Abstract
The need for wide local excision (WLE) versus intralesional (IL) treatment of low-grade chondrosarcomas (CS) of the appendicular skeleton remains controversial. We sought to perform a systematic review and meta-analysis to compare different conventional types of surgical treatments for grade I CS in terms of: (1) rate of local recurrence (LR) and metastases, (2) functional outcome as measured by the Musculoskeletal Tumor Society (MSTS) score, (3) complication rate. Eighteen studies enrolling 695 patients met our criteria. Studies reported on WLE versus IL treatment (n = 7), and IL treatment with or without different adjuvants (N = 11). The LR rate was not significantly different between WLE and IL treatment (OR 2.31; 95% CI, 0.85–6.2; P = 0.1). On the contrary, complication rates were significantly lower in favor of IL treatment (OR 2.27; 95% CI, 0.07–0.72; P = 0.012). The mean reported MSTS score ranged from 21.8 to 28.2 for WLE and from 26.5 to 29.7 for IL treatment, with a significant difference in favor of IL treatment. IL treatment as an alternative to WLE does not greatly increase the risk of LR or metastasis and has lower complication rates with better functional scores. In light of the retrospective nature of the studies available, our findings should be interpreted with caution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chondrosarcoma (CS) is the second most common primary bone sarcoma, accounting for one-fourth of these tumors [1, 2], and is classified into three histological grades based on the presence of cellular atypia, mitotic figures and cellularity [3,4,5,6,7].
Histological grading of low-grade chondral tumors represents an extremely challenging task, and even among experienced pathologists an intraobserver variability has been witnessed [8, 9]. The clinical course cannot always be predicted on the basis of the histological grade alone [3, 10, 11]. Similarly, the distinction between a benign enchondroma and low-grade CS based on imaging studies can be exceedingly difficult [9, 10]. Chondral lesions of the appendicular skeleton have a better prognosis and should be considered separately from lesions of the axial skeleton, since recurrence have been previously reported to be greater in certain locations in the axial skeleton such as the pelvis [12, 13]. Therefore, the diagnosis and the choice of treatment should rely upon a combination of radiologic and pathologic features as well as tumor location among other clinical factors [10, 14, 15]. Five-year patient survival rates of 85–90% have been reported for these low-grade tumors, and distant metastases were found to be very rare [3, 16,17,18].
Surgery is the only curative option for low-grade CS, since chemotherapy and radiation are not effective [19,20,21]. The most common treatment options described are extended curettage with different local adjuvants, and segmental resection with or without reconstruction [1, 19, 22,23,24].
Recently, low-grade CS of long bones has increasingly been treated with IL treatment and local adjuvant therapy due to its slow growth and low metastatic tendency [1, 11, 15, 19, 22, 24,25,26,27,28,29,30,31]; however, there is still no consensus regarding the best surgical strategy, in terms of the type of surgical resection, and the best adjuvant therapy to be used.
This review aims to identify which surgical intervention (1) has the lowest local recurrence (LR) rate and relapse-free survival, (2) offers the best functional outcome measured by the Musculoskeletal Tumor Society (MSTS) score, and (3) has the lowest complication rate for the treatment of low-grade CS.
Materials and methods
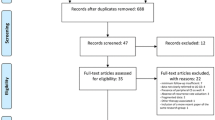
This study was performed in accordance with the PRISMA statement [32, 33]. The Population, Intervention, Comparison and Outcome (PICO) framework was used to define the search strategy [34] (Table 1). The study’s protocol was registered before data collection was done and is accessible at the international prospective register of systematic reviews (PROSPERO CRD42017052600) [35]. The detailed flow of the search strategy and selection process is shown in Fig. 1.
Search strategy and eligibility
A systematic literature search was performed on January 2, 2017, using PubMed, Embase®, and Cochrane databases (Fig. 1). The following search strings for all fields were used in PubMed: ((low[All Fields] AND grade[All Fields]) AND (“chondrosarcoma”[MeSH Terms] OR “chondrosarcoma”[All Fields])) OR (grade[All Fields] AND (“chondrosarcoma”[MeSH Terms] OR “chondrosarcoma”[All Fields])) AND ((“surgery”[Subheading] OR “surgery”[All Fields] OR “surgical procedures, operative”[MeSH Terms] OR (“surgical”[All Fields] AND “procedures”[All Fields] AND “operative”[All Fields]) OR “operative surgical procedures”[All Fields] OR “surgery”[All Fields] OR “general surgery”[MeSH Terms] OR (“general”[All Fields] AND “surgery”[All Fields]) OR “general surgery”[All Fields]) OR (“therapy”[Subheading] OR “therapy”[All Fields] OR “treatment”[All Fields] OR “therapeutics”[MeSH Terms] OR “therapeutics”[All Fields]) OR resection[All Fields] OR (“curettage”[MeSH Terms] OR “curettage”[All Fields])) AND (“1980/01/01”[PDAT] : “2016/12/31”[PDAT]). And the string: grade AND I AND (‘chondrosarcoma’/exp OR chondrosarcoma) OR (low AND grade AND (‘chondrosarcoma’/exp OR chondrosarcoma)) AND (‘treatment’/exp OR treatment OR ‘surgery’/exp OR surgery OR ‘resection’/exp OR resection OR ‘curettage’/exp OR curettage) AND [1980–2016]/py was used in Embase® (‘Broad search’ mode).
This search yielded 755 and 729 titles from PubMed and Embase®, respectively. Two independent reviewers (SSS and JMP) examined the citation information for each result from both databases for relevant studies; subsequently, the same two reviewers screened the full texts and also scanned the reference lists of the included articles for additional studies that met the inclusion criteria.
Inclusion and exclusion criteria
Studies were eligible for inclusion if they met the following criteria (Table 1): (1) studies describing treatment of patients with pathologically verified low-grade CS, (2) articles published in English after 1/1/1980, (3) minimum duration of follow-up of 2 years for more than 80% of patients included; (4) at least five patients with a low-grade CS per study. (5) The proportions of adults (age ≥ 18 years old) was over 80 percent. (6) The location of tumor was in the appendicular skeleton only.
Studies including other CS subtypes (e.g., mesenchymal, clear cell, periosteal, myxoid, dedifferentiated, borderline), grade II and higher lesions, or secondary CS were excluded, if the cases of low-grade CS could not be independently investigated. Also, radiofrequency ablation without surgery, as well as case reports, simulation studies, animal studies, letters, editorials, notes, congress abstracts.
Data extraction
All eligible studies were assessed for methodological quality by two independent reviewers (SSS, JPM). The study design, methodology, patient population parameters and outcomes for all studies included in the systematic review were extracted into a pre-specified grid. Data extraction was performed by a single individual with independent verification by a second reviewer, with disagreements resolved by consensus or third reviewer (JDA) arbitration. In cases where the level of evidence was not specified by the authors, two independent reviewers (SS and JPM) assigned levels of evidence to each eligible study using the Centre for Evidence-Based Medicine in Oxford guidelines for therapeutic studies [36] (Table 2).
Quality appraisal
The quality of the included studies was assessed using STROBE for the assessment of observational studies [37]. Since all of the studies included in this systematic review were observational studies, we found this tool to be the more appropriate for their evaluation. We utilized 9 out of the 22 items of the STROBE checklist for the methodological assessment (Tables 3, 4). These items relate to items 5–8 and 12–16 in the original STROBE list. As part of the quality appraisal, we also analyzed the quality of pathologic evaluation of specimens (Table 5). Specifically, we sought whether there was a description of the pathologic criteria used for diagnosis, cited reference to consensus criteria used for diagnosis, and whether or not the diagnosis was established by an experienced/board-certified/certified musculoskeletal pathologist. In cases where the level of expertise of the pathologist was not specified, we contacted the corresponding authors [38, 39]. The quality appraisal score was comprised of 12 items (9 STROBE plus 3 pathologic criteria). Each item was scored as: well described (+), partly described (±), or poorly/not described (−). The final score was rounded off downward (e.g., an item that consisted of 1 well described [+] and 1 partly described [±] subitem was scored as partly described [±]). In cases of disagreement, consensus was sought between the two investigators (SS and JPM). Articles were included if 75% of items were well described (+). Two partly described items (±) counted as one well-described item (+). Quality assessments were conducted from the perspective of the populations and outcomes of interest to this review. After calculating and weighting the STROBE and pathologic criteria, all 18 studies were found to be relevant and eligible for inclusion in the systematic review.
The following data were collected for all eligible studies: name of the journal, author and the year of publication, study type, number of patients, age, gender, pathology criteria used for diagnosis, follow-up length, modality used for treatment, local recurrence rate, occurrence of metastases, complications related to modality treatment, and Musculoskeletal Tumor Society (MSTS) scores [40]. The primary outcome measure targeted for analysis was LR. The secondary outcome measures were metastasis, tumor-related mortality, complications, and functional outcome in terms of MSTS scores.
Outcome measurements
-
1.
Oncological outcomes
The oncological analysis was based on the presence of local recurrences (LR), metastases, or death related to the tumor. LR was defined as any recurrence of tumor following surgical treatment, as reported by the authors, regardless of the imaging modality used for surveillance. Relapse-free survival was defined as the length of time after primary treatment, that the patient survived without evidence to suggest a LR. In case there was a clear distinction between a residual tumor and local recurrence, the residual tumor cases were not included in the analysis. For every case of LR, were collected the following data: time to LR (for further calculation of relapse-free survival), whether or not it resulted in reoperation and pathology from reoperation.
-
2.
Functional outcome
The mean MSTS score with its SD was extracted or, if appropriate, calculated from individual patient data. In case of missing MSTS score standard deviations [22, 38, 41], we contacted the authors, of whom one responded [22] and provided us with the requested additional data. MSTS scores reported as percentage were converted to points.
-
3.
Complications
For each study included in the analysis, we retrieved the description of every complication specified in the text and the overall complication rate. We evaluated the following complications: infections, fracture, subluxation, dislocation, component loosening, non-union, joint stiffness, nerve deficit, wound complications, and systemic complications. If specified by the authors, we also retrieved the time to complication and whether or not it necessitated a reoperation. Owing to the limited information available, we narratively reported the data regarding different complications.
-
2.
Study characteristics
Eighteen retrospective cohort studies were included for final review and analysis following the selection process. There were seven (nonrandomized) comparative studies [1, 22, 23, 25, 27, 42, 43] reporting on WLE versus IL treatment and 11 single-arm studies [2, 8, 11, 12, 28, 29, 38, 39, 41, 43, 44] reporting solely on IL treatment (Table 2). The number of patients included in each study, basic demographics, and the type of adjuvant used are listed in Table 2. There were two level III studies and one level II study, and the remainder was level IV studies.
Statistical analysis
The Comprehensive Meta-analysis Software package (version 3.0, Biostat, Englewood, NJ) was used to execute the meta-analysis. Other descriptive statistical analysis was carried out using IBM SPSS (V.24; SPSS, Chicago, Illinois, USA).
For dichotomous outcomes, odds ratio (OR) and 95% confidence interval (CI) were calculated. For continuous outcomes, standard difference in means and 95% CI were calculated. The heterogeneity of treatment effect among trials was assessed using the I 2 statistics. This describes the percentage of total variation across studies that is due to heterogeneity rather than chance or random error [45]. A value greater than 50 percent reflects significant heterogeneity owing to real differences in study populations, protocols, interventions and outcomes. A random effects (DerSimonian–Laird) model was used for all analyses, as the data were accumulated from a series of studies that had been performed by researchers operating independently, using different modalities of treatment and follow-up methods. For single-arm studies, the event rate for LR and complication rate were computed and compared.
Relapse-free survival was calculated with the Kaplan–Meier method with the log-rank test used to compare between survival estimates of IL and WLE. We tested for publication bias by calculating funnel plot asymmetry with respect to recurrence rates. All P values were two-sided, and an α level of ≤ 0·05 was used to determine statistical significance.
Publication bias
Publication bias in the literature was assessed with a funnel plot (Fig. 2). The funnel plot asymmetry indicated the possibility of some missing studies.
Therefore, we utilized the Duval and Tweedie’s trim and fill method. Here, the asymmetric outlying part of the funnel plot was trimmed off, and the number of studies in this asymmetric part was estimated. These studies were used to estimate the true center of the funnel. This estimate suggested that missing studies would not significantly affect the results, which indicated the influence of the publication bias was small. The asymmetry of the funnel plots seems to reflect the clinical and methodologic heterogeneity rather than publication bias.
Results
Study population
A total of 18 studies, comprising a total number of 695 patients (range 9–108 per study) with a male-to-female ratio of 1:1.8 were included in this review. The mean patient age per study ranged from 35 to 55.5 years.
There were 444 tumors confined to the lower extremity (63.8%) and 253 to the upper extremity (36.4%). Aggregated data showed that the femur was the bone most commonly involved (314 cases, 45.1%), followed by the humerus (241, 34.6%) and the tibia (102, 14.6%).
Pooled individual patient data retrieved from seven studies, [2, 22, 23, 29, 42, 44, 46], reporting a total of 137 patients, were available for analysis.
Oncological outcomes
-
A.
Local recurrence
The local recurrence rate for intralesional treatment was obtained for each of the 18 studies [1, 2, 8, 11, 12, 22, 23, 25, 27,28,29, 38, 39, 41,42,43,44, 46] and varied from 1.4 to 13.3%. A one-arm meta-analysis of all IL subgroups from all 18 studies showed a mean event rate of 0.089 (95% CI 0.065–0.120) for IL treatment, regardless of the adjuvant therapy used, with low heterogeneity (I 2 = 0.0%, P = 0.68) (Fig. 3a). Meta-analysis of seven available comparative studies [1, 22, 23, 25, 27, 42, 47] (Fig. 4a) showed that the recurrence rate was not significantly different between the WLE group and the IL treatment group (OR 2.31; 95% CI, 0.85–6.26; P = 0.1) with low heterogeneity (P = 0.775; I 2 = 0.00%). Pooled individual patient data from seven studies [2, 22, 23, 29, 42, 44, 46], reporting on a total of 137 patients, was analyzed for LR between comparing WLE (N = 45) to IL treatment (N = 92). LR rates of 8.6% (8 cases) in the IL group and 4.4% (2 cases) in the WLE group were found, and this difference was found not to be statistically significant (Fisher’s exact test, P = 0.497)
Fig. 3 Forrest plots of event rates for LR (a) and complication rates (b) with IL therapy for single-arm studies and IL subgroups from comparative studies, using the random effects model. The areas of the squares are proportional to the weights used for combining the data; diamonds represent overall risk estimates; horizontal lines represent 95% CI. CI confidence interval
Adjuvant therapy
There most commonly reported adjuvants in our review were phenol and cryotherapy. Therefore, we carried out another analysis for LR, including only studies reporting either one of these two adjuvants (Fig. 5a). Phenol was found to have a comparable LR rate compared to cryotherapy [event rate 0.085 (95% CI 0.058–0.134) versus 0.078 (95% CI 0.036–0.159), respectively, P = 0.741].
Another analysis of pooled individual participant data (N = 80) was then carried out to compare between different adjuvants utilized in intralesional surgery [no adjuvant (N = 29), phenol (N = 6), cryotherapy (N = 38), polymethyl methacrylate (PMMA) (N = 6), and hydrogen peroxide (N = 1)]. There were a total of six recurrences described (three with cryotherapy, two with no adjuvant, and one with PMMA), with no statistically significant difference between the different local adjuvants (P = 0.85).
Relapse-free survival
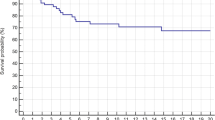
Pooled individual participant data was available for Kaplan–Meier analysis (Fig. 6a). The relapse-free survival at 5 years was 90.8% for the entire cohort, 93.5% for WLE, and 88.9% for IL treatment. Comparison between WLE and IL treatment showed no statistically significant difference in relapse-free survival (log-rank = 0.392, P = 0.531) (Fig. 6b).
-
B.
Metastases
Metastases from low-grade CS were reported in two comparative studies [1, 25], with a total of five patients. The other 16 studies reported no metastases at final follow-up. Therefore, the overall rate of metastatic disease in the entire cohort can be estimated to be 0.71%. A meta-analysis of the seven comparative studies [1, 22, 23, 25, 27, 42, 47] comparing the rate of metastases demonstrated no statistically significant difference between WLE and IL treatment (OR 0.88; 95% CI, 0.097–8.17; P = 0.91), with a low between-study heterogeneity observed (I 2 = 21.77%, P = 0.258) (Fig. 7). The mean time from the initial surgery to diagnosis with metastases was 27.4 months (range 4–72 months). One patient had metastases on multiple sites, while the other four patients had a single-site metastases. The most common site was the lung (5 out of 5 patients). In two of the patients, metastases associated with recurrent tumors upgraded to either grade II CS or dedifferentiated CS.
Of the five patients with metastatic disease, two patients with metastasis involving the lung were treated with metastectomy. Systemic treatment was not specified by the authors. Three of the patients with metastatic disease died at final follow-up.
Functional outcomes
MSTS scores were reported on 11 studies [2, 8, 12, 22, 28, 29, 38, 41, 42, 44, 46]. However, two studies did not report the standard deviation and therefore were not included in the analysis [38, 41]. The mean reported MSTS score ranged from 21.8 to 28.2 for WLE and from 26.5 to 29.7 for IL. Three comparative studies reported MSTS scores for both WLE and IL treatment [22, 42, 46]. A meta-analysis of those studies revealed a standardized difference in means of 1.39 points (SE 0.27, range 0.84–1.93, P < 0.001) with low heterogeneity (P = 0.44; I 2 = 0.0%) (Fig. 8).
Individual participant data from six studies [2, 22, 29, 42, 44, 46] were available for analysis of MSTS scores, reporting individual scores for a total of 103 patients. The mean MSTS score was 28.12 for the IL treatment group (N = 70) compared to 23.88 for the WLE group (N = 33), finding that was statistically significant in favor of the IL treatment group (mean difference 4.23, SE = 0.722, 95% CI 2.8–5.6, P < 0.001).
Complication rates
All studies except two [23, 41] reported on complications. Overall, there were 62 reported complications (61 patients) in 616 patients (excluding the two studies in which report complication rate was not reported), giving an overall complication rate of 10.06% (61/616).
Meta-analysis of data obtained from five comparative studies (Fig. 4b) reporting on complication rates demonstrated a significantly lower rate in favor of the IL group (OR 2.27; 95% CI, 0.07–0.72; P = 0.012) with low heterogeneity (P = 0.62; I 2 = 0.00%).
A separate one-arm meta-analysis of all IL subgroups from all 18 studies showed a mean event rate of 0.108 (95% CI 0.08–0.146) for IL treatment, regardless of the adjuvant used, with low heterogeneity (I 2 = 4.86%, P = 0.39) (Fig. 3b).
Individual participant data reporting on complications showed that the most common complications were fracture (50%), followed by dislocation (11.1%) and non-union (11.1%) within the WLE group. For patients undergoing IL treatment, fractures (73.81%) were found to be the most common complication, followed by infection (7.14%) and joint stiffness (7.14%) (Fig. 9).
Adjuvant therapy
Single-arm meta-analysis comparing between phenol and cryotherapy showed a higher complication rate for cryotherapy. However, the difference between these two modalities was not statistically significant (0.089; 95% CI 0.041–0.183 vs. 0.126, 95% CI 0.069–0.220, respectively, P = 0.587) (Fig. 5b).
Reoperation rate
We further analyzed individual participant data available to compare the likelihood of requiring a reoperation, between WLE and IL. The overall reoperation rate following a complication was 71%. The reoperation rate was found to be 68% for IL treatment versus 77.7% for WLE, a difference that was not statistically significant (Fisher’s exact test, P = 0.58). Likewise, the reoperation rate following a fracture was not significantly different (70.9% for IL vs. 71.4% for WLE, P = 0.68).
Discussion
The diagnosis and treatment of low-grade CS continues to be controversial. Differentiation between chondral lesions of different histological grades on preoperative imaging alone is complicated by interobserver variability [9]. Nevertheless, several recent studies have demonstrated the safety of surgical treatment for low-grade CS, diagnosed based purely on preoperative imaging, without biopsy [48, 49] as long as strict criteria are adhered to in the MRI diagnosis (high-grade features include: peritumoral edema, cortical destruction, cortical expansion, periostitis, and soft tissue extension). The role of biopsy in the preoperative assessment of low-grade chondral tumors is questionable. Disadvantages of biopsy include sampling errors, delay in treatment, increased risk of local recurrence from seeding, morbidity and cost, and a risk of undergrading or overgrading the tumor [49, 50]. Brown et al. [48] reported a series of 53 patients and demonstrated the safety of intralesional treatment for low-grade CS when relying on preoperative radiology with or without additional needle biopsy, with only a small proportion showing high-grade features on final histology. The authors suggest that a biopsy should be reserved only for cases of chondral lesions demonstrating atypical or inconclusive features on MRI. This stresses the importance of a multidisciplinary approach, with combined clinical and radiological assessment for planning the surgical treatment of these tumors.
While the traditional treatment with wide resection ensures total removal of tumor cells, these procedures may require complex reconstructions and are associated with a higher degree of morbidity [2]. In a previous meta-analysis, curettage has been shown to not greatly increase the risk of local recurrence and metastases [14]. Different adjuvants have been proposed as methods to extend the zone of tumor kill, among which are: phenol, alcohol, electrocautery, PMMA, and cryotherapy. However, there are currently no clinical studies to compare the effectiveness of different modalities for extended curettage, to our knowledge. As there is no consensus regarding the best surgical treatment of this low-grade malignancy, performing a systematic review of the literature in the absence of quality randomized prospective trials might offer some insight into the best treatment option.
We aimed to identify which surgical approach provides the lowest recurrence rate, lowest complications rate and the best functional outcome measured by the MSTS score. Our findings suggest no statistically significant advantage to WLE in terms of local recurrence or metastases. On the other hand, we demonstrated a significantly lower rate of complications and higher mean functional scores, with IL treatment. These findings are in line with a previous meta-analysis by Hickey et al. [14] who included five comparative studies and found an odds ratio of 2.26 for IL treatment (95% confidence interval, 0.41–12.62) for local recurrence, which is very similar to our finding (OR 2.31; 95% CI, 0.85–6.26; P = 0.1).
Functionally, IL treatment was found to yield better mean MSTS scores at final follow-up when compared with WLE, bases on a meta-analysis of three comparative studies. Obviously, functional outcome improves dramatically when limbs and joints are spared.
With regard to the specific type adjuvant therapy used during IL treatment, we sought to compare between the two most commonly reported types of adjuvants, cryosurgery and phenol, which were used in five and five studies, respectively. Owing to the limited information available, the current collected data did not allow for subgroup analysis of other adjuvants used as means to extend the margins of IL curettage, such as ethanol [39], hydrogen peroxide [22], PMMA [41], and thermal cauterization [8].
Fractures have been described as a common complication following cryosurgery to weight-bearing bones, due to the production of a large cavity with a rim of eggshell-like dead bone [51]. Yun et al. compared the extent of necrosis created with cryosurgery versus phenol in dogs’ femurs. They found a significantly larger area of damage with cryosurgery, while the effect of phenol was negligible in that only microscopic areas of superficial focal necrosis were found around the cavity wall [52]. We would therefore expect to find a greater recurrence rate with phenol, and perhaps more complications with cryosurgery. Our analysis showed no considerable differences between those two modalities with regard to local recurrence rates, complication rates, or reoperation rates. However, these results should be interpreted with caution since there are no actual comparative studies, to our knowledge, between these two modalities of treatment.
There are several limitations to this study, inherent to the nature of the available data. The studies included are all retrospective cohort design with a relatively small sample sizes subject to systematic and random biases. Unfortunately, this is true in general for orthopedic oncology, as retrospective studies continue to be the dominant form of evidence for the surgical management of primary bone tumors of the extremities [53, 54]. The results presented consist of aggregated as well as pooled individual participant data extracted from many smaller studies with various treatment modalities employed and varying patient populations. However, the low incidence of CS in general makes comparative trials extremely difficult to perform. Since the pathologic criteria and diagnostic measures employed in these studies were critically evaluated, the likelihood of confounding variables and bias was minimized. With respect to possible selection bias, the demographics from pooled data available showed that patients in the intralesional group were significantly younger than patients undergoing WLE (42.9 ± 14.7 vs. 49.43 ± 12.93, P = 0.036). This is likely due to the tendency to preserve joint function and therefore avoid arthroplasties in younger patients. Another limitation of the study is the selection bias with regard to the surgical treatment chosen, as WLE was probably reserved for cases with a more aggressive radiologic appearance. What would have been the recurrence rate with curettage in this patient population remains unknown.
The small number of included studies and their relatively small sample sizes are a possible reason for failing to detect study heterogeneity if it did exist, as the test for heterogeneity is low powered in this type of setup. In addition, the number of events for primary and secondary outcomes was low, especially metastases.
We believe there is a need for methodologically high-quality studies with more uniform study design and more uniform reporting. Wise decisions with regard to the choice for WLE or IL treatment will require trials of higher quality. Despite these limitations, we believe our findings represent the best available evidence.
Conclusions
The results of the current systematic review and meta-analysis indicate better functional results and a lower complication rate for IL treatment, with no significant difference in risk of local recurrence or metastasis, compared to WLE.
Further collaboration in the field of surgical oncology, using randomized controlled trials, is required to establish the superiority of any particular adjuvant treatment used during IL treatment for low-grade CS.
References
Leerapun T, Hugate RR, Inwards CY et al (2007) Surgical management of conventional grade I chondrosarcoma of long bones. Clin Orthop Relat Res 463:166–172
Mohler DG, Chiu R, McCall DA, Avedian RS (2010) Curettage and cryosurgery for low-grade cartilage tumors is associated with low recurrence and high function. Clin Orthop Relat Res 468:2765–2773
Evans HL, Ayala AG, Romsdahl MM (1977) Prognostic factors in chondrosarcoma of bone: a clinicopathologic analysis with emphasis on histologic grading. Cancer 40:818–831
Inwards CY, Unni KK (1995) Classification and grading of bone sarcomas. Hematol Oncol Clin North Am 9:545–569
Mirra JM, Gold R, Downs J, Eckardt JJ (1985) A new histologic approach to the differentiation of enchondroma and chondrosarcoma of the bones. A clinicopathologic analysis of 51 cases. Clin Orthop Relat Res 201:214–237
Rozeman LB, Cleton-Jansen AM, Hogendoorn PC (2006) Pathology of primary malignant bone and cartilage tumours. Int Orthop 30:437–444
Welkerling H, Kratz S, Ewerbeck V, Delling G (2003) A reproducible and simple grading system for classical chondrosarcomas. Analysis of 35 chondrosarcomas and 16 enchondromas with emphasis on recurrence rate and radiological and clinical data. Virchows Arch 443:725–733
Mermerkaya MU, Bekmez S, Karaaslan F et al (2014) Intralesional curettage and cementation for low-grade chondrosarcoma of long bones: retrospective study and literature review. World J Surg Oncol 12:336
Skeletal Lesions Interobserver Correlation among Expert Diagnosticians Study G (2007) Reliability of histopathologic and radiologic grading of cartilaginous neoplasms in long bones. J Bone Joint Surg Am 89:2113–2123
Errani C, Tsukamoto S, Ciani G et al (2017) Risk factors for local recurrence from atypical cartilaginous tumour and enchondroma of the long bones. Eur J Orthop Surg Traumatol
Verdegaal SH, Brouwers HF, van Zwet EW et al (2012) Low-grade chondrosarcoma of long bones treated with intralesional curettage followed by application of phenol, ethanol, and bone-grafting. J Bone Joint Surg Am 94:1201–1207
Meftah M, Schult P, Henshaw RM (2013) Long-term results of intralesional curettage and cryosurgery for treatment of low-grade chondrosarcoma. J Bone Joint Surg Am 95:1358–1364
Normand AN, Cannon CP, Lewis VO et al (2007) Curettage of biopsy-diagnosed grade 1 periacetabular chondrosarcoma. Clin Orthop Relat Res 459:146–149
Hickey M, Farrokhyar F, Deheshi B et al (2011) A systematic review and meta-analysis of intralesional versus wide resection for intramedullary grade I chondrosarcoma of the extremities. Ann Surg Oncol 18:1705–1709
van der Geest IC, de Valk MH, de Rooy JW et al (2008) Oncological and functional results of cryosurgical therapy of enchondromas and chondrosarcomas grade 1. J Surg Oncol 98:421–426
Lee FY, Mankin HJ, Fondren G et al (1999) Chondrosarcoma of bone: an assessment of outcome. J Bone Joint Surg Am 81:326–338
Fiorenza F, Abudu A, Grimer RJ et al (2002) Risk factors for survival and local control in chondrosarcoma of bone. J Bone Joint Surg Am 84:93–99
Bjornsson J, McLeod RA, Unni KK et al (1998) Primary chondrosarcoma of long bones and limb girdles. Cancer 83:2105–2119
Etchebehere M, de Camargo OP, Croci AT et al (2005) Relationship between surgical procedure and outcome for patients with grade I chondrosarcomas. Clinics (Sao Paulo) 60:121–126
Eriksson AI, Schiller A, Mankin HJ (1980) The management of chondrosarcoma of bone. Clin Orthop Relat Res 153:44–66
de Camargo OP, Baptista AM, Atanasio MJ, Waisberg DR (2010) Chondrosarcoma of bone: lessons from 46 operated cases in a single institution. Clin Orthop Relat Res 468:2969–2975
Aarons C, Potter BK, Adams SC et al (2009) Extended intralesional treatment versus resection of low-grade chondrosarcomas. Clin Orthop Relat Res 467:2105–2111
Bauer HC, Brosjo O, Kreicbergs A, Lindholm J (1995) Low risk of recurrence of enchondroma and low-grade chondrosarcoma in extremities. 80 patients followed for 2–25 years. Acta Orthop Scand 66:283–288
Donati D, Colangeli S, Colangeli M et al (2010) Surgical treatment of grade I central chondrosarcoma. Clin Orthop Relat Res 468:581–589
Gunay C, Atalar H, Hapa O et al (2013) Surgical management of grade I chondrosarcoma of the long bones. Acta Orthop Belg 79:331–337
Funovics PT, Panotopoulos J, Sabeti-Aschraf M et al (2011) Low-grade chondrosarcoma of bone: experiences from the vienna bone and soft tissue tumour registry. Int Orthop 35:1049–1056
Campanacci DA, Scoccianti G, Franchi A et al (2013) Surgical treatment of central grade 1 chondrosarcoma of the appendicular skeleton. J Orthop Traumatol 14:101–107
Souna BS, Belot N, Duval H et al (2010) No recurrences in selected patients after curettage with cryotherapy for grade I chondrosarcomas. Clin Orthop Relat Res 468:1956–1962
Schreuder HW, Pruszczynski M, Veth RP, Lemmens JA (1998) Treatment of benign and low-grade malignant intramedullary chondroid tumours with curettage and cryosurgery. Eur J Surg Oncol 24:120–126
Ozaki T, Lindner N, Hillmann A et al (1996) Influence of intralesional surgery on treatment outcome of chondrosarcoma. Cancer 77:1292–1297
Okada K, Nagasawa H, Chida S, Nishida J (2009) Curettage with pasteurization in situ for grade 1 chondrosarcoma—long-term follow up study of less invasive surgical procedure. Med Sci Monit 15:Cs44–Cs48
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62:e1–34
Hutton B, Catala-Lopez F, Moher D (2016) The PRISMA statement extension for systematic reviews incorporating network meta-analysis: PRISMA-NMA. Med Clin (Barc) 147:262–266
Schardt C, Adams MB, Owens T et al (2007) Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak 7:16
Booth A, Clarke M, Dooley G et al (2012) The nuts and bolts of PROSPERO: an international prospective register of systematic reviews. Syst Rev 1:2
Wright JG, Swiontkowski MF, Heckman JD (2003) Introducing levels of evidence to the journal. J Bone Joint Surg Am 85-A:1–3
von Elm E, Altman DG, Egger M et al (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370:1453–1457
Di Giorgio L, Touloupakis G, Vitullo F et al (2011) Intralesional curettage, with phenol and cement as adjuvants, for low-grade intramedullary chondrosarcoma of the long bones. Acta Orthop Belg 77:666–669
Kim W, Han I, Kim EJ et al (2015) Outcomes of curettage and anhydrous alcohol adjuvant for low-grade chondrosarcoma of long bone. Surg Oncol 24:89–94
Enneking WF, Dunham W, Gebhardt MC et al (1993) A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res 286:241–246
Hanna SA, Whittingham-Jones P, Sewell MD et al (2009) Outcome of intralesional curettage for low-grade chondrosarcoma of long bones. Eur J Surg 35:1343–1347
Chen YC, Wu PK, Chen CF, Chen WM (2016) Intralesional curettage of central low-grade chondrosarcoma: A midterm follow-up study. J Chin Med Assoc
Dierselhuis EF, Gerbers JG, Ploegmakers JJ et al (2016) Local treatment with adjuvant therapy for central atypical cartilaginous tumors in the long bones: analysis of outcome and complications in one hundred and eight patients with a minimum follow-up of two years. J Bone Joint Surg Am 98:303–313
Ahlmann ER, Menendez LR, Fedenko AN, Learch T (2006) Influence of cryosurgery on treatment outcome of low-grade chondrosarcoma. Clin Orthop Relat Res 451:201–207
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Donati D, Colangeli S, Colangeli M et al (2010) Surgical treatment of grade I central chondrosarcoma. Clin Orthop Relat Res 468:581–589
Donati D, Yin JQ, Colangeli M et al (2008) Clear cell chondrosarcoma of bone: long time follow-up of 18 cases. Arch Orthop Trauma Surg 128:137–142
Brown M, Gikas P, Bhamra J et al (2014) How safe is curettage of low-grade cartilaginous neoplasms diagnosed by imaging with or without pre-operative needle biopsy? Bone Joint J 96:1098–1105
Berber O, Datta G, Sabharwal S et al (2012) The safety of direct primary excision of low-grade chondral lesions based on radiological diagnosis alone. Acta Orthop Belg 78:254–262
Jennings R, Riley N, Rose B et al (2010) An evaluation of the diagnostic accuracy of the grade of preoperative biopsy compared to surgical excision in chondrosarcoma of the long bones. Int J Surg Oncol 2010:270195
Pritsch T, Bickels J, Wu CC et al (2007) The risk for fractures after curettage and cryosurgery around the knee. Clin Orthop Relat Res 458:159–167
Yun YH, Kim NH, Han DY, Kang ES (1993) An investigation of bone necrosis and healing after cryosurgery, phenol cautery or packing with bone cement of defects in the dog femur. Int Orthop 17:176–183
Lerman DM, Cable MG, Thornley P et al (2016) Has the level of evidence of podium presentations at the Musculoskeletal Tumor Society annual meeting changed over time? Clin Orthop Relat Res 1–8
Evaniew N, Nuttall J, Farrokhyar F et al (2014) What are the levels of evidence on which we base decisions for surgical management of lower extremity bone tumors? Clin Orthop Relat Res 472:8–15
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Shemesh, S.S., Acevedo-Nieves, J.D. & Pretell-Mazzini, J. Treatment strategies for central low-grade chondrosarcoma of long bones: a systematic review of the literature and meta-analysis. Musculoskelet Surg 102, 95–109 (2018). https://doi.org/10.1007/s12306-017-0507-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12306-017-0507-7