Abstract
Work was performed to distinguish the role of sulfonate (–SO3 −) and sulfate (–OSO3 −) with respect to the micellization and clouding phenomenon in ionic surfactant solutions. The clouding phenomenon is a recent addition to the conventional one observed with nonionic surfactants. Three ionic surfactants [sodium dodecylsulfate (SDS), sodium dodecylbenzenesulfonate (SDBS), and sodium dodecylsulfonate (SDSo)] are chosen and the effects of added tetra-n-pentylammonium bromide (TPeAB) and benzyl tributylammonium bromide (BTAC) have been studied on micellization and clouding behaviors in aqueous solution. Based on critical micelle concentration (CMC) and cloud point (CP) measurements, the following order has been observed: SDBS < SDS < SDSo. Though both SDBS and SDSo contain sulfonate groups, they are found at the two ends of the ordering. Therefore, the role of the phenyl ring is also having importance in clouding phenomena. For a typical surfactant, TPeAB was found to be more effective than BTAC. Based on the CP studies, two compositions of SDSo + TPeAB/BTAC were chosen and the effects of different additives (carbohydrate, amino acid, and l-ascorbic acid) on the CP were investigated. Additive may either decrease or increase CP, depending on the structure of the counterion or additive. The present work shows a few novelties: (1) headgroup/counterion dependence of CP and (2) hydrophobicity of counterion/surfactant has an important bearing on the phenomenon. The data can be utilised in improving cloud point extraction methodologies (CPEMs).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surfactant-based systems are widely used for various applications ranging from extraction of DNA to remediation of soil [1–3]. Surfactant monomers aggregate in solution due to their amphiphilic nature via hydrophobic interactions [4]. Solution properties have been found to be dependent on the nature of surfactant monomers (ionic or nonionic), which are also responsible for a particular morphology [5–8]. A few ionic surfactant systems show an unusual thermo responsive behavior on heating [9–12].
Ionic and nonionic surfactant solutions respond to heating in a contrasting fashion. Solutions of nonionic surfactant cannot withstand heating and undergo clouding at a particular temperature known as the cloud point (CP) [13]. However, the CP is not common with ionic surfactants. Reports on clouding phenomenon in ionic surfactant solutions have appeared recently [14–17]. Most of the work on clouding of ionic surfactant solutions is made when quaternary counterions (e.g.. tetra-n-butyl ammonium; TBA+) is added or was part of the surfactant monomer [18–22].
Ion specificity plays an important role in surface and colloid science. In a pioneer work, Franz Hofmeister observed that the effectiveness of counterions in denaturing proteins can be related to their water structuring tendency [23]. A serious effort has been made to include surfactant headgroups in order to widen the scope of the Hofmeister series [24]. In a recent work [17], we attempted to correlate the nature of the headgroup to clouding phenomenon in the presence of TBA+. However, the study was performed with only TBA+ by taking anionic surfactants having different head groups. Sodium dodecylbenzene sulfonate [SDBS] has been found to show clouding at lower [TBA+] when compared to other surfactants of the same charge class. The results were interpreted in light of the combined effect of the aromatic benzene ring and the nature of the sulfonate group. However, the effects of the benzene ring and the sulfonate head group could not be separated out. Various amphiphilic drugs have also been reported to exhibit clouding phenomenon like conventional surfactants [25]. The clouding behaviour has also been affected by the nature of the additive and emulsifier [26, 27]. Conditions required to have clouding in colloidal systems (i.e., micellar solutions), are of great significance both from the academic and industrial points of view [28, 29]. Various pollutants have also been extracted using the clouding principle in the presence of anionic surfactants containing various headgroups [30]. The clouding phenomenon in aqueous ionic surfactant systems may be the consequence of coulombic attraction between oppositely charged headgroups and counterions.
The present study is designed to understand the interplay of the head group and counterion added in order to widen the applicability of Hofmeister-like series in the context of clouding phenomenon in ionic surfactant solutions. Therefore, various quaternary salts (tetra-n-butylammonium bromide, TBAB; tetra-n-butylphosphonium bromide, TBPB; benzyltributylammonium chloride, BTAC; tetraphenylphosphonium bromide, TPhPB; and tetra-n-pentylammonium bromide, TPeAB) have been added to a 30 mM solution and CP measurements were conducted. Critical micelle concentration (CMC) measurements of SDSo surfactants have also been performed with a fixed concentration of quaternary bromide (1/0.5 mM) at different temperatures to support the CP data. CMC and CP data were compared with the data obtained with tetra-n-butylammonium bromide (TBAB) + anionic surfactant systems. The study may single out the effect of the –SO3 head group from the combined effect of the –SO3 head and the phenyl ring studied in an earlier work [17]. CP data were also collected with different classes of additives (carbohydrates, amino acids, and vitamins).
Experimental Section
Materials
SDSo (≥99 % Sigma-Aldrich, St. Louis, MO, USA), SDS (≥99 % Sigma-Aldrich, St. Louis, MO, USA), SDBS (99 % TCI, Tokyo, Japan), TBAB (≥99 % Sigma-Aldrich, St. Louis, MO, USA), TBPB (>99 %, Sigma-Aldrich, St. Louis, MO, USA), BTAC (≥98 %, Sigma-Aldrich, St. Louis, MO, USA), TPhPB (>98 %, TCI, Tokyo, Japan), TPeAB (≥99 %, Sigma-Aldrich, St. Louis, MO, USA), glycine (≥99 %, Sigma-Aldrich, St. Louis, MO, USA), l-leucine (≥98 %, Sigma-Aldrich, St. Louis, MO, USA), l-phenylalanine (≥99 %, Sigma-Aldrich, St. Louis, MO, USA), l-ascorbic acid (≥99 %, Sigma-Aldrich, St. Louis, MO, USA), α-cyclodextrin (98 %, Spectrochem, Mumbai, India), β-cyclodextrin (99 %, Spectrochem, Mumbai, India), d-glucose (Fisher Scientific) and sucrose (Fisher Scientific) were used as received. The water, used in preparing the sample solution, was double-distilled in an all-glass distillation setup. The specific conductivity of the water was in the range of 1–2 µS cm−1.
Various SDSo + quaternary salts samples were prepared by taking requisite amounts of surfactant and quaternary salt and making up the volumes with distilled water. The structures of various anionic surfactants and different quaternary salts are given in Scheme 1.
Methods
Critical Micelle Concentration (CMC) Measurement
Conductometric measurements were carried out using a conductivity meter, an EUTECH cyberscan CON510 (cell constant 1 cm−1) with an inbuilt temperature sensor. A pre-calibrated conductivity cell was used to obtain specific conductance at an appropriate concentration range. The temperature of the sample solution was precisely controlled by a SCHOTT CT 1650 thermostat with an accuracy of ±0.1 °C. The cell with an appropriate amount of the solvent (or water) in a vessel was thermostated for at least 30 min prior to the measurement. The conductivity runs were carried out by adding a concentrated surfactant solution to the water (or salt + water). The CMC and degree of counterion dissociation (α) values for the SDSo were determined from the intersection point of nearly two straight lines (in the plot of the [surfactant] vs specific conductance (κ)), and the ratio of the slopes of the post-micellar to that of the pre-micellar portions of the plot, respectively.
Cloud Point (CP) Measurement
The CP values were obtained by placing sample tubes containing surfactant solutions with a fixed composition into a temperature-controlling water bath. The onset of turbidity temperature (visual observation) was noted. The system was allowed to cool, and the temperature at which the disappearance of turbidity took place was also noted. The average of these two temperatures was taken as the cloud point (CP). This procedure was repeated for the same sample and nearly two concurrent values (within ±0.1 °C) were considered as the final CP. Similar CP measurements were made by diluting the samples using double distilled water to collect CP data at various concentrations.
Results and Discussion
Micellization of Anionic Surfactant with and Without Salts
The CMC and α values of pure and mixed (surfactant + quaternary salt) surfactant systems are summarized in Table 1. The CMC and α value of SDSo have been found to be in close agreement with the literature data [31]. All the three anionic surfactants (SDS, SDBS, and SDSo) have different CMC values, though they have same dodecyl chain in the molecule. The difference is due to the nature of the head group present in each surfactant. It is known that the nature of the head group affects the phenomenon of micelle formation [17]. Presence of a quaternary salt (1 mM) causes a decrease in the CMC value of SDSo. This is similar to what is observed with other anionic surfactants (at 4.4 and 1 mM with SDS and SDBS, respectively) [32, 33]. The micellization phenomenon is facilitated by the presence of the quaternary counterion, which can interact with the micellar surface electrostatically and hydrophobically. However, the effect was more pronounced with SDSo in the presence of TPeAB (even at 0.5 mM), which may be due to the presence of longer alkyl chains present in the salt.
Since clouding phenomenon depends on temperature, it is worthwhile to study micellization of SDSo in the presence of 1 mM quaternary salt at different temperatures (30–50 °C). Figure 1 shows the variation of CMC with temperature. CMC decreases and then increases with continuous increase in temperature (U-shaped behavior). The initial temperature increase decreases the hydration of the polar head, which favours a decrease in the CMC. Further, temperature increase may also break the structure of water around the hydrocarbon part of the SDSo, which opposes micelle formation. These two competing factors are responsible for U-shaped behaviour (Fig. 1). However, the nature of the counterion has only a weak influence on the temperature at the minimum CMC.
Clouding Phenomenon
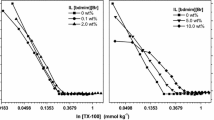
Figure 2 shows the variation of CP with [TPeAB] for different anionic surfactants (SDS, SDBS, and SDSo). The TPeAB concentration required to produce clouding is headgroup dependent: SDSo > SDS > SDBS. The appearance of clouding in the presence of quaternary counterions has been discussed in detail in earlier studies [17, 19]. These counterions can interact electrostatically and hydrophobically with anionic headgroups in a typical micelle. These interactions affect the interaction of water with the micellar surface. All the surfactants have the same dodecyl chain, and; hence, headgroup hydration seems to play a role in overall clouding. In an earlier study it has been proposed that ordering of headgroups can be given based on their cosmotropic (hydrophilic)/chaotropic (less hydrophilic) nature [24]. Sulfate and sulfonate headgroups have been put together in the above ordering. However, the CMC values (Table 1) and CP data (Fig. 2) show that –SO3 − behaves antagonistically towards micellization/clouding in comparison to –OSO3 −. This antagonistic effect of –SO3 − , towards CP may be due to lower α values (Table 1), which may be responsible for less binding with TPeA+. As binding of counterions with headgroup is an important factor in observing clouding with anionic surfactants, the CP is expected at higher [TPeAB]. This indeed is observed in the present case (Fig. 2). The headgroup structural difference seems to influence the micellization process and micellar degree of dissociation resulting in different interactions with TPeA+. Overall clouding phenomenon involves the combined effect of the above factors. This trend could further be understood in the light of basicity and phase data of the headgroups [34]. However, with SDBS, the above ordering was not followed (it shows lower CMC and more prone to clouding) though it also has –SO3 −. Probably, the presence of the benzene ring in SDBS plays a role as it can provide more hydrophobicity to the molecule, which is responsible for increased hydrophobic interactions and lower CMC. The same can be true in the case of clouding. Figure 3 shows the interplay of [SDSo]–[TPeAB] on the clouding behavior. It can be seen that CP decreases on increasing [TPeAB]. At lower [SDSo], the requirement of TPeAB is also lower, which is inconsonant with our earlier study [35]. The perusal of data (Fig. 3a) indicates that the amount of salt required to produce CP increases with an increase in surfactant concentration.
CP measurements have also been performed with BTAC –SDSo aqueous systems (Fig. 3b). CP decreases and then increases with the continuous increase in [BTAC] at different fixed SDSo concentrations. This behavior is different than what we found in case of TPeAB (Fig. 3a). The initial decrease and leveling off in CP with [BTAC] is similar to other anionic surfactants-quaternary salt combinations [16, 35]. However, a novel CP increase was observed at higher [BTAC]. This behaviour has not been found in any of the studies on the clouding phenomenon in ionic surfactant solutions. However, it has been reported that CP increases with quaternary salt concentration when added to a nonionic surfactant. Therefore, the BTAC-SDSo system shows the signatures of both ionic (initial part of Fig. 3b) and nonionic surfactants (latter part of Fig. 3b). This is hard to understand how a surfactant can behave both ways. In a separate small angle neutron scattering (SANS) study, it has been shown that the charge on the anionic micelle decreases on addition of quaternary ammonium salts [36]. In the light of the above fact, at higher [BTAC], the micelle can be considered nearly uncharged (pseudo-nonionic micelle) [12]. If it is true, additional BTAC could be used to re-charge the pseudo-nonionic micelle to a cationic micelle. These cationic micelles may feel electrostatic repulsion, which may be the reason CP increases at higher [BTAC].
Based on the above CP studies (Fig. 3a, b), two samples were chosen (30 mM SDSo + 16 mM TPeAB and 30 mM SDSo + 150 mM BTAC) to study the effect of various additives on the CP. Figure 4 shows the variation of CP with carbohydrate concentration (sugar or cyclodextrin). A decrease in CP with both the sugars (glucose and sucrose) in the presence of TPeAB or BTAC has been observed (Fig. 4). Sugars are known to decrease critical micelle concentration of surfactant due to their water structure-making property [37]. The temperature range in which the single phase exists is reduced, indicating a salting out effect of a typical sugar. However, cyclodextrins (α-cyclodextrin and β-cyclodextrin) are more effective in decreasing the CP with TPeAB in comparison to other sugars. Since cyclodextrins have a hydrophobic cavity in the molecular structure, they can interact hydrophobically with the surfactant monomer [38]. This may assist in the dehydration of the headgroup and lower the CP. A CP decrease in the presence of hydrophobic alkanols has been interpreted by taking hydrophobic interactions into consideration [21]. Contrary to this, with BTAC, cyclodextrins show an increasing effect of CP (Fig. 4b). Probably, the benzyl group in BTAC brings the cyclodextrin molecule deeper into the micellar palisade layer, which draws water from the background solution. This additional water in the headgroup region is responsible for the CP increase.
Figure 5 shows the variation of CP with the addition of an amino acid (glycine, l-leucine and l-phenylalanine) in the presence of TPeAB or BTAC. All amino acids have the same functional group with structurally different side chains. In a separate study, it has been shown that the rate of reaction increases with the hydrophobicity of the side chain [39]. Glycine prefers a polar environment, and would prefer to partition near the headgroup region. In doing so, it may replace a fraction of the water, which is responsible for the CP decrease. This indeed is observed in Fig. 5. Hydrophobic amino acids (leucine or phenylalanine) would prefer a micellar core and may compete with hydrophobic chains of quaternary salts. Since quaternary salts interact electrostatically and hydrophobically with the micellar surface/interior, this interaction would be weakened in the presence of leucine or phenylalanine. Therefore, one can expect a decrease in CP, which indeed is observed (Fig. 5).
Figure 6 shows the variation of CP with l-ascorbic acid with both the systems. With TPeAB or BTAC, a decrease in CP has been observed. l-Ascorbic acid (or vitamin C) molecules contain four hydroxyl groups, which may help to remove water from the headgroup region (due to the water structure-forming nature) and behave similar to sugars. However, with TPeAB, the CP decrease was more pronounced, which may be due to the presence of pentyl chains that are more hydrophobic in nature than butyl chains present in BTAC. These pentyl chains can effectively interact with the allyl cyclic ring of ascorbic acid and be responsible for the larger decrease in CP.
Conclusion
Micellization and CP data allow the ordering of headgroups: SDSo > SDS > SDBS. However, SDBS is effective due to the extra phenyl ring in the molecular structure. Sulfonate comes before sulfate, which is consistent with earlier reported work [24, 34]. TPeAB is found to be more effective in producing CP than BTAC. The hydrophobicity of the counterion has a role to play in clouding phenomenon of anionic surfactant solutions. With SDSo, even TBAB failed to produce clouding, which again delineates the role of nature of the headgroup in clouding phenomena. Effects of additives mostly follow the similar trends as reported in earlier studies on the topic [21, 40].
References
Zhang W, Dai X, Zhao Y, Lu X, Gao P (2009) Comparison of the different types of surfactants for the effect on activity and structure of soybean peroxidase. Langmuir 25:2363–2368
Bernandeg LA (2008) Investigation on the locus of solubilization of polycyclic aromatic hydrocarbons in nonionic surfactant micelles with 1H NMR spectroscopy. Colloids Surf A 324:77–78
Yeom IT, Ghosh MM, Cox CD, Robinson KG (1995) Micellar solubilization of polynuclear aromatic hydrocarbons in coal tar-contaminated soils. Environ Sci Technol 29:3015–3021
Chandler D (2005) Interfaces and the driving force of hydrophobic assembly. Nature 437:640–647
Gabor KH, Naomi C, Ray VW (1996) Changes in polarity and aggregation number upon clouding of a nonionic detergent: effect of ionic surfactants and sodium chloride. Langmuir 12:916–920
Kabir-ud-Din, Siddiqui US, Kumar S (2007) Viscometric studies on aqueous Gemini micelles in the presence of additives. Colloids Surf A 301(2007):209–213
Kumar S, David SL, Aswal VK, Goyal PS, Kabir-ud-din (1997) Growth of sodium dodecyl sulfate micelles in aqueous ammonium salts. Langmuir 13:6461–6464
Sein A, Engberts JBNF (1995) Micellar to lamellar aggregate transition of an anionic surfactant in dilute aqueous solution induced by alkali metal chloride and tetraalkylammonium chloride salts. Langmuir 11:455–465
Yin H, Zhan Z, Huang J, Zheng R, Zhang Y (2003) Temperature-induced micelle to vesicles transition in the sodium dodecylsulfate/dodecyltriethylammonium bromide system. Angew Chem Int Ed 42:2188–2191
Davis TS, Ketner AM, Raghavan SR (2006) Self-assembly of surfactant vesicles that transform into viscoelastic wormlike micelles upon heating. J Am Chem Soc 128:6669–6675
Kumar S, Bhadoria A, Patel H, Aswal VK (2012) Morphologies near cloud point in aqueous ionic surfactant: scattering and NMR studies. J Phys Chem B 116:3699–3703
Bhadoria A, Kumar S, Aswal VK, Kumar S (2015) Mechanistic approach on heat induced growth of anionic surfactants: a clouding phenomenon. RSC Adv 5:23778–23786
Dong R, Hao J (2010) Complex fluids of ploy(oxyethylene) monoalkyl ether nonionic surfactants. Chem Rev 110:4978–5022
Kumar S, Sharma D, Kabir-ud-Din (2003) Temperature-[salt] compensation for clouding in ionic micellar systems containing sodium dodecyl sulfate and symmetrical quaternary bromides. Langmuir 19:3539–3541
Mitra D, Chakraborty I, Bhattacharya SC, Moulik SP (2007) Interfacial and solution properties of tetraalkylammonium bromides and their sodium dodecyl sulfate interacted products: a detailed physicochemical study. Langmuir 23:3049–3061
Rout DK, Chauhan S, Agarwal A (2009) Cloud point and microemulsion phase behaviour of sodium linear alkylbenzene sulfonate with tetrabutyl- and benzyltributyl-substituted ammonium halides. Ind Eng Chem Res 48:8842–8847
Kumar S, Patel H, Patil SR (2013) Test of hofmeister-like series of anionic headgroups: clouding and micellar growth. Colloid Polym Sci 291:2069–2071
Bales BL, Zana R (2004) Cloud point of aqueous solutions of tetrabutylammonium dodecyl sulfate is a function of the concentration of counterions in the aqueous phase. Langmuir 20:1579–1581
Kumar S, Bhadoria A (2012) Thermodynamic energetics of charged micellar solutions with and without salts at the cloud point. J Chem Eng Data 57:521–525
Bales BL, Benrraou M, Tiguida K, Zana R (2005) Effect of the nature of the counterion on the properties of anionic surfactants. 4. Characterizing micelles of tetraalkylammonium dodecyl sulfate as reaction media. J Phys Chem B 109:7987–7997
Ahmad T, Kumar S, Khan ZA, Kabir-ud-Din (2007) Additives as CP modifiers in an anionic micellar solution. Colloids Surf A 294:130–136
Zana R, Schmidt J, Talmon Y (2005) Tetrabutylammonium alkyl carboxylate surfactants in aqueous solution: self-association behaviour, solution nanostructure, and comparison with tetrabutylammonium alkyl sulfate surfactants. Langmuir 21:11628–11636
Kunz W, Henle J, Ninham BW (2004) ‘Zur lehre von der wirkung der salze’(about the science of the effect of salts): franz hofmeister’s historical papers. Curr Opin Colloid Interface Sci 9:19–37
Vlachy N, Jagoda-Cwiklik B, Vacha R, Touraud D, Jungwrith P, Kunz W (2009) Hofmeister series and specific interactions of charged head groups with aqueous ions. Adv Colloid Interface Sci 146:42–47
Kim EJ, Shah DO (2002) Cloud point phenomenon in amphiphilic drug solutions. Langmuir 18:10105–10108
Kumar S, Alam MS, Parveen N, Kabir-ud-Din (2006) Influence of additives on the clouding behaviour of amphiphilic drug solutions. Colloid Polym Sci 284:1459–1463
Alam MS, Kumar S, Naqvi AZ, Kabir-ud-Din (2006) Effect of electrolytes on the cloud point of chlorpromazine hydrochloride solutions. Colloids Surf B 53:60–63
Ballesteros-Gomez A, Sicilia MD, Rubio S (2010) Supramolecular solvents in the extraction of organic compounds. Anal Chim Acta 677:108–130
Yazdi AS (2011) Surfactant-based extraction methods. Trends Anal Chem 30:918–929
Casero I, Sicilia D, Robio S, Perez-Bendito P (1999) An acid induced phase cloud point separation approach using anionic surfactants for the extraction and preconcentration of organic compounds. Anal Chem 71:4519–4526
Yan Z, Bai X, Liu R, Wu S, Wang J (2013) Effect of dipeptides on the micellization and thermodynamic parameters of sodium dodecyl sulfonate: conductometric and fluorimetric studies. J Mol Liquids 177:78–84
Kumar S, Sharma D, Kabir-ud-din (2002) Micellization of sodium dodecyl benzenesulfonate in aqueous quaternary bromides. J Surf Sci Technol 18:25–33
Lin JH, Chen WS, Hou SS (2013) NMR studies of effects of tetralkylammonium bromides on micellization of sodium dodecylsulfate. J Phys Chem B 117:12076–12085
Laughlin RG (1978) Solvation and structural requirements of surfactant hydrophilic groups. Adv Liquid Cryst 3:41–98
Kumar S, Sharma D, Khan ZA, Kabir-ud-Din (2001) Occurrence of cloud points in sodium dodecyl sulfate-tetra-n-butylammonium bromide system. Langmuir 17:5813–5816
Kumar S, Aswal VK, Goyal PS, Kabir-ud-Din (1998) Micellar growth in the presence of quaternary ammonium salts: a SANS study. J Chem Soc Faraday Trans 94:761–764
Schwuger MJ (1971) Effect of structure-forming and structure-disturbing agent on the adsorption and diffusion of surfactants in water. Ber Bunsen-Gen Phys Chem 75:167
Liu Y, Zou C, Wang T, Li M (2014) Density, surface tension and cloud point of aqueous solutions of β-cyclodextrin-polyethylene glycol. J Chem Eng Data 59:3773–3778
Kabir-ud-Din, Salem JK, Kumar S, Khan Z (2000) Effect of cationic surfactants on the addition-elimination type interaction between aspartic acid and ninhydrin. Colloids Surf A 168(3):241–250
Kumar S, Sharma D, Khan ZA, Kabir-ud-Din (2002) Salt-induced cloud point in anionic surfactant solution: role of the headgroup and additives. Langmuir 18:4205–4209
Acknowledgments
The authors are thankful to Solvay Specialties, India Pvt. Limited, for providing financial support. S K Y is also thankful for a research fellowship. We also appreciate the help extended by Heads, Chemistry and Applied Chemistry Departments, The Maharaja Sayajirao University of Baroda, Vadodara, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that there are no conflicts of interest.
About this article
Cite this article
Yadav, S.K., Yadav, R.B. & Kumar, S. Tuning of Cloud Point by the Nature of Surfactant Headgroup: Influence of Counterion and Additives. J Surfact Deterg 19, 1063–1069 (2016). https://doi.org/10.1007/s11743-016-1860-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-016-1860-5