Abstract
Purpose
This study investigated the prevalence of abnormally cleaved embryos and determined which types of abnormally cleaved embryos (1-3c, 2-4c, 3-5c, 4-6c), might be suitable for transfer based on live birth data.
Methods
One hundred seventy-one women (whose transferred embryos were confirmed to be either fully implanted or fully unimplanted) provided 1256 embryos, which were analyzed.
Results
Of these embryos, 320 embryos were transferred, of these transferred embryos, 291 embryos were normal and 29 embryos were abnormal, which five embryos were not analyzed because each one was presented one abnormal cleavage type. These 24 embryos were divided into four groups. Inclusion criteria were as follows: women under 37 years of age undergoing first fresh in vitro fertilization (IVF) treatment with a basal antral follicle count of 5–15, body mass index (BMI) of 18–25 kg/m2, number of retrieved oocytes between 5 and 20, and tubal factors as the cause of infertility. Time-lapse imaging analysis software was used to compare temporal parameters of normal cleavage and abnormal cleavage groups (there were four abnormal groups, based on the prevalence of abnormal cleavage embryos). Cleavage times were analyzed before the abnormal cleavage occurred, and time intervals were analyzed after the abnormal cleavage based upon the types of abnormal cleavage. In addition, the time intervals of t4-t3 and t8-t5 were also analyzed; corresponding time parameters were measured in the normal group as well. Implantation rate, clinical pregnancy rate, ongoing pregnancy rate, and live birth rate were also measured in the normally cleaved and abnormally cleaved embryos. The prevalence of abnormal cleavage was 15.92 % (200/1256). T8-t5 was the most important parameter in the prediction of potential development (production of a live-born baby) of abnormally cleaving embryos.
Conclusions
Abnormally cleaving embryos were able to produced live births with T8-t5 the best parameter to predict the developmental potential of abnormally cleaving embryos.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The main challenges in assisted reproductive technology (ART) are identifying and selecting embryos with the highest developmental competence and ultimately improving the probability of pregnancy and live birth rate. Currently, most IVF laboratories in the world believe that standard morphologic assessment is the gold standard; this is based upon the number and symmetry of blastomeres, the degree of fragmentation, presence and number of nuclei, and size per embryo at a few static time points [1, 2]. However, the development of embryos is a dynamic process, and embryonic morphology may change markedly over a few hours; subtle changes, then, may decide the fate of the embryos without being detected [3, 4]. To remedy this situation, time-lapse imaging, a tool that can continuously and non-invasively monitor embryos, is emerging [5–7].
Time-lapse imaging is a non-invasive system that allows for continuous embryonic monitoring, maintenance of optimal culture conditions, and detection of abnormal events. Since Payne and colleagues [8] applied the technique to humans, many parameters have been proposed for embryonic selection in order to improve success rates. Using time-lapse technology, we can clearly investigate abnormal events. Abnormally cleaving embryos are defined as having more than two cells that originated from a single cell division event (newly created blastomeres are completely separated by confluent cell membranes at the end of the division) [9]. We defined 1-3c abnormal cleavage (AC1-3C) as one-cell embryos developing to three cells directly; 2-4c abnormal cleavage (AC2-4C) was defined as embryos developing from two cells to four cells directly; 3-5c abnormal cleavage (AC3-5C) was defined as embryos going from three cells to five cells directly; and 4-6c abnormal cleavage (AC4-6C) was defined as embryos going from four cells to six cells directly (Fig. 1). Implantation was measured using serum hCG concentrations 14 days after embryo transfer. Clinical pregnancy was confirmed by the presence of gestational sacs using ultrasonographic examination at week 7. Ongoing pregnancy was confirmed at 12 weeks. The live birth was also used as a clinical outcome. There are no reports in the literature that correlate abnormal cleavage embryos with implantation rate, clinical pregnancy rate, ongoing pregnancy rate, or live-birth rate.
In this study, outcome rates were reported for both normally and abnormally cleaving embryos. The four most prevalent types of abnormally cleaving embryos were investigated, and these were analyzed to determine their relationship with implantation rate, clinical pregnancy rate, ongoing pregnancy rate, and live birth rate, with the aim of uncovering an index that can be used to improve potential development in abnormally cleaving embryos.
Materials and methods
Overall study design
The inclusion date were women under 37 years of age undergoing first fresh in vitro fertilization (IVF) treatment with a basal antral follicle count of 5–15, body mass index (BMI) of 18–25 kg/m2, number of retrieved oocytes between 5 and 20, with tubal factors as the cause of infertility, and men had normal sperm were enrolled in our study; conditions such as genetic disease and ovarian and uterine factor infertility were excluded. Embryos that passed a standard morphologic assessment were transferred [1, 2]. Only when the oocyte was fertilized (assessed as two pronuclear embryos after IVF), the embryo(s) was selected to be imaged. Embryo assessment was according to the criterion of Dale [10]. Embryos with more than seven cells on day 3, fairly equal size blastomeres, few or no cytoplasmic fragments were defined as good quality embryos (grade 1). According to Dale criteria, embryos with grades 1~3 were transferrable embryos.
All the embryos were transferred on day 3. The staff members performing the transfer were blinded to the time-lapse imaging results, and all cleavage times were recorded by a single observer.
This was a retrospective study using a time-lapse imaging system (Primo Vision, Vitrolife kft, Sweden). A total of 171 patients undergoing IVF treatment, whose transferred embryos showed complete implantation or complete failure of implantation between January 2014 and December 2014, were monitored in the study. All procedures and protocols were approved by the ethics committee of the Chongqing Obstetric and Gynecology Hospital. A total of 1256 derived embryos were monitored to analyze the prevalence and the composition of abnormal cleavage. Among them, 320 transferred embryos showed complete implantation or complete failure of implantation; and of these, 291 embryos were normal, and 29 embryos were abnormal. These embryos were then measured to compare temporal parameters (five embryos were excluded because each showed one abnormal cleavage type). Of the 320 transferred embryos, 176 embryos were implanted, and 144 embryos were not implanted. The 24 abnormally cleaving embryos were divided into the AC1-3C group, AC2-4C group, AC3-5C group, and AC4-6C group to compare temporal parameters with normally cleaved embryos. The five types of abnormally cleaving embryos were rare (1-4c; 1-3c, 4-6c; 1-5c, 7-9c; 3-5c-7c; 6-8c), and because each type had only one embryo, these were not analyzed statistically, although 6-8c embryos implanted and eventually produced a single live-birth baby. Cleavage times were analyzed before the abnormal cleavage, and time intervals after cleavage based on the types of abnormal cleavage were also analyzed; in addition, the time intervals of t4-t3 and t8-t5 (duration of cultured development) that reflected the synchronicity of the embryos were also analyzed. We then calculated the implantation rate per embryo, the clinical pregnancy rate per patient, the ongoing pregnancy rate per patient, and the live birth rate per patient for each normal cleavage group and abnormal cleavage group.
Ovarian stimulation and oocyte retrieval
Patient ovarian stimulation was carried out as previously described [11]. Briefly, when at least three follicles have reached a mean diameter of 18 mm, 250-mcg HCG (Ovidrel, Merck Serono, Italy) was administered intramuscularly to induce final follicular and oocyte maturation. Oocyte retrieval was carried out by transvaginal ultrasound-guided follicle aspiration 36 h after HCG injection.
Embryo culture
After retrieval, the cumulus-corona-oocyte complexes were incubated in culture for 2–3 h with G-IVF medium (Vitrolife, Kungsbacka, Sweden) at 37.0 °C, in 6.0 % CO2 and 5.0 % O2 in compressed air at high humidity. Oocytes were inseminated with 10,000 motile spermatozoa/ml medium based on IVF procedures which were defined as the fertilization time, and after 4 h, the cumulus cells were mechanically removed to achieve denudation. After confirming fertilization, the zygotes were cultured in microdroplets of culture medium (Vitrolife) in the time-lapse system individually until day 3 of development.
Time-lapse analysis and recording of kinetic parameters
We calculated the time from insemination of spermatozoa for each embryo. Time-lapse images of each embryo were retrospectively analyzed using an Embryo Viewer software workstation. Images were achieved every 5 min in 10 different focal planes during 45 h of culture. In the abnormal groups, cleavage times and intervals were analyzed before the abnormal cleavage and after the abnormal cleavage based on the types of abnormal cleavage; additionally, we measured the time reflecting synchronicity of the embryos. In the normal group, the corresponding time parameters were also measured as well.
Statistical analyses
The Student’s t test was used for comparing the age, FSH, LH, AMH, and the time points,Mann-Whitney U test was used for the time intervals. Data are presented as mean ± SD. P < 0.05 was considered to be statistically significant. Statistical analysis was performed using the Statistical Package for the Social Science 17.0 (SPSS, Inc.).
Results
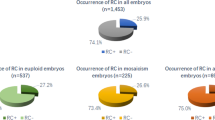
A total of 1256 embryos were cultured inside the time-lapse system to analyze the prevalence and the proportion of abnormal cleavage. The prevalence of abnormal cleavage was 15.92 % (200/1256), and the percentages of AC1-3C, AC2-4C, AC3-5C, AC4-6C, others embryos were 32 % (64/200), 22.50 % (45/200), 19.50 % (39/200), 7.5 % (15/200), 18.5 % (37/200), respectively (Fig. 2). The age, FSH, LH, and AMH in normal cleavage (NC) group and abnormal cleavage (AC) groups were listed as Table 1. The implantation rate and clinical pregnancy rate, ongoing pregnancy rate, live birth rate in the normal cleavage, and abnormal cleavage groups are shown in Table 2. The timing of morphokinetic events, including cell division timing (t2cb, the beginning of the 2-cell stage; t2ce, the end of the 2-cell stage; t3, time to the 3-cell stage; t4, time to the 4-cell stage; t5, time to the 5-cell stage; t6, time to the 6-cell stage; t4-t3, time interval between the 3- and 4-cell stages; t5-t4, time interval between the 4- and 5-cell stages; t6-t5, time interval between the 5- and 6-cell stages; t7-t6, time interval between the 6- and 7-cell stages; t8-t7, time interval between the 7- and 8-cell stages; s2 = t4-t3, from 2-cell to 4-cell; and s3 = t8-t5, from 4-cell to 8-cell) for all embryos, and corresponding parameters in the normal group, were also measured (Table 3). We compared normally cleaving with abnormally cleaving embryos, and found t8-t5, the cleavage synchronicity from four to eight cells) to differ significantly: 13.37 ± 6.68 vs. 4.99 ± 4.73 (P ≤ 0.00); 13.80 ± 9.63 vs. 4.99 ± 4.73 (P ≤ 0.00); 12.06 ± 6.70 vs. 4.99 ± 4.73 (P ≤ 0.00); 13.80 ± 9.63 vs. 4.99 ± 4.73 (P ≤ 0.00); 2.61 ± 0.48 vs. 4.99 ± 4.73 (P ≤ 0.32) in 1-3c, 2-4c, 3-5c, and 4-6c, respectively. Of the transferred embryos only, the implantation rate, clinical pregnancy rate, ongoing pregnancy rate, and live birth rate in AC1-3C, AC2-4C, AC3-5C, and AC4-6C are shown in Table 4. In the AC1–3C group, t3, t4-t3, and t5-t4 were found to be significantly different from the normal cleavage group; in the AC2–4C group, t4-t3 was found to be significantly different from the normal cleavage group and in the AC3-5C group, t3 and t5 were found to be significantly different from the normal cleavage group.
Discussion
The objective of this study was to review embryonic time-lapse videos (1256 embryos) to investigate the prevalence of abnormally cleaving embryos and evaluate four categories of abnormally cleaving embryos (1-3c, 2-4c, 3-5c, 4-6c), particularly to detect whether all types are not suitable for transfer. In this study, based on the prevalence of abnormal cleavage, time-lapse technology was combined with observation of these four groups to compare the morphokinetic behavior of normal cleavage and abnormally cleaving embryos, and then compared the implantation rate, clinical pregnancy rate, ongoing rate, and live-birth rate in the four types of abnormally cleaving embryos, respectively.
Embryos showing an abnormal cleavage phenotype have been previously shown to correlate with poor developmental and implantation potential [9, 12, 13], and our results are consistent with these studies. Meseguer et al. observed that several abnormal division behaviors including the direct cleavage from the zygote into a three-blastomere embryo (1C–3C), uneven blastomere size at the two-cell stage, and multinucleation at the four-cell stage were detrimental for the further development of cleavage embryos to the blastocyst stage [12]. Rubio et al. observed 109 of the transferred embryos that directly cleaved (“direct cleavage” defined as less than 5-h in duration from the two- to three-cell stages), but only one DC2–3 embryo was known to result in a clinical pregnancy (1 %), and 80 (73.4 %) DC2–3 embryos did not implant; in addition, the known implantation rate of the DC2–3 embryos was significantly lower than for embryos with a normal cleavage pattern (1.2 vs. 20.2 %, respectively). Kelly et al. observed two independent types of atypical cleavage (AC1 and AC2) and transferred 27 AC embryos, but only two implanted [9]. More recently, Yang et al. reported that abnormal cleavage accounted for a 100 % influence on blastocyst formation [14]. However, the previously mentioned study did not describe the final pregnancy outcome. Our present study adds new insight to the final pregnancy outcome of abnormally cleaving embryos: of the 24 transferred common abnormal cleavage embryos, seven embryos were implanted, all five patients achieved clinical pregnancy, five patients achieved ongoing pregnancy, and, of these, four patients resulted in a live birth.
The mechanism of abnormal cleavage is not clear. The formation of three pronuclei was believed to be related to abnormal cleavage from one cell to more than three blastomeres. During fertilization, penetration of (i) two haploid spermatozoa or (ii) a single diploid spermatozoon (as a result of I or II meiotic division error) into the oocyte causes diandric triploidy [15]. Kola et al. further described that most of the tripronuclear oocytes cleaved directly to three cells at the first cleavage, and all embryos that cleaved to three cells had a severely abnormal chromosomal composition [16]. However, this explanation cannot be applied to our study, since embryos in the present study were identified by time-lapse technology so as to avoid abnormally fertilized zygotes. The previous research indicated that a bipronuclear zygote with two polar bodies (PB) could also cleave directly into three blastomeres [4, 9, 13], and our study appears to be consistent with theirs. Kalatova et al. [17] described one mechanism for pathologic multiplication of centrioles and mitotic poles as one in which the centriole duplicated normally, but there existed an additional microtubule-organizing center as a stand-alone body of pure “empty” pericentriolar matrix (i.e., without centrioles) in addition to two normal centrosomes. This resulted in a tripolar spindle, with a centriole distribution in mitosis of 2:2:0. Ledbetter et al. [18] previously reported that the rate of mosaicism at the cleavage stage varies greatly from 15 to 90 %; however, the rate of mosaicism observed in prenatal diagnosis ranges only from 1 to 2 %. This would indicate a selection mechanism against mosaicism in the latter stages of development. Taylor et al. [19] reported that the clinical consequences of mosaicism depended upon which chromosome was involved, when an error(s) occurred, and whether the error(s) continued to propagate. Such a mechanism could explain our results, but further future studies need to be undertaken to elucidate the molecular nature of the mechanism(s). Campbell et al. [20] further depicted no significant differences between aneuploid and euploid embryos in irregular division patterns (“direct” or “rapid” division defined as being less than of 5-h duration), which may further support our contention. We additionally studied the implantation rate, clinical pregnancy rate, ongoing pregnancy rate, and live birth rate in the AC1-3c, AC2-4c, AC3-5c, and AC4-6c groups as illustrated in Table 4. We found that the AC4-6c group possessed a higher implantation rate, clinical pregnancy rate, ongoing pregnancy rate, and live birth rate. These results may illustrate that the later the AC, the higher the implantation rate, clinical pregnancy rate, ongoing pregnancy rate, and live birth rate, which is consistent with a previous study [9, 19].
We found intriguing results regarding the time parameter “t8-t5” when comparing the four types of AC embryos with NC embryos. Specifically, t8-t5 manifested significant differences in the AC1-3c, AC2-4c, and AC3-5c groups compared with the NC group, but lacked significance between the AC4-6c and the NC groups. However, the implantation rate, clinical pregnancy rate, ongoing pregnancy rate, and live birth rate in the AC4-6c group were significantly higher than in the other three groups, indicating that t8-t5 might predict the potentially successful development of AC embryos. A previous study indicated that the time-parameter t8-t5 was significant between day 3 embryos possessing optimal morphology and day 3 embryos without optimal morphology, and this was also the case for day-5 blastocysts [21]. Desai et al. [22] investigated day-5 embryos and found that t8-t5 was significantly different between blast-transferred and blast-frozen blastocysts compared with poor-quality blastocysts/embryos. Another study indicated that day-3 embryos satisfying our t8-t5 criteria (0.7–30.8) would develop into blastocysts, but that the implantation rate was not significantly different with respect to t8-t5 [23]. Results also showed that “t3” in AC1–3C group and “t5” in AC3–5C group were significantly different from the normal control group. This was consistent with the hypothesis of this work, but “t4” in the AC2–4C group and “t6” in the AC4–6C group had no significant difference from the normal control group. Results also showed “t4-t3, t5-t4” in the AC1–3C group and t3-t5 in AC3–5C group in to be significantly different from the normal control group. To explain these discrepancies, the sample size will be expanded for further study. Meseguer et al. found “t4-t3” to be one of the most predictive parameters of embryos’ implantation potential, and this might explain the results found here that “t4-t3” in the AC1–3C group was significantly different from the normal control group [12].
Our results indicate that abnormally cleaving embryos can also achieve a live birth. We observed that the later the AC occurs, the better the clinical consequences, and t8-t5 may be a reliable index for abnormally cleaving embryos so as to select normally developing embryos. However, as our sample size was very small, we will next expand the sample size and continue the study further.
References
Racowsky C, Vernon M, Mayer J, Ball GD, Behr B, Pomeroy KO, et al. Standardization of grading embryo morphology. Fertil Steril. 2010;94:1152–3.
Scott L, Finn A, O’Leary T, McLellan S, Hill J. Morphologic parameters of early cleavage-stage embryos that correlate with fetal development and delivery: prospective and applied data for increased pregnancy rates. Hum Reprod. 2007;22:230–40.
Gardner DK, Sakkas D. Assessment of embryo viability: the ability to select a single embryo for transfer—a review. Placenta. 2003;24:S5–S12.
Hlinka D, Kalatova B, Uhrinova I, Dolinska S, Rutarova J, Rezacova J, et al. Time-lapse cleavage rating predicts human embryo viability. Physiol Res. 2012;61:513–25.
Conaghan J, Chen AA, Willman SP, Ivani K, Chenette PE, Boostanfar R, et al. Improving embryo selection using a computer-automated time-lapse image analysis test plus day 3 morphology: results from a prospective multicenter trial. Fertil Steril. 2013;100:412–9.
Herrero J, Meseguer M. Selection of high potential embryos using time-lapse imaging: the era of morphokinetics. Fertil Steril. 2013;99:1030–4.
Kirkegaard K, Agerholm IE, Ingerslev HJ. Time-lapse monitoring as a tool for clinical embryo assessment. Hum Reprod. 2012;27:1277–85.
Payne D, Flaherty SP, Barry MF, Matthews CD. Preliminary observations on polar body extrusion and pronuclear formation in human oocytes using time-lapse video cinematography. Hum Reprod. 1997;12:532–41.
Wirka KA, Chen AA, Conaghan J, Ivani K, Gvakharia M, Behr B, et al. Atypical embryo phenotypes identified by time-lapse microscopy: high prevalence and association with embryo development. Fertil Steril. 2014;101:1637–48.
Dale B, Elder K. In vitro fertilization. United Kingdom: Cambrige University; 1997. p. 115–6.
Ye H, Huang GN, Zeng PH, Pei L. IVF/ICSI outcomes between cycles with luteal estradiol (E2) pre-treatment before GnRH antagonist protocol and standard long GnRH agonist protocol: a prospective and randomized study. J Assist Reprod Genet. 2009;26:105–11.
Meseguer M, Herrero J, Tejera A, Hilligsøe KM, Ramsing NB, Remohí J. The use of morphokinetics as a predictor of embryo implantation. Hum Reprod. 2011;26:2658–71.
Rubio I, Kuhlmann R, Agerholm I, Kirk J, Herrero J, Escribá MJ, et al. Limited implantation success of direct-cleaved human zygotes: a time-lapse study. Fertil Steril. 2012;98:1458–63.
Yang ST, Shi JX, Gong F, Zhang SP, Lu CF, Tan K, et al. Cleavage pattern predicts developmental potential of day 3 human embryos produced by IVF. Reprod BioMed Online. 2015;2:1–9.
Yu SL, Lee RK, Su JT, Chih YF, Tsai YC, Lin MH, et al. Distinction between paternal and maternal contributions to the tripronucleus in the human zygotes obtained after in vitro fertilization. Taiwan J Obstet Gynecol. 2006;45:313–6.
Kola I, Trounson A, Dawson G, Rogers P. Tripronuclear human oocytes: altered cleavage patterns and subsequent karyotypic analysis of embryos. Biol Reprod. 1987;37:395–401.
Kalatova B, Jesenska R, Hlinka D, Dudas M. Tripolar mitosis in human cells and embryos: occurrence, pathophysiology and medical implications. Acta Histochem. 2015;117:111–25.
Ledbetter DH, Zachary JM, Simpson JL, Golbus MS, Pergament E, Jackson L, et al. Cytogenetic results from the US collaborative study on CVS. Prenat Diagn. 1992;12:317–45.
Taylor TH, Gitlin SA, Patrick JL, Crain JL, Wilson JM, Griffin DK. The origin, mechanisms, incidence and clinical consequences of chromosomal mosaicism in humans. Hum Reprod Update 2014. doi:10.1093/humupd/dmu016.
Campbell A, Fishel S, Bowman N, Duffy S, Sedler M, Thornton S. Retrospective analysis of outcomes after IVF using an aneuploidy risk model derived from time-lapse imaging without PGS. Reprod BioMed Online. 2013;27:140–6.
Herrero J, Tejera A, Albert C, Vidal C, de los Santos MJ, Meseguer M. A time to look back: analysis of morphokinetic characteristics of human embryo development. Fertil Steril. 2013;100:1602–9.
Desai N, Ploskonka S, Goodman LR, Austin C, Goldberg J, Falcone T. Analysis of embryo morphokinetics, multinucleation and cleavage anomalies using continuous time-lapse monitoring in blastocyst transfer cycles. Reprod Biol Endocrinol. 2014;12:54.
Chamayou S, Patrizio P, Storaci G, Tomaselli V, Alecci C, Ragolia C, et al. The use of morphokinetic parameters to select all embryos with full capacity to implant. J Assist Reprod Genet. 2013;30:703–10.
Acknowledgments
The authors gratefully acknowledge Lihong Wu for her technical support regarding the management of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures and protocols were approved by the ethics committee of the Chongqing Obstetric and Gynecology Hospital ethical number RGIEA-201405.
Additional information
Capsule Abnormally cleaving embryos were able to produced live births with T8-t5 the best parameter to predict the developmental potential of abnormally cleaving embryos.
Rights and permissions
About this article
Cite this article
Fan, Y.L., Han, S.B., Wu, L.H. et al. Abnormally cleaving embryos are able to produce live births: a time-lapse study. J Assist Reprod Genet 33, 379–385 (2016). https://doi.org/10.1007/s10815-015-0632-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-015-0632-x