Abstract
Background
The low aggressiveness of Grade I chondrosarcomas is compatible with conservative surgical treatment.
Questions/purpose
We asked whether combined curettage and cryotherapy would yield low rates of recurrence and whether supplemental internal fixation would retain function with low rates of complications in patients with Grade I central chondrosarcomas of the proximal humerus or distal femur.
Methods
We retrospectively reviewed 15 patients: nine women and six men with a mean age of 45 years (range, 26–70 years). All patients underwent curettage and cryosurgery through a cortical window; we replaced the window and plated the region with at least three screws beyond the curetted area. None of the patients was lost to followup, and 14 patients (93%) were reexamined by us after a minimum of 5 years (mean, 8 years; range, 5–11 years).
Results
There were no perioperative anesthetic, neurologic, hardware, or healing complications. None of the patients had local recurrence or metastases develop. At last followup, the Musculoskeletal Tumor Society score was 27.9 (range, 22–30) and all patients had resumed their previous activities. No complications were associated with this simplified cryotherapy technique.
Conclusions
The data confirm the appropriateness of conservative surgery for central low-grade chondrosarcomas of the proximal humerus and distal femur based on a combination of intralesional curettage and cryogenic parietal sterilization. Candidates for this approach should be chosen on the basis of the affected bone site, local extension staging, and clinicopathologic grading. We recommend supplementary internal fixation.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biopsy-based diagnosis of Grade I central chondrosarcoma poses a thorny problem for the surgeon. Simple radiographic monitoring and followup provide one alternative but often are a source of concern for the patient and surgeon [21]. Surgery is the only curative treatment option [11]. Extensive excision minimizes the possibility of recurrences and metastases but often is mutilating; diaphyseal tumors require allograft reconstruction [9] and metaphyseal and epiphyseal tumors require the use of a prosthesis [12, 14]. Such aggressive treatment contrasts with the generally indolent growth of these low-grade tumors, which often are discovered by chance [15] and sometimes are mistaken for atypical enchondromas [4, 14]. Although there formerly was a tendency to opt for aggressive surgery, there now is a trend toward intralesional treatment of these low-grade chondrosarcomas combining tumor excision with skeletal conservation [1, 14, 18, 19, 24, 28]. After curettage alone, Bauer et al. noted two cases of recurrence among 23 patients with chondrosarcomas [2]. Adjuvant cryosurgery, an approach developed for other tumors [27], reportedly is associated with few recurrences of proximal bone chondrosarcomas [17] and associated with cures for more distal tumors [1, 14, 24]. However, few data are available regarding long-term outcome after combined curettage and adjuvant parietal sterilization [13, 14].
We therefore asked three questions: (1) whether curettage and cryotherapy in patients with Grade I long-bone intramedullary chondrosarcomas would eliminate recurrences and metastases; (2) whether the patients would be able to resume their activities and maintain high functional scores; and (3) whether the approach would have a low complication rate.
Patients and Methods
In 1997, we adopted combined curettage and cryotherapy for Grade I long-bone chondrosarcomas. The selection of the patients was based on a combination of radiographic and histopathologic criteria [7, 17]. The tumor had to be located in the proximal humerus or distal femur or the diaphysis of one of these bones (tumors of the proximal femur, girdles, or spine were excluded) and the patients had not previously received surgical treatment for their tumors. The tumors could not have eroded more than half of the cortical thickness without breaching it. The workup had to include standard radiography, MRI, and radionuclide bone scintigraphy. Radiographic endosteal erosion or a scintigraphic uptake greater than that of the ipsilateral iliac crest was considered suspect for malignancy [17, 22, 26]. The last two examinations were used to ensure the tumor was strictly intracompartmental with no perforation of the cortical bone corresponding to Enneking’s Stage IA [5] and that it was the only skeletal tumor. Chest radiography was used to rule out pulmonary metastasis. We considered cortical perforation, additional sites, or metastasis as contraindications. Prior confirmation of Grade I chondrosarcoma by open surgical biopsy was required. Cytologic and histologic findings were used for tumor grading following the study of O’Neal and Ackerman [23]. Grade I chondrosarcoma has an increased cellularity compared with chondromas and chondrocytes have a slightly enlarged dense nucleus. High cellularity and tissue-architectural features such as host bone entrapment and myxoid changes were useful to differentiate Grade I chondrosarcomas from chondromas [23]. Grade II chondrosarcomas are more cellular lesions with reduced calcification and more pronounced myxoid stroma. Nuclei are enlarged and hyperchromatic and cells more frequently are binucleated.
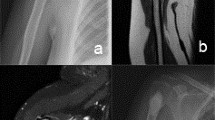
Between 1999 and 2002 we performed biopsies in and treated 55 patients with intraosseous cartilaginous tumors: 25 with benign chondromas (biopsy followed by radiographic monitoring), seven with Grade I chondrosarcomas of the proximal femur or girdles (which did not fulfill our anatomic criteria for cryoblation) and eight with Grade II or III chondrosarcomas (which did not fulfill our radiographic, histologic, or cytologic criteria for cryoblation); the remaining 15 patients with Grade I chondrosarcomas of the proximal humerus, diaphyseal or distal femur form the basis for this report. Among the 15 patients, there were nine women and six men with a mean age at diagnosis of 45 years (± 3; range, 26–70 years). Five tumors were painful and 10 were discovered by chance during rheumatologic investigations (Fig. 1). There were no pathologic fractures. On scintigraphy, all the tumors had an uptake greater than that for the ipsilateral iliac crest, which we considered as an internal standard [22]. There were 10 tumors of the proximal humerus and five of the distal femur (four metaphyseal and one diaphyseal). The mean long dimension of the tumors on radiographs was 41 mm (± 14 mm; range, 27–110 mm). We observed endosteal erosion in all 15 patients but always less than 50% of the cortical thickness (Fig. 2). An enhancement of the central portion of the tumor with gadolinium was seen in 12 of the 15 cases. No patients were lost to followup but one patient did not attend the final visit; however, he was seen by his general practitioner who reported the patient had no pain or local abnormalities. The other 14 patients (93%) attended the final visit after a minimum followup of 5 years (mean ± SD, 8 ± 1.5 years; range, 5–11 years).
The surgical biopsies and excisions were performed by the same team. During biopsy, we made a cortical window marked and fixed by interference screws (Fig. 3). At least three distinct tumor sites were biopsied proximal, central, and distal relative to the topographic center of the tumor (shown by fluoroscopy). The biopsy specimens were frozen and examined histologically and cytologically by the same trained musculoskeletal pathologist (GL). All samples (biopsy specimens and excised material) were reexamined by a second pathologist (HD) during the review in 2008. None of the chondrosarcomas (all Grade I on initial biopsy) was upgraded after initial examination of the curettage material. On second review for this study, the second pathologist (HD) concluded the lack of invasion of surrounding bone, myxoid changes, and nuclear atypia were inconsistent with the diagnosis of Grade I chondrosarcoma in two original biopsy samples; however, the increased cellularity and clinical and radiographic features were inconsistent with chondroma. After surgery, microscopic examination of the curettage samples underlined architectural features of malignancy and the diagnosis of Grade I chondrosarcoma finally was confirmed for these two patients.
All patients underwent surgery under general anesthesia. The limbs were draped free for mobility. The surgical approach was chosen to excise the biopsy track and to avoid neurovascular pedicles. The interference screws were removed and the cortical window enlarged with a rectangular shape to allow extensive manual curettage and reaming of the tumor cavity walls. After curettage, the tumor cavity was flooded with liquid nitrogen three times, once every 10 minutes. A temperature probe was not used. The free limb segment was mobilized to swirl the liquid nitrogen around the entire surface of the cavity wall. The cut surfaces of the muscle and skin were protected with three thicknesses of woven surgical sheet, which were changed after each cycle. We replaced the cortical window and plated the area with at least three screws proximal and three distal to the area of bone exposed to liquid nitrogen. We supplemented the fixation with a nonvascularized fibular graft in one patient with extensive humeral involvement.
For patients with humeral tumors, we immobilized the arm with a sling and began immediate passive motion for 3 to 5 weeks. For patients with femoral tumors, we recommended crutch walking with no weightbearing for 3 months. We instituted no other treatment or physiotherapy apart from analgesia.
The patients were seen after 3, 6, and 12 months and then every year for 5 years and finally in 2008. At each visit, they had a functional assessment based on the Musculoskeletal Tumor Society (MSTS) scoring system [6] and a physical examination and radiography of the tumor site and chest. The radiographic evaluation was based on analysis of the area of curettage. We considered the lesion remodeled when the wall of the cavity progressed to a well-defined margin without any scalloping or “moth-eaten” zone associated with thickening of the cortex. The area had to progress toward mineralization and restoration of a central homogenous trabeculation. Quantitative values are expressed as mean, SD, and range.
Results
No patients had recurrence or metastases. No patient had local pain, but three continued to have joint pain related to the initial rheumatologic disorder that led to discovery of the tumor. All the chest radiographs were normal. Radiographs showed the treatment region was stable. No osteolysis or secondary fractures occurred. Bone remodeling and densification were noted in half of the patients (Fig. 4). One patient had three scintigraphic examinations for another indication, and the preoperative area of increased uptake disappeared 1 year after surgery.
All patients had resumed their normal personal and professional activities. The MSTS score was 27.9 points (± 3; range, 22–30 points).
There were no perioperative anesthetic complications and no neurologic, healing, or septic disorders. Prominent screws from the internal fixation were removed from two patients. One was located subcutaneously above a femoral condyle, and the other was located in the subacromial bursa. All the internal fixation plates were left in place. One patient (the one who did not return for followup at our clinic) had an early fracture distal to the humeral plate beyond the region of bone cryotherapy. The fracture healed after the patient used a sling for 3 months. When seen by his general practitioner for a followup for this study, he reported no pain or local abnormalities.
Discussion
The decision to deescalate surgical treatment of low-grade central chondrosarcomas is difficult for experienced teams who are aware of the difficulties of treating patients with local recurrences and metastases [12–14]. However, the damages associated with extensive surgical resection are incommensurate with the low-aggressive nature of these tumors [14, 17]. We therefore asked three questions: (1) whether curettage and cryotherapy in patients with Grade I long-bone intramedullary chondrosarcomas would eliminate recurrences and metastases; (2) whether the patients would be able to resume their activities and maintain high functional scores; and (3) whether the approach would have a low complication rate.
The limitations of this study were a small study group and a second histologic analysis (HD), which was not blinded because the samples were not compared with those of a control group of patients with typical benign chondromas. Nevertheless, we included all patients meeting our anatomic and histopathologic criteria and there was complete agreement of the two pathologists for the diagnosis of low-grade sarcomas on the curettage samples (Fig. 5). We believe the minimal followup of 5 years is essential to draw conclusions regarding the evolution of low-grade tumors.
Host bone entrapment in well-differentiated cartilaginous tumor is a major architectural feature for the diagnosis of Grade I chondrosarcoma, especially if there is a lack of nuclear atypia. Presence of an area with higher cellularity and myxoid changes in other fields leads more easily to a diagnosis of malignancy (Stain, hematoxylin and eosin-safran; original magnification, ×100).
At a mean followup of 8 years, none of the 15 patients had signs of tumor recurrence or metastases. These findings therefore support those of others series (Table 1). The results seem to depend on two key points: proper selection of the patients and complete destruction of the tumor by the combination of extensive curettage with adjuvant cytotoxic therapy. Candidates for this approach should be chosen on the basis of the affected bone site, local extension staging, and clinicopathologic grading. The skeletal location of these tumors is the first criterion. In this series, the conservative technique was used only for distal femur, diaphyseal femur and humerus, and proximal humerus. Although Ahlmann et al. [1] reported successful curettage-cryotherapy of two scapular tumors and one acetabular tumor, followup was too short (3.2 years) to conclude the appropriateness of this technique for axial tumors, which tend to be of a higher grade [14] and more progressive [25, 27, 29]. The intracompartmental nature of the tumor is determined on imaging studies and especially MRI. For this treatment, we selected tumors that had eroded, at the most, half of the cortical thickness without breaching it. Even if determining the cortical thickness analysis is not reliable from one or two projected images, thinning of 50% or less could be considered an indicator of low-grade sarcomas [10, 26]. The second criterion is the intralesional excision, which may require enlargement of the cortical window [3]. In this series, parietal sterilization was achieved with a simplified cryotherapy technique based on that described by Marcove et al. [18]. We did not use an injector or a temperature probe as described in other series [1, 28], but this did not seem to compromise the ability to destroy the tumor. This technique seemed as effective as others (Table 1). Moreover, the results suggest filling of the residual cavity with cement [1] to prevent tumor spillage is useless. The third criterion is clinicopathologic grading. The histologic findings must be interpreted in view of clinical and radiographic features of the tumor [8] after studying its skeletal location and its local extension staging (Enneking stage). Grading is difficult for these tumors [8, 20, 23]. We believe open surgical biopsy is crucial before deciding on this conservative treatment [8], because it can provide sufficient material from several areas of the tumor.
In this series, all the patients resumed their activities and had a functional score close to normal. These findings support those of all the series that use conservative treatments with adjacent bone and joint preservation (Table 1) and are far more satisfying than those obtained with large resections [9, 12, 13].
The approach used in this series had no complications. We identified no patients with air embolism or nerve palsy. The rate reported in a recent comparative study is approximately 2% for those two last complications [30]. It is crucial when choosing the surgical approach to avoid neurovascular structures. Although we have no data, we believe the sheets helped protect the incised skin and muscle and we replaced the sheets after each of the three instillations of liquid nitrogen into the tumor cavity. The risk of fracture was reported from 1% (four of 440) by Meller et al. [19] to 20% by Marco et al. [17]. Van der Geest et al. reported a rate of 14% in a series of 130 enchondromas and Grade I chondrosarcomas, six fractures in the proximal humerus and 12 in the distal femur [30]. In their series [30], prophylactic internal fixation was used in only 11 of 130 cases when it was systematic in our series, which had no fractures at the same anatomic sites. A second explanation could be the simplified technique we used obtained frozen margins probably inferior to the 15-mm thickness estimated by Van der Geest et al. [30] or the 7 to 12 mm observed in a dog model by Malawer et al. [16]. This bone necrosis appeared sufficient to kill residual tumor cells and prevent recurrence of the tumor but did not seem to have penetrated the entire cortical bone, because the treated region had the long-term behavior of viable rather than devitalized bone with radiographic progressive mineralization. An autologous graft was used in one patient but in retrospect, we suspect was unnecessary. Polymethylmethacrylate cement has been used in some series [1, 16] as a coadjuvant therapy and to obtain immediate bone strength but could impair the mineralization observed in half of the cases of our series and even with a tumor cavity left empty.
With a minimum followup of 5 years, these data confirm the use of combined treatment with intralesional curettage and parietal sterilization for central low-grade chondrosarcomas. Candidates for this conservative treatment must be chosen on the basis of the affected bone site, macroscopic stage (IA), and histocytologic Grade I. Cryotherapy associated with internal fixation achieved tumor sterilization, allowed preservation of function, and precluded complications.
References
Alhmann ER, Menendez LR, Fedenko AN, Learch T. Influence of cryosurgery on treatment outcome of low-grade chondrosarcoma. Clin Orthop Relat Res. 2006;451:201–207.
Bauer HC, Brosjo O, Kreicbergs A, Lindholm J. Low risk of recurrence of enchondroma and low-grade chondrosarcoma in extremities: 80 patients followed for 2–25 years. Acta Orthop Scand. 1995;66:283–288.
Bickels J, Meller I, Shmookler BM, Malawer MM. The role and biology of cryosurgery in the treatment of bone tumors: a review. Acta Orthop Scand. 1999;70:308–315.
Eefting D, Schrage YM, Geirnaerdt MJ, Le Cessie S, Taminiau AH, Bovée JW, Hogendoorn PC; EuroBoNeT consortium. Assessment of interobserver variability and histologic parameters to improve reliability in classification and grading of central cartilaginous tumors. Am J Surg Pathol. 2009;33:50–57.
Enneking WF. A system of staging musculoskeletal neoplasms. Clin Orthop Relat Res. 1986;204:9–24.
Enneking WF, Dunham W, Gebhardt MC, Malawar MM, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246.
Evans HL, Ayala AG, Romsdahl MM. Prognostic factors in chondrosarcoma of bone: a clinicopathologic analysis with emphasis on histologic grading. Cancer. 1977;40:818–831.
Flemming DJ, Murphey MD. Enchondroma and chondrosarcoma. Semin Musculoskelet Radiol. 2000;4:59–71.
Fuchs B, Ossendorf C, Leerapun T, Sim FH. Intercalary segmental reconstruction after bone tumor resection. Eur J Surg Oncol. 2008;34:1271–1276.
Geirnaerdt MJ, Hermans J, Bloem JL, Kroon HM, Pope TL, Taminiau AH, Hogendoorn PC. Usefulness of radiography in differentiating enchondroma from central grade 1 chondrosarcoma. AJR Am J Roentgenol. 1997;169:1097–1104.
Healey JH, Lane JM. Chondrosarcoma. Clin Orthop Relat Res. 1986;204:119–129.
Langlais F, Lambotte JC, Collin P, Thomazeau H. Long-term results of allograft composite total hip prostheses for tumors. Clin Orthop Relat Res. 2003;414:197–211.
Lee FY, Mankin HJ, Fondren G, Gebhardt MC, Springfield DS, Rosenberg AE, Jennings LC. Chondrosarcoma of bone: an assessment of outcome. J Bone Joint Surg Am. 1999;81:326–338.
Leerapun T, Hugate RR, Inwards CY, Scully SP, Sim FH. Surgical management of conventional grade I chondrosarcoma of long bones. Clin Orthop Relat Res. 2007;463:166–172.
Levy JC, Temple HT, Mollabashy A, Sanders J, Kransdorf M. The causes of pain in benign solitary enchondromas of the proximal humerus. Clin Orthop Relat Res. 2005;431:181–186.
Malawer MM, Marks MR, McChesney D, Piasio M, Gunther SF, Schmookler BM. The effect of cryosurgery and polymethylmethacrylate in dogs with experimental bone defects comparable to tumor defects. Clin Orthop Relat Res. 1988;226:299–310.
Marco RA, Gitelis S, Brebach GT, Healey JH. Cartilage tumors: evaluation and treatment. J Am Acad Orthop Surg. 2000;8:292–304.
Marcove RC, Stovell PB, Huvos AG, Bullough PB. The use of cryosurgery in the treatment of low and medium grade chondrosarcoma: a preliminary report. Clin Orthop Relat Res. 1977;122:147–156.
Meller I, Weinbroum A, Bickels J, Dadia S, Nirkin A, Merimsky O, Issakov J, Flusser G, Marouani N, Cohen N, Kollender Y. Fifteen years of bone tumor cryosurgery: a single-center experience of 440 procedures and long-term follow-up. Eur J Surg Oncol. 2008;34:921–927.
Mirra JM, Gold R, Downs J, Eckardt JJ. A new histologic approach to the differentiation of enchondroma and chondrosarcoma of the bones: a clinicopathologic analysis of 51 cases. Clin Orthop Relat Res. 1985;201:214–237.
Müller PE, Dürr HR, Wegener B, Pellengahr C, Maier M, Jansson V. Solitary enchondromas: is radiographic follow-up sufficient in patients with asymptomatic lesions? Acta Orthop Belg. 2003;69:112–118.
Murphey MD, Flemming DJ, Boyea SR, Bojescul JA, Sweet DE, Temple HT. Enchondroma versus chondrosarcoma in the appendicular skeleton: differentiating features. Radiographics. 1998;18:1213–1237.
O’Neal LW, Ackerman LV. Chondrosarcoma of bone. Cancer. 1952; 5:551–577.
Pidhorz L, Le Nay P, Boscher Y, François H. [Treatment of chondrosarcoma of the tibia by cryosurgery: apropos of 2 cases] [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1987;73:401–406.
Pring ME, Weber KL, Unni KK, Sim FH. Chondrosarcoma of the pelvis: a review of sixty-four cases. J Bone Joint Surg Am. 2001;83:1630–1642.
Rosenthal DI, Schiller AL, Mankin HJ. Chondrosarcoma: correlation of radiological and histological grade. Radiology. 1984;150:21–26.
Sanerkin NG, Gallagher P. A review of the behaviour of chondrosarcoma of bone. J Bone Joint Surg Br. 1979;61:395–400.
Schreuder HW, Pruszczynski M, Veth RP, Lemmens JA. Treatment of benign and low-grade malignant intramedullary chondroid tumors with curettage and cryosurgery. Eur J Surg Oncol. 1998;24:120–126.
Tsuchiya H, Ueda Y, Morishita H, Nonomura A, Kawashima A, Fellinger EJ, Tomita K. Borderline chondrosarcoma of long and flat bones. J Cancer Res Clin Oncol. 1993;119:363–368.
van der Geest IC, de Valk MH, de Rooy, Pruszczynski M, Veth RP, Schreuder HW. Oncological and functional results of cryosurgical therapy of enchondromas and chondrosarcomas grade 1. J Surg Oncol. 2008;98:421–426.
Acknowledgments
We thank Gérard Lancien MD, for the first histopathologic evaluation (1997–2002).
Author information
Authors and Affiliations
Corresponding author
Additional information
Frantz Langlais—Deceased.
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangement, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the reporting of these cases, that all investigations were conducted in conformity with ethical principles of research, and that informed consent was obtained.
About this article
Cite this article
Souna, B.S., Belot, N., Duval, H. et al. No Recurrences in Selected Patients after Curettage with Cryotherapy for Grade I Chondrosarcomas. Clin Orthop Relat Res 468, 1956–1962 (2010). https://doi.org/10.1007/s11999-009-1211-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-009-1211-1