reach full blastocyst stage (tB) as major predictors of euploidy. Our data stress that planar embryos with incomplete cleavage also belong to the group of aneuploid embryos that might be identified by the help of time-lapse imaging.
However, with all the effort which is made to deselect potentially aneuploid embryos, one should not forget that some abnormally cleaving embryos are able to produce live-births [18, 45, 46]. Such results might raise awareness of avoiding hasty disposal of embryos with suboptimal early cleavage events.
References
Wirleitner B, Schuff M, Stecher A, Murtinger M, Vanderzwalmen P (2016) Pregnancy and birth outcomes following fresh or vitrified embryo transfer according to blastocyst morphology and expansion stage, and culturing strategy for delayed development. Hum Reprod 31:1685–1695
Ajduk A, Zernicka-Goetz M (2015) Polarity and cell division orientation in the cleavage embryo: from worm to human. Mol Hum Reprod 22:691–703
Erenus M, Zouves C, Rajamahendran P, Leung S, Fluker M, Gomel V (1991) The effect of embryo quality on subsequent pregnancy rates after in vitro fertilization. Fertil Steril 56:707–710
Giorgetti C, Terriou P, Auquier P, Hans E, Spach SL, Salzmann J et al (1995) Embryo score to predict implantation after in vitro fertilization: based on 957 single embryo transfers. Hum Reprod 10:2427–2431
De Cássia Savio Figueira R, Souza Setti A, Paes De Almeida Ferreira Braga D, Iaconelli A Jr, Borges E Jr (2010) Blastomere multinucleation: contributing factors and effects on embryo development and clinical outcome. Hum Fertil (Camb) 13:143–150
Hardarson T, Hanson C, Sjögren A, Lundin K (2001) Human embryos with unevenly sized blastomeres have lower pregnancy and implantation rates: indications for aneuploidy and multinucleation. Hum Reprod 16:313–318
Alikani M, Cohen J, Tomkin G, Garrisi GJ, Mack C, Scott RT (1999) Human embryo fragmentation in vitro and its implications for pregnancy and implantation. Fertil Steril 71:836–842
Ebner T, Shebl O, Moser M, Sommergruber M, Tews G (2008) Developmental fate of ovoid oocytes. Hum Reprod 23:62–66
Ebner T, Tews G, Sommergruber M, Moser M (2005) Cytoplasmic pitting has a negative influence on implantation outcome. J Assist Reprod Genet 22:239–244
Alpha Scientists in Reproductive Medicine, ESHRE Special Interest Group of Embryology (2011) The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Reprod Biomed Online 22:632–646
Rhenman A, Berglund L, Brodin T, Olovsson M, Milton K, Hadziosmanovic N et al (2015) Which set of embryo variables is most predictive for live birth? A prospective study in 6252 single embryo transfers to construct an embryo score for the ranking and selection of embryos. Hum Reprod 30:28–36
Lundin K, Bergh C, Hardarson T (2001) Early embryo cleavage is a strong indicator of embryo quality in human IVF. Hum Reprod 16:2552–2557
Moriwaki T, Suganuma N, Hayakawa M, Hibi H, Katsumata Y, Oguchi H et al (2004) Embryo evaluation by analyzing blastomere nuclei. Hum Reprod 19:152–156
Saldeen P, Sundström P (2005) Nuclear status of four-cell preembryos predicts implantation potential in in vitro fertilization treatment cycles. Fertil Steril 84:584–589
Lemmen JG, Agerholm I, Ziebe S (2008) Kinetic markers of human embryo quality using time-lapse recordings of IVF/ICSI-fertilized oocytes. Reprod Biomed Online 17:385–391
Ciray HN, Campbell A, Agerholm IE, Aguilar J, Chamayou S, Esbert M et al (2014) Time-Lapse User Group. Proposed guidelines on the nomenclature and annotation of dynamic human embryo monitoring by a time-lapse user group. Hum Reprod 29:2650–2660
Rubio I, Kuhlmann R, Agerholm I, Kirk J, Herrero J, Escribá MJ et al (2012) Limited implantation success of direct-cleaved human zygotes: a time-lapse study. Fertil Steril 98:1458–1463
Almagor M, Or Y, Fieldust S, Shoham Z (2015) Irregular cleavage of early preimplantation human embryos: characteristics of patients and pregnancy outcomes. J Assist Reprod Genet 32:1811–1815
Liu Y, Chapple V, Roberts P, Matson P (2014) Prevalence, consequence, and significance of reverse cleavage by human embryos viewed with the use of the embryoscope time-lapse video system. Fertil Steril 102(1295–1300):e2
Cruz M, Garrido N, Herrero J, Pérez-Cano I, Muñoz M, Meseguer M (2012) Timing of cell division in human cleavage-stage embryos is linked with blastocyst formation and quality. Reprod Biomed Online 25:371–381
Ebner T, Maurer M, Shebl O, Moser M, Mayer RB, Duba HC et al (2011) Planar embryos have poor prognosis in terms of blastocyst formation and implantation. Reprod Biomed Online 25:267–272
Paternot G, Debrock S, De Neubourg D, D’Hooghe TM, Spiessens C (2014) The spatial arrangement of blastomeres at the 4-cell stage and IVF outcome. Reprod Biomed Online 28:198–203
Liu Y, Chapple V, Roberts P, Matson P (2015) Clinical significance of intercellular contact at the four-cell stage of human embryos, and the use of abnormal cleavage patterns to identify embryos with low implantation potential: a time-lapse study. Fertil Steril 103(1485–1491):e1
Ebner T, Oppelt P, Radler E, Allerstorfer C, Habelsberger A, Mayer RB et al (2016) Morphokinetics of vitrified and warmed blastocysts predicts implantation potential. J Assist Reprod Genet 34:239–244
Gardner RL (2002) Experimental analysis of second cleavage in the mouse. Hum Reprod 12:3178–3189
Cauffman G, Verheyen G, Tournaye H, Van de Velde H (2014) Developmental capacity and pregnancy rate of tetrahedral-versus non-tetrahedral-shaped 4-cell stage human embryos. J Assist Reprod Genet 31:427–434
Piotrowska-Nitsche K, Zernicka-Goetz M (2005) Spatial arrangement of individual 4-cell stage blastomeres and the order in which they are generated correlate with blastocyst pattern in the mouse embryo. Mech Dev 122:487–500
Piotrowska-Nitsche K, Perea-Gomez A, Haraguchi S, Zernicka-Goetz M (2005) Four-cell stage mouse blastomeres have different developmental properties. Development 132:479–490
Antczak M, Van Blerkom J (1997) Oocyte influences on early development: the regulatory proteins leptin and STAT3 are polarized in mouse and human oocytes and differentially distributed within the cells of the preimplantation stage embryo. Mol Hum Reprod 3:1067–1086
Edwards RG, Beard HK (1997) Oocyte polarity and cell determination in early mammalian embryos. Mol Hum Reprod 3:863–905
Hiiragi T, Solter D (2004) First cleavage plane of the mouse egg is not predetermined but defined by the topology of the two apposing pronuclei. Nature 430(6997):360–364
Motosugi N, Bauer T, Polanski Z, Solter D, Hiiragi T (2005) Polarity of the mouse embryo is established at blastocyst and is not prepatterned. Genes Dev 19:1081–1092
Van de Velde H, Cauffman G, Tournaye H, Devroey P, Liebaers I (2008) The four blastomeres of a 4-cell stage human embryo are able to develop individually into blastocysts with inner cell mass and trophectoderm. Hum Reprod 23:1742–1747
De Paepe C, Krivega M, Cauffman G, Geens M, Van de Velde H (2014) Totipotency and lineage segregation in the human embryo. Mol Hum Reprod 20:599–618
Ebner T, Oppelt P, Mayer RB, Shebl O (2014) Developmental capacity and pregnancy rate of tetrahedral-versus non-tetrahedral-shaped 4-cell stage human embryos. J Assist Reprod Genet 31:621
Goodman LR, Goldberg J, Falcone T, Austin C, Desai N (2016) Does the addition of time-lapse morphokinetics in the selection of embryos for transfer improve pregnancy rates? A randomized controlled trial. Fertil Steril 105:275–285
Ergin EG, Calişkan E, Yalçinkaya E, Oztel Z, Cökelez K, Ozay A et al (2014) Frequency of embryo multinucleation detected by time-lapse system and its impact on pregnancy outcome. Fertil Steril 102(1029–1033):e1
Balakier H, Sojecki A, Motamedi G, Librach C (2016) Impact of multinucleated blastomeres on embryo developmental competence, morphokinetics, and aneuploidy. Fertil Steril 106(608–614):e2
Joergensen MW, Agerholm I, Hindkjaer J, Bolund L, Sunde L, Ingerslev HJ et al (2014) Altered cleavage patterns in human tripronuclear embryos and their association to fertilization method: a time-lapse study. J Assist Reprod Genet 31:435–442
Yang Z, Zhang J, Salem SA, Liu X, Kuang Y, Salem RD et al (2014) Selection of competent blastocysts for transfer by combining time-lapse monitoring and array CGH testing for patients undergoing preimplantation genetic screening: a prospective study with sibling oocytes. BMC Med Genom 7:38
Chawla M, Fakih M, Shunnar A, Bayram A, Hellani A, Perumal V et al (2015) Morphokinetic analysis of cleavage stage embryos and its relationship to aneuploidy in a retrospective time-lapse imaging study. J Assist Reprod Genet 32:69–75
Kramer YG, Kofinas JD, Melzer K, Noyes N, McCaffrey C, Buldo-Licciardi J et al (2014) Assessing morphokinetic parameters via time lapse microscopy (TLM) to predict euploidy: are aneuploidy risk classification models universal? J Assist Reprod Genet 31:1231–1242
Rienzi L, Capalbo A, Stoppa M, Romano S, Maggiulli R, Albricci L et al (2015) No evidence of association between blastocyst aneuploidy and morphokinetic assessment in a selected population of poor-prognosis patients: a longitudinal cohort study. Reprod Biomed Online 30:57–66
Campbell A, Fishel S, Bowman N, Duffy S, Sedler M, Hickman CF (2013) Modelling a risk classification of aneuploidy in human embryos using non-invasive morphokinetics. Reprod Biomed Online 26:477–485
Stecher A, Vanderzwalmen P, Zintz M, Wirleitner B, Schuff M, Spitzer D et al (2014) Transfer of blastocysts with deviant morphological and morphokinetic parameters at early stages of in vitro development: a case series. Reprod Biomed Online 28:424–435
Fan YL, Han SB, Wu LH, Wang YP, Huang GN (2016) Abnormally cleaving embryos are able to produce live births: a time-lapse study. J Assist Reprod Genet 33:379–385
Acknowledgements
The authors would like to thank G. Schappacher-Tilp for statistical expertise.
Author information
Authors and Affiliations
Contributions
TE: manuscript writing, data management. AH: data collection. PO: data management, manuscript editing. ER: manuscript editing. SHE: data collection. RBM: data collection. EP: protocol development, data analysis. OS: manuscript editing, project development.
Corresponding author
Ethics declarations
Funding
To ensure objectivity and transparency in research it is stated that no funding was received for this study.
Conflict of interest
None of the authors/coauthors declared a conflict of interest related to this study.
Ethical approval
As mentioned in the “Methods” section time-lapse technology in our lab is routinely applied, therefore we refrained from applying for IRB approval for the present observational study.
Informed consent
Patients gave informed consent and were not charged for time-lapse imaging. In no case affected embryos were transferred. No animals were involved.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Video 1. Planar arrangement of cells. It should be noted that oocyte presents 1PN between 14h and 30h before 2Pn formed (35h). t2 (44.8h), t3 (57.8h), and t4 (58.5h) are delayed and embryo development stopped at 6-cell stage (MP4 17760 kb)
Video 2. Regularly fertilized zygote (2Pn) resulting in planar arrangement of cells (43.4h). At 112.6h early morula stage is reached upon which development stopped (MP4 38041 kb)
Rights and permissions
About this article
Cite this article
Ebner, T., Höggerl, A., Oppelt, P. et al. Time-lapse imaging provides further evidence that planar arrangement of blastomeres is highly abnormal. Arch Gynecol Obstet 296, 1199–1205 (2017). https://doi.org/10.1007/s00404-017-4531-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-017-4531-5