Abstract
Background
The traditional treatment for chondrosarcoma is wide local excision (WLE), as these tumors are resistant to chemotherapy and radiation treatment. While achieving negative margins has traditionally been the goal of chondrosarcoma resection, multiple studies have demonstrated good short-term results after intralesional procedures for low-grade chondrosarcomas (LGCS) with curettage and adjuvant treatments (phenol application, cauterization or cryotherapy) followed by either cementation or bone grafting. Due to the rarity of this diagnosis and the recent application of this surgical treatment modality to chondrosarcoma, most of the information regarding treatment outcomes is retrospective, with short or intermediate-term follow-up. The aim of this study was to assess the long-term results of patients with LGCS of bone treated with intralesional curettage (IC) treatment versus WLE. This retrospective analysis aims to characterize the oncologic outcomes (local recurrence, metastases) and functional outcomes in these two treatment groups at a single institution.
Methods
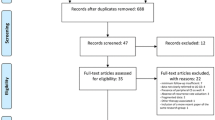
Using an institutional musculoskeletal oncologic database, we retrospectively reviewed medical records of all patients with LGCS of the appendicular skeleton that underwent surgical treatment between 1985 and 2007. Thirty-two patients (33 tumors) were identified with LGCS; 17 treated with IC and 15 with WLE.
Results
Seventeen patients (18 tumors) with a minimum clinical and radiologic follow-up of 10 years were included. Nine patients were treated with IC (four with no adjuvant, three with additional phenol, one with liquid nitrogen and one with H2O2) with either bone graft or cement augmentation, and nine others were treated with WLE and reconstruction with intercalary/osteoarticular allograft or megaprosthesis. The mean age at surgery was 41 years (range 14–66 years) with no difference (p = 0.51) between treatment cohorts. There was a mean follow-up of 13.5 years in the intralesional cohort (range 10–19 years) and 15.9 years in the WLE cohort (range 10–28 years, p = 0.36). Tumor size varied significantly between groups and was larger in patients treated with WLE (8.2 ± 3.1 cm versus 5.4 ± 1.2 cm, at the greatest dimension, p = 0.021). There were two local recurrences (LR), one in the intralesional group and one in the wide local excision group, occurring at 3.5 months and 2.9 years, respectively, and both required revision. No further LR could be detected with long-term follow-up. The MSTS score at final follow-up was significantly higher for patients managed with intralesional procedures (28.7 ± 1.7 versus 25.7 ± 3.4, p = 0.033). There were less complications requiring reoperation in the intralesional group compared with the wide local excision group, although this difference was not found to be statistically significant (one versus four patients, respectively; p = 0.3).
Conclusion
This series of low-grade chondrosarcoma, surgically treated with an intralesional procedures, with 10-year follow-up, demonstrates excellent local control (88.9%). Complications were infrequent and minor and MSTS functional scores were excellent. Wide resection of LGCS was associated with lower MSTS score and more complications. In our series, the LR in both groups were detected within the first 3.5 years following the index procedure, and none were detected in the late surveillance period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chondrosarcoma is the second most common primary bone sarcoma, accounting for one-fourth of these tumors [1, 2], and occurs most commonly in the fourth and fifth decades of life [3]. Chondrosarcomas are further classified according to histological grades based on the presence of cellular atypia, mitotic figures and cellularity [4,5,6,7].
Histological grading of low-grade chondral tumors is a very challenging task, and even among experienced pathologist, high interobserver variability has been witnessed [8, 9]. The clinical course cannot always be predicted on the basis of the histological grade alone [4, 10, 11] and the distinction between a benign enchondroma and a low-grade chondrosarcoma (LGCS) can be extremely difficult on radiographs [9, 11]. As such, the diagnosis is best determined utilizing pathologic, radiologic, and clinical findings. Studies over the last 10 years have reported on the treatment of LGCS with extended intralesional curettage (EIC) [3, 8, 10, 12,13,14,15]. These studies demonstrate good functional results and short-term oncologic outcomes. In a recent systemic review and meta-analysis, Chen et al. [14] analyzed 10 studies involving 394 LGCS patients with a minimum 2-year follow-up. Two hundred and fourteen treated with EIC, and 180 with wide local excision (WLE), and found IC to be associated with less complications, and better MSTS scores, without increasing the risk for local recurrence (LR) or metastases. Others, have also found intralesional treatment consisting of curettage and local adjuvant treatment such as phenol application [1, 10, 12, 16, 17], cauterization [8], or cryotherapy [2, 13, 18, 19], followed by either bone grafting or polymethyl methacrylate (PMMA) applied to the cavity, to have similar or lower LR risk and metastasis compared with WLE. However, complications rates as well as functional outcomes seems to be worse with the latter [14, 20].
Although several studies have investigated the results of both treatment options for the treatment of LGCS, it is unclear if these findings are sustained in the long term, as most studies present an average follow-up of less than 7 years [1, 2, 8, 10, 12, 15,16,17,18, 21,22,23,24]. Two recent systemic reviews and meta-analysis describing studies with an average follow-up of 4.7 and 8.5 years, have reported LR rates of 8.6–9.8% for EIC and 4.4–5% for WLE, without a significant difference between groups. Five-year relapse-free survival was reported to be 88.9% and 93.5% for both groups, respectively, without a significant difference. Metastases rates were low, 0.7%, within 2.3 years from the index surgery [14, 20]. In the current study, we present a minimum 10-years follow-up on this cohort, and report on the long-term oncological and functional outcomes.
Materials and methods
Following institutional review board approval, we retrospectively reviewed medical, surgical and radiographic records of patients treated for LGCS between 1985 and 2007. The study included a consecutive series of patients surgically treated at our institution (a multidisciplinary sarcoma center) and evaluated prospectively, and whose data were reviewed retrospectively. The diagnosis of LGCS was made on the basis of the clinical history, physical examination, and radiographic findings which was confirmed by post-operative final pathology assessment. Thirty-one patients (32 tumors) were identified with a diagnosis of LGCS in the original report [25]. Nine patients were lost to follow-up at a mean follow-up period of 4.1 years (ranging from 2–7 years). Five were treated with IC and 4 with WLE. Only one of whom, who presented with an LGCS of the distal femur and was treated with WLE and a cemented megaprosthesis, had a post-operative complication (a patellar fracture). None of these patients had an LR or evidence of metastases. At their last follow-up their MSTS score was 27.9 ± 3.2.
Seven additional patients died of unrelated causes (last follow-up at 4.8 ± 1.9 years), leaving 15 patients (16 tumors) available for clinical and radiographic follow-up. Two additional patients, who were not included in the original study, were added to the present study. Both were diagnosed with LGCS and treated surgically, with clinical and radiologic follow-up longer than 10 years. Therefore, the final cohort included 17 patients (18 tumors), with a minimum follow-up of 10 years, and a mean follow-up of 14.8 years.
Following diagnosis, the patients were operated with either IC or WLE, based upon the surgeon’s preference. For intralesional excision, allograft powder, cancellous allograft or cement were used for grafting, and for WLE, intercalary allograft, or megaprosthesis with cement were used for reconstruction. For intralesional resections, adjuvant therapy was used, either phenol, H2O2 or liquid nitrogen. Following surgery all patients participated in physiotherapy sessions to encourage full range of motion. Patient continued follow-up at the musculoskeletal oncology outpatient clinic.
The histopathologic, and radiologic diagnosis of LGCS was made on the basis of the criteria proposed by Mirra et al. [5] and Murphey et al. [26], respectively. Specific characteristics such as: moderate cellularity with bilacunal nuclei, entrapment of normal lamellar bone, rare or absent mitoses, minimal pleomorphism, absent necrosis, and scant myxoid stroma were identified in all specimens on pathologic analysis. Similarly, all tumors had radiographic evidence of Grade 2–3 endosteal scalloping, no overt cortical breakthrough or soft tissue mass on MRI, and bone scintigraphy radiotracer uptake greater than the anterior–superior iliac spine with or without focal heterogeneity in the lesion. The final pathologic diagnosis was rendered by a musculoskeletal pathologist following formal multidisciplinary review of the surgical pathology to ensure clinic–radio–pathologic correlation.
The retrieved data included age and gender, tumor location and size (in centimeters as measured by maximal radiographic linear dimension), surgical treatment (wide local excision versus intralesional treatment) including the local adjuvant used and reconstructive techniques, post-operative complications (hardware failure, fracture and allograft fracture, non-union, deep infections and reoperations), functional outcome evaluated by Musculoskeletal Tumor Society (MSTS) scores [27], and oncological outcomes including: incidence of LR, metastasis, and death. Follow-up was done in intervals of 3–6 months during the first 5 years, and yearly after this.
No patient in either group had metastatic disease develop or died of their chondrosarcoma. All deaths (patients who were excluded due to insufficient follow-up duration, four patients following intralesional resections and three patients WLEs) were unrelated to chondrosarcoma or the surgery, and none had LR or metastases at the final follow-up encounter.
Statistical analysis
Statistical analysis was conducted using SPSS (Version 15.0; SPSS Inc, Chicago, IL, USA), with a 2-tailed alpha of 0.05. Continuous variables were analyzed using paired Student’s t test after testing for normality and equal variance. Categorical analysis was conducted with Chi-square and Fisher’s exact test where appropriate.
Results
The final cohort included 17 patients (18 tumors), 11 females and 6 males, treated surgically with LGCS of the extremities. The mean age at diagnosis was similar between cohorts, 41 ± 12 years. Tumor size varied significantly between groups and was larger in patients treated with WLE (8.2 ± 3.1 cm versus 5.4 ± 1.2 cm, at the greatest dimension, p = 0.021). All lesions involved the appendicular skeleton.
Of the 18 tumors, nine were treated with intralesional procedures (four with no adjuvant, three with additional phenol, one with liquid nitrogen and one with H202) with either bone graft or cement augmentation, while nine others were treated with WLE and reconstruction with intercalary/osteoarticular allograft or megaprosthesis. There was a mean follow-up of 13.5 years in the intralesional cohort (range 10–19 years) and 15.9 years in the wide local excision cohort (range 10–28 years), p = 0.36, with a mean follow-up of 14.8 ± 5.3 years, ranging from 10 to 28 years for the whole cohort.
There were two LRs, one in the intralesional group and one in the wide local excision group (p = 1), occurring at 3.5 months and 2.9 years, respectively, and both required revision. No further LRs could be detected with long-term follow-up.
There were no cases of metastatic disease. The MSTS score at final follow-up was significantly higher for patients managed with intralesional procedures (28.7 ± 1.7 versus 25.7 ± 3.4, p = 0.033).
There were less complications requiring reoperation in the intralesional group compared with the WLE group, although this difference was not found to be statistically significant (one versus four patients, respectively; p = 0.3). In the intralesional group, there was a single case of fracture within 1 month of the initial surgery, treated with open reduction and internal fixation (ORIF). The patient eventually underwent removal of hardware 2.7 years after the index procedure. In the WLE group, there were overall four complications. Two patients with LGCS of the proximal humerus were diagnosed with allograft fractures within 2 years from the initial procedure. Another patient had shoulder instability 1 month following surgery and was treated surgically. The fourth patient had hardware failure 16 months following resection and reconstruction with intercalary allograft in the mid-shaft femur, and was also complicated with hardware-related infection, and thus required several reoperations. He subsequently underwent distal femoral replacement 4.9 years after his index procedure.
Discussion
LGCS is a slowly growing, locally aggressive tumor with an indolent course and recurrent growth potential. Differentiating between an enchondroma and an LGCS can be extremely difficult and has led to these tumors being categorized together in the WHO classification as atypical cartilaginous tumor/chondrosarcoma grade I [14]. LGCS are considered a tumor of low–intermediate malignant potential, most often behaving in a locally aggressive fashion [17, 24, 28].
LGCS are often considered less aggressive, and surgery is the treatment modality of choice since chemotherapy and radiation are not effective. This condition in the limbs, can be managed by curettage with or without adjuvant therapy (e.g., phenol, cement, cryotherapy) with a high chance of success [27]; even though, whether or not to use adjuvant therapy as well as the type of adjuvant is under debate. Care must be taken not to overtreat benign tumors or undertreat malignant ones [29]. The current literature consists mainly of retrospective chart reviews reporting a variable sample size [17, 20] that do not include a long follow-up period for every individual reported, with a mean surveillance period of 2–13 years. There is also great variability in the diagnostic measures, the surveillance methods and treatment strategies [20].
A negative impact on survival as a result of LRs has been seen in previous studies. In their retrospective review on 164 patients surgically treated for LGCS, Schwab et al. [30] found an LR in 21 patients (13%), with an average time to recurrence of 3.2 years (range 4 months to 9 years) [30]. All six mortalities were in patients with LR, and in 5 of whom distal metastases were also noted. As LR of LGCS in the long bones of the extremity was associated with a substantially worse overall survival when compared with patients without LR, they concluded that recurrence signifies a more aggressive tumor phenotype. A more recent study by Dierselhuis et al. retrospectively reviewed 108 LGCS treated surgically with curettage and adjuvant phenolization, at a mean follow-up of 4 years, and found no LRs, yet five patients were diagnosed with a residual tumor [17]. The authors found the residual tumors to have no impact on the patient survival, since neither actual LR nor upgrading in the local residue occurred.
The large variability in the reported rates of LR, as well as the differences in the reported impact of LRs on patient survival, raises fundamental questions about the duration of follow-up and the desired surveillance methods. To our knowledge , there is no acceptable follow-up regimen for these tumors, with no consensus stopping point for follow-up (Figs. 1, 2).
A 41-year-old female who presented with worsening pain in her left thigh, associated with weight bearing. Anteroposterior radiograph (a) coronal T1-weighted MRI section (b) demonstrate a chondral lesion involving the diaphyseal–metaphyseal junction of the left femur. She subsequently underwent intercalary resection and reconstruction with intercalary allograft and long IMN (c). Bisected femur contained cartilaginous tumor with focal chalk-like gritty areas (d). Low power field microscopy showed an infiltrative growth pattern of low-grade chondrosarcoma with engulfment of the adjacent cancellous bone (e). Following surgery, the patient has fully recovered functionally, and followed up for 11 years. AP (f) and lateral (g) radiographs of the left femur at the latest follow-up show that the allograft was fully incorporated and there are no signs of LR
A 55-year-old male who presented with persistent left shoulder pain. Anteroposterior (a) and axial (b) radiographs, as well as sagittal T2-weighted MRI section (c) demonstrate a chondral lesion involving the metaphyseal left proximal humerus. Bone scan (Tc-99m) (d) demonstrated high uptake corresponding with the radiographic finding. He subsequently underwent intralesional treatment which included extended curettage with phenol, followed by bone grafting. Low power field microscopy showed an infiltrative growth pattern of low-grade chondrosarcoma with engulfment of the adjacent cancellous bone (e). Following surgery, the patient has fully recovered functionally, and followed up for 10 years. AP (f) radiograph and coronal T1-weighted MRI section (g) at the latest follow-up show that the allograft was fully incorporated and there are no signs of LR
Both the European sarcoma network working group (ESMO) and the British sarcoma group clinical guidelines state that for low-grade bone sarcomas, the frequency of follow-up visits can be reduced to 4–6 monthly for 2 years and then annually. Late metastases as well as LRs and failure of reconstructions may occur more than 10 years after diagnosis in all tumors and there is no universally accepted stopping point for follow-up [27, 29]. Hence the importance of reporting oncologic and clinical outcomes with long-term follow-up for this particular malignancy. The present study adds valuable data to the limited existing evidence about time to detection of LRs. Looking at 18 tumors reported herein, we found two LRs. One was identified in a humerus following intralesional curettage (Table 1, patient 3), and another found following WLE and reconstruction with intercalary allograft (Table 1, patient 13), occurring at 3.5 months and 2.9 years, respectively, and both required revision. No further LRs could be detected with long-term follow-up in any of the patients. These findings are in line with several other case series, reporting LRs as early as 2 months from the initial surgery and as late as 9 years [1, 2, 10, 12, 13, 15, 16, 21, 22, 31].
As for the functional results following surgery, we found a mean MSTS score of 27.3 ± 3.8 for our entire cohort at a mean follow-up of 14 years, suggesting an overall satisfying functional outcome with surgical treatment, at long term. We did find better functional results in the group of patients treated with intralesional curettage (28.7 ± 1.7 versus 25.7 ± 3.4, p = 0.033), as one would expect given lesser extent of these procedures compared to WLE and reconstruction. These functional results resemble the ones reported in the previous report from our group (29.5 for intralesional versus 25.1 for WLE), suggesting there was no significant decline in the functional outcomes in our cohort. Regarding complications, we did not find ones necessitating reoperations later than 5 years following the index procedure.
We acknowledge the several limitations of the present study. We had relatively small numbers of patients in both treatment groups. Additionally, there was also a significant number of patients lost to follow-up from the initial cohort of patients presented in the previous study from our group; however, with such a considerable follow-up term set as an inclusion criterion, this could be something expected. Furthermore, half of the patients lost to follow-up died from unrelated causes without an evidence of recurrence or metastatic disease. Finally, the heterogeneity of the surgical treatment of the initial lesion in our study precludes us from making treatment recommendations based on our data.
Conclusions
This series of low-grade chondrosarcoma, surgically treated with intralesional procedures, with 10-year follow-up, demonstrates excellent local control (88.9%). Complications were infrequent and minor, and MSTS functional scores were excellent. Wide resection of LGCS was associated with lower MSTS score and more complications. There were no late LR or metastases in neither group. Further larger-scale multicentric studies are needed in order to establish a stopping point for surveillance in this malignancy.
References
Leerapun T, Hugate RR, Inwards CY, Scully SP, Sim FH (2007) Surgical management of conventional grade I chondrosarcoma of long bones. Clin Orthop Relat Res 463:166–172. https://doi.org/10.1097/BLO.0b013e318146830f
Mohler DG, Chiu R, McCall DA, Avedian RS (2010) Curettage and cryosurgery for low-grade cartilage tumors is associated with low recurrence and high function. Clin Orthop Relat Res 468:2765–2773. https://doi.org/10.1007/s11999-010-1445-y
Hickey M, Farrokhyar F, Deheshi B, Turcotte R, Ghert M (2011) A systematic review and meta-analysis of intralesional versus wide resection for intramedullary grade I chondrosarcoma of the extremities. Ann Surg Oncol 18:1705–1709. https://doi.org/10.1245/s10434-010-1532-z
Evans HL, Ayala AG, Romsdahl MM (1977) Prognostic factors in chondrosarcoma of bone: a clinicopathologic analysis with emphasis on histologic grading. Cancer 40:818–831
Mirra JM, Gold R, Downs J, Eckardt JJ (1985) A new histologic approach to the differentiation of enchondroma and chondrosarcoma of the bones. A clinicopathologic analysis of 51 cases. Clin Orthop Relat Res 214–237
Rozeman LB, Hogendoorn PCW, Bovee JVMG (2002) Diagnosis and prognosis of chondrosarcoma of bone. Expert Rev Mol Diagn 2:461–472. https://doi.org/10.1586/14737159.2.5.461
Welkerling H, Kratz S, Ewerbeck V, Delling G (2003) A reproducible and simple grading system for classical chondrosarcomas. Analysis of 35 chondrosarcomas and 16 enchondromas with emphasis on recurrence rate and radiological and clinical data. Virchows Arch 443:725–733. https://doi.org/10.1007/s00428-003-0896-x
Mermerkaya MU, Bekmez S, Karaaslan F, Danisman M, Kosemehmetoglu K, Gedikoglu G et al (2014) Intralesional curettage and cementation for low-grade chondrosarcoma of long bones: retrospective study and literature review. World J Surg Oncol 12:336. https://doi.org/10.1186/1477-7819-12-336
Skeletal Lesions Interobserver Correlation among Expert Diagnosticians Study (2007) Reliability of histopathologic and radiologic grading of cartilaginous neoplasms in long bones. J Bone Joint Surg Am 89:2113–2123. https://doi.org/10.2106/JBJS.F.01530
Verdegaal SHM, Brouwers HFG, van Zwet EW, Hogendoorn PCW, Taminiau AHM (2012) Low-grade chondrosarcoma of long bones treated with intralesional curettage followed by application of phenol, ethanol, and bone-grafting. J Bone Joint Surg Am 94:1201–1207. https://doi.org/10.2106/JBJS.J.01498
Errani C, Tsukamoto S, Ciani G, Akahane M, Cevolani L, Tanzi P et al (2017) Risk factors for local recurrence from atypical cartilaginous tumour and enchondroma of the long bones. Eur J Orthop Surg Traumatol 27:805–811. https://doi.org/10.1007/s00590-017-1970-4
Di Giorgio L, Touloupakis G, Vitullo F, Sodano L, Mastantuono M, Villani C (2011) Intralesional curettage, with phenol and cement as adjuvants, for low-grade intramedullary chondrosarcoma of the long bones. Acta Orthop Belg 77:666–669
Meftah M, Schult P, Henshaw RM (2013) Long-term results of intralesional curettage and cryosurgery for treatment of low-grade chondrosarcoma. J Bone Joint Surg Am 95:1358–1364. https://doi.org/10.2106/JBJS.L.00442
Chen X, Yu LJ, Peng HM, Jiang C, Ye CH, Zhu SB et al (2017) Is intralesional resection suitable for central grade 1 chondrosarcoma: a systematic review and updated meta-analysis. Eur J Surg Oncol 43:1718–1726. https://doi.org/10.1016/j.ejso.2017.05.022
Hanna SA, Whittingham-Jones P, Sewell MD, Pollock RC, Skinner JA, Saifuddin A et al (2009) Outcome of intralesional curettage for low-grade chondrosarcoma of long bones. Eur J Surg Oncol 35:1343–1347. https://doi.org/10.1016/j.ejso.2009.06.001
Campanacci DA, Scoccianti G, Franchi A, Roselli G, Beltrami G, Ippolito M et al (2013) Surgical treatment of central grade 1 chondrosarcoma of the appendicular skeleton. J Orthop Traumatol 14:101–107. https://doi.org/10.1007/s10195-013-0230-6
Dierselhuis EF, Gerbers JG, Ploegmakers JJW, Stevens M, Suurmeijer AJH, Jutte PC (2016) Local treatment with adjuvant therapy for central atypical cartilaginous tumors in the long bones: analysis of outcome and complications in one hundred and eight patients with a minimum follow-up of two years. J Bone Joint Surg Am 98:303–313. https://doi.org/10.2106/JBJS.O.00472
Schreuder HW, Pruszczynski M, Veth RP, Lemmens JA (1998) Treatment of benign and low-grade malignant intramedullary chondroid tumours with curettage and cryosurgery. Eur J Surg Oncol 24:120–126
Souna BS, Belot N, Duval H, Langlais F, Thomazeau H (2010) No recurrences in selected patients after curettage with cryotherapy for grade I chondrosarcomas. Clin Orthop Relat Res 468:1956–1962. https://doi.org/10.1007/s11999-009-1211-1
Shemesh SS, Acevedo-Nieves JD, Pretell-Mazzini J (2018) Treatment strategies for central low-grade chondrosarcoma of long bones: a systematic review of the literature and meta-analysis. Musculoskelet Surg 102:95–109. https://doi.org/10.1007/s12306-017-0507-7
Bauer HC, Brosjo O, Kreicbergs A, Lindholm J (1995) Low risk of recurrence of enchondroma and low-grade chondrosarcoma in extremities. 80 patients followed for 2–25 years. Acta Orthop Scand 66:283–288
Gunay C, Atalar H, Hapa O, Basarir K, Yildiz Y, Saglik Y (2013) Surgical management of grade I chondrosarcoma of the long bones. Acta Orthop Belg 79:331–337
Kim W, Han I, Kim EJ, Kang S, Kim H-S (2015) Outcomes of curettage and anhydrous alcohol adjuvant for low-grade chondrosarcoma of long bone. Surg Oncol 24:89–94. https://doi.org/10.1016/j.suronc.2015.04.001
Ahlmann ER, Menendez LR, Fedenko AN, Learch T (2006) Influence of cryosurgery on treatment outcome of low-grade chondrosarcoma. Clin Orthop Relat Res 451:201–207. https://doi.org/10.1097/01.blo.0000229293.98850.5d
Aarons C, Potter BK, Adams SC, Pitcher JDJ, Temple HT (2009) Extended intralesional treatment versus resection of low-grade chondrosarcomas. Clin Orthop Relat Res 467:2105–2111. https://doi.org/10.1007/s11999-008-0691-8
Murphey MD, Flemming DJ, Boyea SR, Bojescul JA, Sweet DE, Temple HT (1998) Enchondroma versus chondrosarcoma in the appendicular skeleton: differentiating features. Radiographics 18:1213–1215. https://doi.org/10.1148/radiographics.18.5.9747616
Group ESESNW (2014) Bone sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 25(Suppl 3):iii113– iii 23
Czerniak B. Dorfman, Czerniak S (2015) Bone tumors E-book. Elsevier Health Sciences
Gerrand C, Athanasou N, Brennan B, Grimer R, Judson I, Morland B et al (2016) UK guidelines for the management of bone sarcomas. Clin Sarcoma Res 6:7. https://doi.org/10.1186/s13569-016-0047-1
Schwab JH, Wenger D, Unni K, Sim FH (2007) Does local recurrence impact survival in low-grade chondrosarcoma of the long bones? Clin Orthop Relat Res 462:175–180. https://doi.org/10.1097/BLO.0b013e3180caac2c
Donati D, Colangeli S, Colangeli M, Di Bella C, Bertoni F (2010) Surgical treatment of grade I central chondrosarcoma. Clin Orthop Relat Res 468:581–589. https://doi.org/10.1007/s11999-009-1056-7
Funding
There is no funding source.
Author information
Authors and Affiliations
Contributions
All authors have demonstrated (1) substantial contributions to research design, or the acquisition, analysis or interpretation of data; (2) drafting the paper or revising it critically; (3) approval of the submitted and final versions.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Waived.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shemesh, S.S., Pretell-Mazzini, J., Quartin, P.a.J. et al. Surgical treatment of low-grade chondrosarcoma involving the appendicular skeleton: long-term functional and oncological outcomes. Arch Orthop Trauma Surg 139, 1659–1666 (2019). https://doi.org/10.1007/s00402-019-03184-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-019-03184-w