Abstract
Two ionic surfactants (sodium dodecylsulphate (SDS) and sodium dodecylbenzenesuphonate (SDBS)) are chosen, and effect of addition of quaternary bromide or chloride (tetra-n-butylammonium bromide, tetra-n-butylphosphonium bromide, tetraphenylphosphonium bromide, tetra-npentylammonium bromide and benzyl tributylammonium chloride) has been studied on the cloud point (CP) behaviour in aqueous solution. CP behaviour was observed due to dehydration of headgroup region in presence of quaternary counterion. Order of counterion to decrease CP is as follows: TPeA+ > BTA+ > TBP+ > TPhP+ > TBA+ for SDS and TPeA+ > TPhP+ > BTA+ > TBP+ > TBA+ for SDBS. CP data have been used to select surfactant + salt system. Effect of additives (carbohydrate, amino acid or vitamin), on the CP, has been seen on above such selected systems. Additive may either decrease or increase the CP, depending upon the structure of counterion and/or the additive. Counterion (cations) can be arranged in an order like Hofmeister series.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is well known that solution behaviours of ionic and non-ionic surfactant solutions, at elevated temperature, are different [1]. Clouding phenomenon is a principle feature in non-ionic surfactant solution while it is less common with aqueous ionic surfactant. Recently, clouding phenomenon has also been reported with ionic surfactant solution under variety of experimental conditions (pH, special counterion (quaternary) or special headgroup, molecular architecture, etc.) [2–7]. Dehydration of the headgroup and counterion due to their mutual presence seem important for the occurrence of clouding phenomenon. Many amphiphilic drugs also show clouding phenomenon under specific conditions [8–10].
Surfactant and polymer are frequently investigated systems in the context of Hofmeister series [11–13]. However, progress in that field has been restricted due to lack of systematic work on Hofmeister effects with respect to phenomenon related to surface and colloid science [14]. Collins et al. [15] proposed a “law of matching water affinity” and correlated with the association of oppositely charged ions in aqueous solution. Clouding phenomenon in charged micellar solution is the consequence of the interaction between oppositely charged counterion and headgroup [3–6]. Clouding at lower temperature/concentration may facilitate the cloud point extraction methodology (CPEM) for the extraction of thermally labile moieties [16, 17].
Above facts prompted us to study Hofmeister effect in the context of clouding phenomenon in an anionic surfactant solution. In a previous study, we have established Hofmeister-like series of anionic headgroups with respect to a single quaternary ammonium counterion, i.e. tetra-n-butylammonium (TBA+) [18]. Cloud point (CP) data fitted in the ordering of headgroups proposed by Vlachy et al. [19]. Further, sulphate and sulphonate headgroups are more prone to produce clouding with TBA+ [18]. To generalise the clouding effect, we have chosen few symmetrical/unsymmetrical quaternary counterions and CP measurements have been performed in aqueous solutions of two anionic surfactants (Scheme 1), namely, sodium dodecylsulphate (SDS) and sodium dodecylbenzenesuphonate (SDBS). The quaternary salts chosen (Scheme 1), for driving the corresponding counterions, are as follows: tetra-n-butylammonium bromide (TBAB), tetra-n-butylphosphonium bromide (TBPB), tetraphenylphosphonium bromide (TPhPB), tetra-n-pentylammonium bromide (TPeAB) and benzyl tributylammonium chloride (BTAC). This study would provide an insight, about counterion specificity, in producing clouding in anionic surfactant solution. Further, carbohydrate, vitamins and amino acids are used in pharmaceuticals, medicinal and food preparations as they are non-toxic, bio-friendly and have fair solubility in water [20]. Present CP data may help to develop an alternative methodology for the extraction of charged organic moieties [1]. This would enlarge the spectrum of CPEM [16, 17, 21, 22] by including biological excipients. The CP plays a significant role in suggesting the limit of aqueous solubility and hence the use in physicochemical processes. The practical importance of the clouding phenomenon reflects in its application to solute extraction/ pre-concentration and detergency (it reaches to maximum just below the CP) [23, 24]. The present surfactant systems can be used to extract/pre-concentration both hazardous metal ions and hydrophobic organic material, in a single step, which is not possible with conventional solvent extraction techniques (with hazardous non-polar solvents).
Material and methods
Materials
SDS (≥99% Sigma-Aldrich, St. Louis, MO, USA), SDBS (99% TCI, Tokyo, Japan), TBAB (≥99% Sigma-Aldrich, St. Louis, MO, USA), TBPB (>99%, Sigma-Aldrich, St. Louis, MO, USA), BTAC (≥98%, Sigma-Aldrich, St. Louis, MO, USA), TPhPB (>98%, TCI, Tokyo, Japan), TPeAB (≥99%, Sigma-Aldrich, St. Louis, MO, USA), l-leucine (≥98%, Sigma-Aldrich, St. Louis, MO, USA), l-phenylalanine (≥99%, Sigma-Aldrich, St. Louis, MO, USA), l-ascorbic acid (≥99%, Sigma-Aldrich, St. Louis, MO, USA), α-cyclodextrin (98%, Spectrochem, Mumbai, India), β-cyclodextrin (99%, Spectrochem, Mumbai, India), d-glucose (Fisher Scientific) and sucrose (Fisher Scientific) were used as received. The water, used in preparing the sample solution, was double-distilled in an all-glass distillation setup (specific conductivity within 1–2 μS cm−1).
Various surfactant + quaternary salt solutions were prepared by taking requisite amounts of surfactant and quaternary salt and making up the volumes with distilled water.
Methods
CP measurement
CP data are acquired by placing samples containing surfactant (SDS or SDBS) solutions, with a fixed concentration of quaternary salt, into a temperature-controlling thermostat (SCHOTT CT 1650). The temperature of the sample solution was precisely controlled with an accuracy of ±0.1 °C. Temperature at the onset of turbidity (visual observation) has been note down. The system was allowed to cool, and the temperature at which the disappearance of turbidity took place has also been noted. The average of these two temperatures was taken as the cloud point (CP). This procedure was repeated for the same sample, and nearly two concurrent values (within ±0.1 °C) were considered as the final CP. Similar CP measurements were made by diluting the samples using double distilled water to collect CP data at various concentrations. The method was also adopted to get CPs in the presence of various biological excipients.
Surface tension measurement
Surface tension measurement have been carried out using a Du Nouy detachment tensiometer (Win–Son & Co., Kolkata) with a platinum (gold joint) ring at 30 ± 1 °C. The tensiometer was calibrated using double-distilled water. A known volume of water was added to a vessel containing stock solution (30 mL) of the quaternary salt. Solutions were agitated and stirred after each dilution.
NMR
NMR spectra were obtained with Bruker Avance 400 Spectrometer at different temperatures. All the sample solutions were prepared in D2O. About 0.6 mL of the solution was transferred to a 5-mm NMR tube and chemical shifts were recorded on the δ (ppm) scale.
Results and discussion
Quaternary counterions have four short alkyl chains in addition to positive charge on the central atom (e.g. N or P). Due to this reason, quaternary counterions can interact with anionic micelles via electrostatic and hydrophobic interactions. Detailed discussions on these interactions are reported elsewhere [2, 5, 6]. Vlachy et al. [25] discussed the role of the headgroup on the counterion specificity related to micelle to vesicle transition. It has been observed that if the sulphate headgroup is replaced by carboxylate headgroup, the order of the ions is reversed, i.e. it follows the reversed Hofmeister series, towards micellar structural transition. Hofmeister series is known in many areas, the mechanism is yet to be settled. This may be due to a complex interplay of direct and indirect effects of ions on the solute molecule and on the water structure. The situation is even more unclear in the case of clouding phenomenon. To shed more light on clouding phenomenon in ionic surfactants solutions, various symmetrical and unsymmetrical quaternary counterions are added to SDS or SDBS solution. Regarding clouding phenomenon in ionic surfactant solutions, dehydration of counterion or headgroup plays a decisive role in controlling the CP [18, 19]. The quaternary counterions consist of four alkyl chains/phenyl ring in addition to positive charge on the central atom (N or P). Therefore, such counterions can interact with the micelle hydrophobically (by penetrating some alkyl chains/ring between monomers of the micelle) and electrostatically (as counterion and micellar surface are oppositely charged). With increase of temperature, the hydration of ionic headgroups decreases with a simultaneous increase in interaction with such counterions. The presence of quaternary counterions near the micellar surface may assist in replacing the water from the headgroup region. Thus, the removal of water attributes the lesser degree of hydration of the micelle surface region. Heating may, further, dehydrate the micellar surface region (containing quaternary counterion and headgroup) and is responsible for the clouding phenomenon. The interaction of TBAB and SDS micelle has been investigated using NMR. Headgroup region protons show distinct shift on heating (Figs. S4–S6). This is an indirect observation of the interaction of quaternary counterion with SDS micellar surface. In a separate NMR study, it has been reported that butyl chain of TBA+ interacts with alkyl chain part of the surfactant [6]. This interaction depends on nature of the headgroup. Similar behaviour is expected for other counterion–SDS/SDBS combination.
Figure 1 shows the variation of CP with the addition of different quaternary salts (TBAB, TBPB, BTAC, TPhPB, TPeAB) in 30 mM SDS solution. Perusal of the data suggests that CP variations are dependent on the nature and structure of the quaternary counterion. For the same alkyl chain length in a typical counterion (TBAB or TBPB), central atom (N or P) plays an important role to produce clouding. The size of TBP+ counterion is bigger than TBA+. Therefore, TBP+ counterion will occupy more micellar surface than TBA+. If it is correct, TBP+ will remove effectively more water from the surface and lower the CP than the TBA+, at the same concentration. This is indeed observed in Fig. 1. However, TPeA+ counterion has found most effective in producing clouding in SDS solution. This counterion has n-pentyl chains which would provide additional hydrophobicity to the counterion (in comparison to TBA+ or TBP+) which obviously would provide more hydrophobicity near the micellar surface and can assist in removing the water from the micellar headgroup region. In a separate study, it has been reported that alkyl chains of the counterion can also be used in linking various micelles which are responsible for the phase separation (at CP) [3]. Above factors seem responsible regarding the effectiveness of TPeA+ (Fig. 1). This explanation found support from the fact that BTA+ (an unsymmetrical counterion) is more effective in producing clouding than butyl chain salt (TBA+ or TBP+). BTA+ and TBA+ or TBP+ contain three similar butyl chains. The fourth butyl chain is substituted by a benzyl group in BTA+ and is responsible for the increased hydrophobicity of the counterion. The CP data (Fig. 1) show that BTAC is more effective than TBA+ or TBP+. If all the butyl chains of the counterion were substituted by phenyl rings (cases of TBP+ and TPhP+), effectiveness to produce clouding decreases. This could be understood in the light of the fact that the phenyl ring is less hydrophobic than a butyl chain [26]. Therefore, hydrophobicity of the counterion has a definite role in addition to the nature of the central atom (N or P). Further, polarizability of quaternary counterion may increase by increasing the alkyl chain or substituting alkyl chain with benzyl ring [11–13]. Ninham and Yaminsky, based on polarizability, have quantified Hofmeister effect [27]. Polarizable ions have greater surface excess on the micelle compared to less polarizable ions and responsible for greater dispersion forces. The presence of N or P as central atom in a quaternary counterion may also influence the polarizability. Therefore, TPeA+ prefers micellar surface and is responsible for effective dehydration of the headgroup region. Hence, TPeA+ and TBA+ appear at the two extremes of effectiveness of producing clouding. This is indeed observed in Fig. 1. Based on data shown in Fig. 1, clouding potential (effectiveness of counterion in producing clouding) can be arranged in the following order: TPeA+ > BTA+ > TBP+ > TPhP+ > TBA+. From the above polarizability trend and order of clouding potential, one can say that counterion polarizabiltiy has definite role in clouding phenomenon. Surface tension vs [salt] plots (Fig. S7) show that rate of decrease of surface tension can be correlated with surface area per polar head of counterion. This will be lowest for TPeAB and highest for TBAB. Surface area follows nearly the same order as obtained for CP. Some discrepancies have been observed with salts having benzyl or phenyl moiety. However, the overall order shows a broad correlation between surface per polar head of counterion and its clouding potential for SDS.
Above ordering was tested with another anionic surfactant, SDBS. Figure 2 shows the variation of CP with quaternary salt concentration in 30 mM SDBS. CP data show that SDBS–quaternary counterion combinations give clouding at lower temperature than the SDS. This may be due to the presence of benzene ring in the SDBS molecule. Due to this, SDBS behaves as a more hydrophobic surfactant as discussed in an earlier study [28]. This increased hydrophobicity is responsible for lower CP. Another point worth noting is the ordering of counterion. In case of SDBS, counterion ordering is similar as in SDS (Fig. 3a) with a difference that TPhP+ does not follow the trend (Fig. 3b). This may be due to that the presence of phenyl ring in SDBS seems responsible for increased interaction with four phenyl rings present in TPhP+ via aromatic–aromatic interactions [29]. These interactions are known for stabilisation of structure of amphiphilic assemblies. The analogy can be drawn in the case of micellar systems which are expected to be stabilised (and hence of bigger size) and responsible for the change in ordering. The ordering is as follows with SDBS: TPeA+ > TPhP+ > BTA+ > TBP+ > TBA+.
On the basis of CP data, Hofmeister-like series for quaternary counterions can be constructed (Fig. 3). The order seems dependent on hydrophobicity of a typical counterion. However, headgroup–counterion interaction has a role in dehydrating the micellar surface with a concomitant occurrence of clouding. Above ordering should be tested with other anionic surfactant–quaternary counterion pairs before reaching any generalisation.
Figure 4a–d shows the variation of CP with carbohydrate addition (sugar or cyclodextrin). The general observation is a decrease in CP with all the additives. However, sucrose addition in the presence of TPhPB shows an increase in CP (Fig. 4c). Sugars are known to decrease critical micelle concentration of surfactant due to their water structure forming property [30]. The temperature range in which single-phase appearance reduces indicates a salting out effect of a typical sugar. Sucrose–TPhPB combination does not fit in above generalisation. Probably, four phenyl rings in TPhP+ bring sucrose molecule in the micellar palisade layer which draws water from the background solution and minimise the salting out effect of sugar due to change in its site of localisation. This additional water in the headgroup region is responsible for the CP increase (Fig. 4c). Among the carbohydrates, cyclodextrins are having better effect for decreasing the CP. Since cyclodextrin is having certain hydrophobic cavity in the molecular structure, it can interact hydrophobically with the surfactant micelles [31]. Further, hydroxyl groups of cyclodextrin may assist to draw headgroup region water. Above interrelated factors may be responsible for CP decrease. Recently, CP decrease is observed in an ionic drug solution and explained in the light of above factors [32]. Similar type of CP decrease in presence of hydrophobic alkanols has been interpreted by taking hydrophobic interactions into consideration [33]. Figure 5 shows the variation of CP with the addition of amino acid (l-leucine and l-phenylalanine) in presence of various quaternary salts. CP variation depends on the nature of amino acid and quaternary salt. However, TPhPB (increase in CP) and TPeAB (decrease in CP) fall at two different extremes. Other salts behave in between of the above. The behaviour of TPhPB is similar as found with carbohydrates (Fig. 4c) and can be understood in the same way. Similarly, TPeAB, being a more hydrophobic quaternary salt, induces hydrophobic interaction both with the amino acids and with the surfactant alky chain. This may be the reason of continuous fall in CP as observed in Fig. 5.
Effect of amino acid on the CP of 30 mM SDS + quaternary salt system (TBPB (22 mM); BTAC (19 mM); TPhPB (20 mM) and TPeAB (11 mM)): , l-leucine (TBPB);
, l-leucine (TBPB); , l-leucine (BTAC);
, l-leucine (BTAC); , l-leucine (TPhPB);
, l-leucine (TPhPB); , l-leucine (TPeAB);
, l-leucine (TPeAB); , l-phenylalanine (TBPB);
, l-phenylalanine (TBPB); , l-phenylalanine (BTAC);
, l-phenylalanine (BTAC); , l-phenylalanine (TPhPB) and
, l-phenylalanine (TPhPB) and , l-phenylalanine (TPeAB)
, l-phenylalanine (TPeAB)
Variation of CP with l-ascorbic acid for 30 mM surfactant + quaternary salt system has also been studied (see supporting information, Fig. S3) without controlling the pH of the solution. With all the salts, a decrease in CP has been observed. l-Ascorbic acid (or vitamin C) molecule contains four hydroxyl groups which help in removing water from the headgroup region (due to water structure forming nature). With TPhPB, CP decreasing effect has been found more pronounced than with other salts (which may be due to the presence of four phenyl rings). These phenyl rings can interact with allyl ring of ascorbic acid [34] and responsible for significant decrease in CP. CP data (see supporting information, Figs. S1 and S2) have also been collected with sugars and amino acids with 30 mM SDBS + quaternary salt systems. Similar CP behaviour has been observed as was observed with SDS and can be interpreted in similar lines.
Conclusion
In this communication, influence of nature of counterion on the clouding behaviour of anionic surfactant has been investigated and data are interpreted in the light of headgroup–counterion interaction and their mutual dehydration. It has been noticed that most CP studies in anionic micellar solution were conducted by adding TBA+. However, present study shows that other quaternary counterions can produce similar effect with even better performance (Fig. 3). Hydrophobicity of the counterion plays a pivotal role in interacting with micellar surface and dehydrating the micelle. This dehydration is responsible for the clouding phenomenon observed. Similar dehydration occurs with non-ionic micelle on heating and responsible for the phenomenon. However, there is a difference in the mechanism of dehydration in the two cases (ionic and non-ionic). In former case (ionic), water is bound to the micellar surface via stronger ion–dipole interaction then the case of latter where water is bound via weaker dipole–dipole interaction. With ionic surfactants, heating may cause dehydration of the counterion and headgroup, which enhances electrostatic attraction between the headgroup and counterion [19] in addition to increased hydrophobic interaction between hydrophobic parts of counterion and micellar interior. A counterion ordering is reported, with respect to clouding phenomenon. The effect of different classes of additives on CP (carbohydrate, amino acid or vitamin) has been found to be dependent on the nature and hydrophobicity of a typical salt. The work may find use in various applications such as metal extraction, solvent extraction and pollution control among others [35, 36].
References
Dong R, Hao J (2010) Complex fluids of poly(oxyethylene) monoalkyl ether non-ionic surfactants. Chem Rev 110:4978–5022
Kumar S, Sharma D, Kabir-ud-Din (2000) Cloud point phenomenon in anionic surfactant + quaternary bromide systems and its variation with additives. Langmuir 16:6821–6824
Bales BL, Zana R (2004) Cloud point of aqueous solutions of tetrabutylammonium dodecyl sulfate is a function of the concentration of counter ions in the aqueous phase. Langmuir 20:1579–1581
Warr GG, Zemb TN, Drifford M (1990) Liquid-liquid phase separation in cationic micellar solutions. J Phys Chem 94:3086–3092
Kumar S, Bhadoria A, Patel H, Aswal VK (2012) Morphologies near cloud point in aqueous ionic surfactant: scattering and NMR studies. J Phys Chem B 116:3699–3703
Bhadoria A, Kumar S, Aswal VK, Kumar S (2015) Mechanistic approach on heating induced growth of anionic surfactants: a clouding phenomenon. RSC Adv 5:23778–23786
Casero I, Sicilia D, Rubio S, Perez-Bendito D (1999) An acid-induced phase cloud point separation approach using anionic surfactants for the extraction and preconcentration of organic compounds. Anal Chem 71:4519–4526
Kumar S, Alam MS, Parveen N, Kabir-ud-Din (2006) Influence of additives on the clouding behaviour of amphiphilic drug solutions. Colloid Polymer Sci 284:1459–1463
Alam MS, Kumar S, Naqvi AZ, Kabir-ud-Din (2006) Effect of electrolytes on the cloud point of chlorpromazine hydrochloride solutions. Colloids Surf B 53:60–63
Goel SK (1998) Measuring detergency of oily soils in the vicinity of phase inversion temperatures of commercial nonionic surfactants using an oil-soluble dye. J Surf Deterg 1:221–226
Manet S, Karpichev Y, Dedovets D, Oda R (2013) Effect of Hofmeister and alkylcarboxylate anionic counterions on the Krafft temperature and melting temperature of cationic gemini surfactants. Langmuir 29:3518–3526
Nostro PL, Ninham BW (2012) Hofmeister phenomenon: an update on ion specificity in biology. Chem Rev 112:2286–2322
Salis A, Ninham BW (2014) Models and mechanisms of Hofmeister effects in electrolyte solutions, and colloid and protein systems revisited. Chem Soc Rev 43:7358–7377
Ninham BW, Nostro PL (2010) Molecular forces and self assembly, in colloid, nano science and biology. Cambridge University Press, Cambridge, U. K
Collins KD, Neilson GW, Enderby JE (2007) Ions in water: characterizing the forces that control chemical process and biological structure. Biophys Chem 128:95–104
Saitoh T, Hinze WL (1991) Concentration of hydrophobic organic compounds and extraction of protein using alkylammoniosulfate zwitterionic surfactant mediated phase separations (cloud point extractions). Anal Chem 63:2520–2525
Carabias-Martınez R, Rodrıguez-Gonzalo E, Moreno-Cordero B, Pérez-Pavón JL, Garcıa-Pinto C, Laespada EF (2000) Surfactant cloud point extraction and preconcentration of organic compounds prior to chromatography and capillary electrophoresis. J Chromatography A 902:251–265
Kumar S, Patel H, Patil SR (2013) Test of Hofmeister-like series of anionic headgroups: clouding and micellar growth. Colloid Polym Sci 291:2069–2077
Vlachy N, Jagoda-Cwiklik B, Vacha R, Touraud D, Jungwrith P, Kunz W (2009) Hofmeister series and specific interactions of charged head groups with aqueous ions. Adv Colloid Interf Sci 146:42–47
Kabir-ud-Din Rub MA, Sheikh MS (2010) Cloud-point modulation of an amphiphilic drug with pharmaceutical excipients. J Chem Eng Data 55:5642–5652
Quina FH, Hinze WL (1999) Surfactant-mediated cloud point extractions: an environmentally benign alternative separation approach. Ind Eng Chem Res 38:4150–4168
Da Silva RC, Loh W (1998) Effect of additives on the cloud points of aqueous solutions of ethylene oxide–propylene oxide–ethylene oxide block copolymers. J Colloid Interface Sci 202:385–390
Helenius A, Simons K (1975) Solubilization of membranes by detergents. Biochim Biophys Acta 415:29–79
Cox MF, Borys NF, Matson TP (1985) Interactions between LAS and nonionic surfactants. J Am Oil Chem Soc 62:1139–1143
Vlachy N, Drechsler M, Verbavatz JM, Touraud D, Kunz W (2008) Role of the surfactant headgroup on the counterion specificity in the micelle-to-vesicle transition through salt addition. J Colloid Interface Sci 319:542–548
Agam G (1994) Industrial chemicals: their characteristics and development. Elsevier (Amsterdam), Chapter 4, p 60
Ninham BW, Yaminsky V (1997) Ion binding and ion specificity: the Hofmeister effect and Onsager and Lifshitz theories. Langmuir 13:2097–2108
Kumar S, Sharma D, Khan ZA, Kabir-ud-Din (2002) Salt-induced cloud point in anionic surfactant solution: role of the headgroup and additives. Langmuir 18:4205–4209
Burley SK, Petsko GA (1985) Aromatic-aromatic interaction: a mechanism of protein structure stabilization. Science 229:23–28
Schwuger MJ (1971) Effect of structure-forming and structure-disturbing agent on the adsorption and diffusion of surfactants in water. Ber Bunsen-Gen Phys Chem 75:167
Liu Y, Zou C, Wang T, Li M (2014) Density surface tension and cloud point of aqueous solutions of β-cyclodextrin-polyethylene glycol. J Chem Eng Data 59:3773–3778
Naqvi AZ, Rub MA, Kabir-ud-Din (2011) Effects of pharmaceutical excipients on cloud points of amphiphilic drugs. J Colloid Interf Sci 361:42–48
Ahmad T, Kumar S, Khan ZA, Kabir-ud-Din (2007) Additives as CP modifiers in an anionic micellar solution. Colloids Surf A Physicochem Eng Asp 294:130–136
Dougherty DA (1996) Cation-pi interactions in chemistry and biology: a new view benzene, Phe, Try, and Trip. Science 271:163–168
Drecher MR, Simnick AJ, Fischer K, Smith RJ, Patel A, Schmidt M, Chilkoli A (2008) Temperature triggered self assembly of polypeptides into multivalent spherical micelles. J Am Chem Soc 130:687–694
Matema K, Goralska E, Sobczynska A, Szymanowski J (2004) Recovery of various phenols and phenyl amines by micellar enhanced ultra filtration and cloud point separation. Green Chem 6:176–182
Acknowledgement
The authors are thankful to Solvay Specialties, India Pvt. Limited, for providing a financial support. SKY is also thankful for a research fellowship. We also appreciate the help extended by Heads, Chemistry and Applied Chemistry Departments, The Maharaja Sayajirao University of Baroda, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that there is no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 523 kb)
Rights and permissions
About this article
Cite this article
Yadav, S.K., Kumar, S. Counterion-specific clouding in aqueous anionic surfactant: a case of Hofmeister-like series. Colloid Polym Sci 295, 869–876 (2017). https://doi.org/10.1007/s00396-017-4074-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-017-4074-0




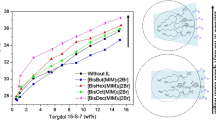


 , TBPB;
, TBPB; , BTAC;
, BTAC; , TPhPB;
, TPhPB; , TPeAB and
, TPeAB and , TBAB
, TBAB
 , TBPB;
, TBPB; , BTAC;
, BTAC; , TPhPB;
, TPhPB; , TPeAB and
, TPeAB and , TBAB
, TBAB

 ,
,  , sucrose (insets are for:
, sucrose (insets are for: , α-cyclodextrin and
, α-cyclodextrin and , β-cyclodextrin)
, β-cyclodextrin)