Abstract
A cloud point evaluation was performed for the nonionic surfactant Tergitol 15-S-7 in aqueous solutions of McIlvaine buffer (pH 7.0). Cloud point temperatures of the selected nonionic surfactant were studied in relation to the effect that the addition of dicationic ionic liquids (ILs) had on the systems. The dicationic ILs with different alkyl spacer lengths were derived from 1, n-bis(3-methylimidazolium-1-yl)alkane bromide ([BisAlk(MIM)2][2Br]) — in which Alk = But, Hex, Oct, and Dec. The dicationic ILs with longer alkyl spacer chains (Alk ≥ 6) led to an increase in the Tergitol’s cloud point values, due to their ability to fold their structures in solution, which increase the interactions of ILs with tergitol in the mixed micelles. This increase was even more pronounced when the concentration of the dicationic ILs was increased. Furthermore, the addition of dicationic ILs increased the micelles size; however, the hydrodynamic diameter of mixed micelles did not depend on the length of the ILs’ alkyl spacer chains. The Tergitol/IL systems with lower critical micellar concentration values will result in higher cloud point values, due to the greater colloidal dispersion stability.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nonionic surfactants have a broad range of applications, from cleaning products to personal care and biopharmaceutical stabilizers [1,2,3,4]. In contrast to other types of surfactants, the solubility of diluted aqueous solutions of nonionic surfactants decreases with an increase in temperature, which leads to phase separation. This temperature-induced phase separation leads to the formation of an aqueous micellar two-phase system (AMTPS), which has great potential in the separation of biocompounds, because the use of water would decrease the current dependence on organic solvents [5,6,7,8,9,10,11].
The lower critical solution temperature (LCST) indicates the maximum solubilization temperature of the surfactant [12, 13]. It can also be defined as the temperature at which the visual aspect of the solution changes from transparent to turbid, which is known as the cloud point. Above the cloud point, these thermo-reversible systems equilibrate in two macroscopic liquid phases: a phase rich in surfactant (colloidal components), and a surfactant-poor phase [5,6,7, 14].
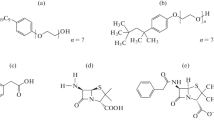
Tergitol 15-S-7 is a linear primary ethoxylated alcohol (see Fig. 1a) and a biodegradable nonionic surfactant that is used in food applications, cleaning, and as an ingredient in meat and poultry processing [15]. As an LCST surfactant, the solubility of Tergitol 15-S-7 decreases with an increase in temperature, due to the increasing of thermal agitation, which leads to the decrease of time of contact between oligo(oxyethylene) headgroups and water. Thus, the characterization of its phase behavior is of great interest, especially considering its use in many industrial processes. Furthermore, changes in physicochemical properties affect the performance of Tergitol 15-S-7 solution near the cloud point temperature [1, 15].
Although the cloud point of a nonionic surfactant is basically determined by its molecular structure [15,16,17], it can be strongly affected by additives. The effects that additives such as inorganic electrolytes, ionic surfactants, ionic liquids (ILs), and alcohols have on the cloud point temperatures of nonionic surfactants have been investigated [15, 18, 19]. These studies confirmed that the surfactant’s phase behavior can be tailored to a target application and/or certain requirements of the application. Conventional ionic surfactants and ILs can modulate the cloud points of nonionic surfactants by decreasing or increasing the LCST in accordance with the surfactant or IL structure [9, 20, 21].
Dicationic ILs consist of a double charge structure linked by a spacer chain (Fig. 1b) [22]. Some of these dicationic ILs are amphiphilic and have surface activity properties in solution and their behavior is comparable to traditional surfactants [23,24,25]. Moreover, the knowledge and characterization of a surfactant’s phase diagrams in the presence of additives such as ILs are crucial for designing temperature-driven extraction processes [9, 26]. Additionally, the presence of two cationic headgroup in ILs improved the properties and allow applications such as reaction media, separations, lubricants, electrolytes in batteries, and applications in pharmaceutical sciences [27,28,29,30,31].
Coutinho et al. [9] determined the impact of structure of ILs on the CP of three kinds of Tergitol (15-S-7, 15-S-9 and NP-10) and the formation of aqueous micellar two-phase systems. They observed that imidazolium and ammonium-based ILs with one cationic headgroup and one alkyl chain, lead to the formation of water-headgroup interactions and, consequently, promote an increase in the cloud points. The phosphonium and ammonium-based ILs with two and four alkyl chains, respectively, present a more hydrophobic micellar environment, which causes a weakened water-headgroup interactions and a decrease in the cloud point and the mixed micelles size. The authors also investigated two dicationic ILs. Nevertheless, the structures are quite different (gemini) from the ones present in this research (bolaform).
Thus, a more deeper and systematic investigation is highly desirable to evaluated the impact of bolaform dicationic ILs on the CP of Tergitol and the formation of mixed micelles, which impact in their separations properties. Thus, the present study aims to investigate the impact that the concentration and alkyl spacer length of dicationic ILs have on the cloud point behavior of the nonionic surfactant Tergitol 15-S-7 (0–16 wt%) in McIlvaine buffer (pH 7.0) aqueous solution. Here, McIlvaine buffer was used at pH 7.0 since the neutral pH is generally the optimal one to perform extraction and purification studies for biomolecules.
Experimental section
Materials
1,4-Dibromobutane, 1,6-Dibromohexane, 1,8-Dibromooctane, 1,10-Dibromodecane, 1-Methylimidazole, and Tergitol 15-S-7 — with mass fraction purity of 99, 96, 98, 97, 99, and 99%, respectively — were purchased from Sigma-Aldrich (St. Louis, MO, USA). Acetonitrile and ethyl ether — with mass fraction purity of 99.9% and HPLC grade, respectively — were purchased from Tedia (Rio de Janeiro, RJ, Brazil). All chemical products were used without further purification. The ILs—1,4-bis(3-methylimidazolium-1-yl)butane bromide; 1,6-bis(3-methylimidazolium-1-yl)hexane bromide; 1,8-bis(3-methylimidazolium-1-yl)octane bromide and 1,10-bis(3-methylimidazolium-1-yl)decane bromide—were synthesized in accordance with methodologies previously described by Shirota et al. [32] and Frizzo et al. [23]. The structures of all products were confirmed by 1H and 13C NMR spectroscopy and mass spectroscopy, and were in accordance with the characterization data depicted in the reference [23].
Methods
Cloud point determination
The phase behavior of the systems composed of the nonionic surfactant Tergitol 15-S-7 and McIlvaine buffer pH 7 (0.18 mol/L, being constituted by 0.2 mol/L of Na2HPO4 and 0.1 mol/L of citric acid, as detailed in Table S1 of SI) was determined in both the absence and presence of ILs, using the cloud point methodology [9, 33]. The amount of IL and tergitol (in mmol) use to prepare the mixtures are showed in the Tables S2-S6 of SI. The cloud point of each surfactant/IL mixture was determined by visually identifying the temperature at which the monophasic phase became turbid during heating at 0.1 ºC/min. Special precautions were used to ensure identical conditions for all measurements. Given the good experimental reproducibility, three determinations were found to be enough for each system, with the average value considered (in which SD not exceeded ± 0.2). Mixtures with 0.5 to 15 wt% of surfactant and 0 or 2 wt% of each IL were prepared in an aqueous solution of 0.18 mol L−1 of McIlvaine buffer (pH 7.0) up to a final volume of 10 mL, as previously established [7]. The solutions were prepared by weighing the well-defined amounts of tergitol, ionic liquid and McIlvaine buffer that composes the final solutions. The systems were heated from 20 to 40 °C in a temperature-controlled water bath with a precision of 0.01 K (ME-18 V Visco-Thermostat, Julabo). These systems were used to evaluate the effect that the nature and concentration of the IL (at 2 and 5.0 wt%) had on the cloud points of the Tergitol 15-S-7.
Dynamic light scattering
Using a Malvern Zetasizer Nano-ZS from Malvern Instruments, dynamic light scattering measurements were taken in order to evaluate the micelle size of the systems studied. Mixtures containing 1, 2.5, 5.0, 7.25, 9.0, 10.0, and 15.0 wt% of Tergitol 15-S-7 and 0 and 2 wt% of IL in water were analyzed in order to observe the impact that the incorporation of the IL had on micelle formation. The measurements were taken at 25 °C to ensure that all systems were below the binodal curve — and, therefore, in the monophasic region — and that the measurements only concerned the size of micelles homogeneously dispersed in solution. Samples were irradiated with red light (HeNe laser, wavelength of 565 nm). The intensity fluctuations of the scattering light were detected at a scattering angle of 173° in order to generate the intensity versus time autocorrelation function. The cumulant analysis of this function (provided by version 7.03 of the DTS software) yielded the particle size (intensity-based Z-average) and its distribution width (polydispersity index). The hydrodynamic diameter (Dh) of the aggregates was subsequently determined using the Stokes − Einstein equation, assuming spherical shape and a low volume fraction of the dispersed particles. The determined values essentially allow a quantitative trend regarding the size of the micelles to be established. At least six measurements were taken for each sample, and the average size and standard deviation were determined.
Surface tension measurements
Surface tension measurements were taken using the Du Noüy ring method. A Krüss GmbH K20 Easy Dyne tensiometer (Hamburg, Germany) coupled to a Brookfield TC-650 refrigerating/heating Julabo F12 circulator bath (Middleborough, United States) was used. A Tergitol 15-S-7 stock solution with 0.58 mmol L−1 concentration was prepared. Analyses were performed at 25 °C in both the absence and presence of ILs. The stock solution was progressively added into pure water or IL aqueous solution (at 3 g L−1) in order to obtain the desired Tergitol 15-S-7 concentration (from 0 to 0.13 mmol L−1), and at this concentration range, the values are both above and below the CMC value for the nonionic surfactant. After each addition of nonionic surfactant, the mixture was homogenized and the surface tension measured after temperature stabilization.
Results and discussion
Effect of cation of ILs on the cloud point curve profile of Tergitol 15-S-7
The cloud points of the nonionic surfactant Tergitol 15-S-7 were determined in both the absence and presence of several dicationic [BisAlk(MIM)2][2Br] ILs, in which Alk = But, Hex, Oct, and Dec. The selected ILs enabled evaluation of the effect that the alkyl chain length of the spacer in the symmetric dicationic ILs — with short side alkyl chains (e.g., methyl) — had on the phase behavior. The binodal curves were determined by visual identification of the cloud points. Initially, the cloud points of Tergitol 15-S-7 without IL — but in the presence of 0.18 mol L−1 of McIlvaine buffer at pH 7.0 — were determined in order to characterize the phase behavior of the surfactant. Subsequently, the cloud points of the Tergitol + IL mixtures in the buffer solution were determined for ILs with a concentration of 2.0 wt%, in order to evaluate the effect of each dicationic IL on the cloud points. The results are shown in Fig. 2.
From the binodal curves shown in Fig. 2, it can be seen that the dicationic ILs have an impact on the nonionic surfactant’s phase behavior. As reported in the literature, the cloud points of nonionic surfactants are strongly influenced by various additives (e.g., electrolytes, organic additives, nonelectrolytes, and ionic surfactants) [18]. In ILs absence, the hydration of ethoxylate groups of Tergitol 15-S-7 promotes the hydration of the polar micellar region and, consequently, dictates the surfactant’s solubility [34, 35]. As amphipathic compounds, it is expected that the IL’s cationic head interacts with the surfactant’s polar portion, while the alkyl spacer chain interacts with the surfactant’s apolar counterpart, which leads to the formation of mixed micelles composed of nonionic surfactant and IL. Consequently, this can influence the surfactant’s cloud points.
In the absence of ILs, the cloud point of Tergitol 15-S-7 shows an almost linear increase as the surfactant concentration increases. In the presence of ILs, the cloud points exhibit different behaviors according to the structure of the dicationic IL. [BisBut(MIM)2][2Br] — with four CH2 groups between the cationic heads — promotes a small decrease in the cloud point; while the remaining [BisAlk(MIM)2][2Br] ILs (in which Alk = Hex, Oct, and Dec) lead to an increase in the cloud point.
For imidazolium-based monocationic ILs, it has been reported that a more hydrophilic character induces an increase in the cloud point [9]. Nevertheless, in this present study, it was observed that the IL with the shortest alkyl spacer chain ([BisBut(MIM)2][2Br]) led to a decrease in the Tergitol’s cloud point; whereas the more hydrophobic ILs (alkyl spacer chains n ≥ 6) induced an increase in the cloud point temperature. These results suggest the following: (i) [BisBut(MIM)2][2Br] has a salting-out effect in the colloidal dispersion and, therefore, it reduces the cloud point temperature; (ii) [BisAlk(MIM)2][2Br] — in which Alk = 6 and 8 — is in a transition region between a salt and a surface-active IL behavior, which leads to a slight increase in cloud point temperature, without any apparent trend; and (iii) [BisDec(MIM)2][2Br] behaves like a usual surface-active IL.
According to a previous study, the CMC of the dicationic ILs in water decreases in accordance with the following carbon number order in the alkyl spacer chain: 4 > 6 > 8 > 10 [24]. In that same study, considerable differences were noted for the CMC of [BisAlk(MIM)2][2Br] (in which Alk = But, Hex, Oct, and Dec) in water, with values of 492, 278, 245, and 23 mM, respectively, which follows the increase of hydrophobic character of these ILs. Thus, it can be seen that dicationic ILs with lower CMC values (e.g., [BisDec(MIM)2][2Br]) lead to higher cloud point temperatures in the colloidal dispersion (mixed micelles).
Previous studies provided that [BisOct(MIM)2][2Br] has the ability to fold the alkyl spacer chain in solution [22, 23]. Additionally, this result was supported by Hennemann et al. [36] suggesting that long dicationic ILs (e.g., [BisAlk(MIM)2][2Br], in which Alk = Hex, Oct, and Dec) have a greater dication-anion interaction strength due to the ability to fold the alkyl spacer chain, which results in a greater proximity between the positive charges.
In this sense, it is reasonable suggested that dicationic ILs with long alkyl spacer chains increase the nonionic surfactant’s cloud point temperature, due to the folded organization of the molecules in solution (cf. the schematic representation of what might be a plausible mixed micelle organization for Tergitol and ILs in Fig. 3a). When the IL molecules were incorporated into the mixed micelles, the interaction between the apolar-apolar and polar-polar portions of both components (IL and Tergitol) was favored, which enabled an increase in the solubility (miscibility) of the mixture. Another interesting aspect is that, with the folding of the ILs (for n = 6, 8, and 10), there are two cationic portions interacting with the water through ion–dipole interactions, which leads to an increase in miscibility (greater hydration sphere) and, consequently, increases the cloud point temperature. Due to the small alkyl spacer chains, [BisBut(MIM)2][2Br] does not allow the folding of cation, which difficults the polar interactions with both Tergitol and the polar solvent, and, consequently, decreasing the cloud point temperature (see Fig. 3b). In addition, the mixed micelles of ILs (for n = 6, 8, and 10) present greater electrostatic repulsion when compared with n = 4. For n = 4, only one cationic portion exhibit effective contact with the water; therefore, the miscibility is lower. This hypothesis can be further corroborated by the critical parameter packing (CPP) of the ILs’ structure, which is related to the cation/anion nature and symmetry, and also to the alkyl chain length. CPP has also been reported to influence the cloud points of nonionic surfactants [9]. The CPP values for [BisAlk(MIM)2][2Br] — in which Alk = But, Hex, Oct, and Dec — were 0.41, 0.23, 0.11, and 0.25, respectively [24]. Then, we can infer that dicationic ILs with a smaller CPP value (CPP < 1/3) lead to a decrease in the effective CPP of the mixed micelle (composed of IL and Tergitol molecules), which leads to an increase in both solubility and the cloud point temperature of the mixture. When this data is combined with the cloud points and the ILs’ tendency to fold, everything seems to be in agreement. [BisBut(MIM)2][2Br] has a higher CPP and, therefore, result in bigger micelles that are less soluble in water, which induces a decrease in the cloud point temperature. By contrast, [BisAlk(MIM)2][2Br] — in which Alk = Hex and Dec — had similar CPP values, which explains why the cloud point temperatures of these two ILs are more similar. As can be seen in Sect. 3.3, the CPP seems to be unrelated to the hydrodynamic diameter, given that a higher CPP should result in a larger hydrodynamic diameter of the micelles [9].
The cloud points of Tergitol 15-S-7 in water have been previously reported in the literature [15]. Recently, Vicente et al. [9] determined the cloud points of this surfactant in McIlvaine buffer at pH 7.0, and they reported a salting-out effect on the phase behavior due to the buffer’s salt content. The cloud points obtained in the present study also exhibited a salting-out effect with the increase in Tergitol concentration (see Fig. S1 in the ESI), although the curve behavior was different. This difference can be attributed to the different ionic strength of the medium due to the pH adjustment of McIlvaine buffer with acid or base.
Effect of IL concentration on the cloud points of Tergitol 15-S-7
The results showed that adding distinct amounts of dicationic ILs can change the cloud points of the nonionic surfactant (cf. ILs molar concentration in Table S2 in ESI). Nevertheless, it is extremely relevant to evaluate the impact of the IL concentration on the cloud points in the mixed systems, in order to find the best condition to attain the desired phase behavior. Thus, an investigation was performed in which the IL concentration was varied from 0 to 5.0 wt%, while maintaining the Tergitol concentration constant at 5 wt%. The results are shown in Fig. 4.
From Fig. 4, it can be seen that, when the concentration of the most hydrophobic ILs [BisAlk(MIM)2][2Br] in which Alk = Hex, Oct, and Dec increases, the cloud point temperatures also increase. In fact, with 5 wt% of [BisAlk(MIM)2][2Br] — in which Alk = Dec, Hex, and Oct — there is an increase in the cloud point temperature of 7, 4, and 3 °C, respectively. This effect is stronger for ILs with longer alkyl spacer chains and is probably due to the alkyl chain’s ability to fold, which increases the contact of the cationic IL portion with water, thus increasing the ability of these ILs to form hydration complexes with water in the mixed micelle. On the other hand, when the concentration of [BisBut(MIM)2][2Br] — the more hydrophilic IL — increases, the cloud point does not change significantly. These outcomes indicate that the increase in the concentration of more hydrophobic ILs has a great impact on the intermolecular interactions with Tergitol and water, and, consequently, on the LCST.
Effect of ILs on the micelle size of Tergitol 15-S-7
In order to determine if the dicationic ILs induce changes in the micelle size of the Tergitol, dynamic light scattering (DLS) measurements were taken at different Tergitol 15-S-7 concentrations in both absence and presence of ILs. Though, it should be stressed that although this technique assumes spherical micelles—which has been shown to occur to Tergitol 15-S-7 [37]—it is not possible to guarantee that in presence of the ILs, the mixed micelles will maintain the same morphology. The DLS experiments were performed not as much as a quantitative analysis but more as a qualitative measurement of these ILs influence over the conventional nonionic micelle size. From the data, it could be seen that there was only one population of aggregates for Tergitol 15-S-7 in either the absence or presence of ILs (Fig. S1 in the ESI). The results were analyzed considering the micelle’s diameter, which is given in nm — see Fig. 5.
In ILs absence, the size of the micelles decreases with the increase in Tergitol concentration. Generally, this is the opposite of what is expected (i.e., an increase in micelle size is expected with the increase in surfactant concentration). However, it is not the first report of such behavior. The data obtained by Vicente et al. [9] also showed that for Tergitol 15-S-7, the micelle size slightly decrease when the surfactant concentration was increased from 1 to 10 wt%. As the surfactant concentration increases, the surfactant molecules are not incorporated into the existing micelle, causing the micelle to increase in size. Hence, the system reorganizes itself to form a larger number of aggregates that are smaller in size. The main difference from the present investigation is that, at 1 wt% of surfactant, the previous work reports a micelle diameter slightly higher than 13 nm whereas the current study shows a diameter ~ 17 nm. The main reason for this lies on the fact that the previous work used a buffered system while here we are working with distilled water, thus not having the salts influence in compacting the micelles.
For a given Tergitol concentration, the systems with dicationic ILs show an increase in the micelle size when compared to the systems without IL, which suggests that the growth of the mixed micelle is induced by the presence of ILs. [BisDec(MIM)2][2Br] promoted the greatest increase in the cloud point values; however, it did not have a pronounced effect on the micelle size (as this IL can be easily folded, the electrostatic repulsion between cationic heads prevents the growth of the mixed micelle). The systems with [BisAlk(MIM)2][2Br] — in which Alk = But, Hex, and Oct — had a larger micelle diameter. The same systems had less changes in cloud point values than those systems with [BisDec(MIM)2][2Br]. Nevertheless, the results observed are not consistent with those reported for monocationic ILs, in which it has been established that compounds that increase the cloud point decrease the micelle size; while those that decrease the cloud point induce significant micellar growth in the nonionic surfactant [9]. The decrease in micelle size in the presence of ionic surfactants and ILs has been attributed to a decrease in the micelle’s aggregation number [9, 38]. In this process, the original nonionic surfactant micelles tend to break down when the IL is added, and the new IL/Tergitol micelles subsequently reorganize with a lower aggregation number, due to the electrostatic repulsions of the IL’s cationic head. The opposite trend (i.e., an increase in micelle diameter with the addition of ionic surfactant) has also been reported as a function of surfactant nature [9].
Considering results reported herein, it is suggested that the dicationic imidazolium-based ILs with short alkyl spacer chains are not capable of promoting the breaking down of the nonionic surfactant micelles. Instead, these ILs only insert some molecules into the Tergitol micelles already formed, thus increasing the hydrodynamic diameter of the mixed micelles (Fig. 3b). This behavior can be attributed to the presence of an additional cationic head, which means a greater possibility of interaction of ILs with the Tergitol’s polar portion. The less pronounced mixed micelle growth for [BisDec(MIM)2][2Br] can be rationalized by considering that its aggregation number is lower than for [BisAlk(MIM)2][2Br], in which Alk = But, Hex, and Oct.
As previously reported, the addition of monocationic imidazolium-based ILs in tergitol solution causes an increase in the cloud point temperature of the mixture, reduce the micelle size and have lower CPP values, due to the greater micellar solubility [9]. In this present study, the addition of dicationic imidazolium-based ILs in Tergitol solution demonstrated that the increase in alkyl chain spacer of [BisAlk(MIM)2][2Br], lead to an increase in the cloud point values as well as an increase in micelle size. However, the micelle size did not show any correlation with CPP values (solutions with larger micelles would be expected to have higher CPP values).
The increase in the micelle size formed by dicationic imidazolium-based ILs for pure ILs in solution was reported by Frizzo et al. [22]. Using small angle X-ray scattering data, authors showed that as greater is the hydrophobicity of the dicationic ILs (related to characteristics of the anions), more closely packed are the aggregates. It has also been shown that the micelle size of the ILs has no relationship with the length of the alkyl spacer chain [24], which was observed in this present study for the mixed micelles.
Additionally, when the concentration of Tergitol is high (15 wt%) all hydrodynamic radius tend to the value of pure Tergitol (without IL). This indicates that at low concentrations of Tergitol the ILs participate effectively in the formation of the mixed micelle. On the other hand, at high Tergitol concentrations, the amount of ILs is lower in relation to tergitol and does not affect the size of the micelles (in which, there is just a few ILs molecules per Tergitol micelle).
ILs influence upon the surface activity properties of Tergitol 15-S-7
Surface tension measurements were taken in order to investigate if the surface activity of Tergitol is modified by the presence of dicationic ILs. Figure 6 shows the plot of surface tension (γ) versus the natural logarithm of Tergitol in both the presence and absence of [BisAlk(MIM)2][2Br] in aqueous solutions, at 25 °C. All curves showed a decrease in surface tension with the increase in Tergitol concentration, followed by a slight change in slope. The position of the discontinuities was determined taking the maximum point of the second derivative of the experimental curves, according with Phillips method [39, 40]. The discontinuities correspond to CMC that is the concentration in which the aggregates start to be formed. The surface activity properties for pure ILs have already been described by Frizzo et al. [24].
The surface activity parameters of Tergitol in the presence and absence of ILs are shown in Table 1. Although we cannot confirm that the mixed aggregates are in fact spherical micelles, is important to mention that the term “micelles” (and “CMC”) was used for both aggregates (in the absence and presence of ILs) for organizational purposes and better understanding. The CMC values for Tergitol without IL were in accordance with values previously described in the literature [15]. The CMC values obtained was lowest for [BisHex(MIM)2][2Br] and highest for [BisOct(MIM)2][2Br], which lets us conclude that there is no relationship between the size of the ILs’ spacer chains and the CMC for the mixed micelle. Therefore, it can be pointed out that the mixed micelles aggregation does not follow the same order to pure ILs aggregation in solution, since the CMC of [BisAlk(MIM)2][2Br] is lower as greater is the ILs alkyl spacer chain [24]. This observation supports the distinct aggregation mechanism of mixed micelles in relation to pure ILs aggregation in solution.
The values obtained for surface tension at the CMC (γcmc) were very similar in the presence of the dicationic ILs, regardless of the variation in the spacer chain. However, when comparing the Tergitol micelles with the mixed micelles, there was a decrease in the γcmc values with the presence of dicationic ILs, which indicates greater surface activity for mixed micelles. The relative surface pressure in the CMC (Πcmc) showed the same behavior, which indicates the effectiveness of surface tension reduction. The mixed micelles had greater surface activity than pure Tergitol micelles and, therefore, could potentially be used in various industrial applications [41, 42].
The maximum excess concentration (Гmax) in the liquid/air interface was obtained from the Gibbs equation, in which the value of the pre-factor m was considered to be 1 for neutral surfactant [43]. The Гmax value for the mixed micelles had a difference of ± 0.10 for the ILs containing butyl, octyl, and decyl spacer chains, and was much larger for Tergitol/[BisHex(MIM)2][2Br]. This could be due to the greater stability of the Tergitol/[BisHex(MIM)2][2Br] system at the interface, since the CMC value in this case was the lowest obtained. The values obtained for Amin follow the expected trend for all of the systems. For Tergitol/[BisHex(MIM)2][2Br], the higher Гmax value is reflected in lower Amin values, since better packing density of the molecules results in a greater concentration of the surfactant at the liquid–air interface, thus decreasing the Amin value.
Conclusions
The cloud points of Tergitol 15-S-7 in McIlvaine buffer were affected by the presence of dicationic ILs. Dicationic ILs with shorter alkyl spacer chain (n = 4) promoted a slight decrease in cloud point temperature, while ILs with longer alkyl spacer chain increased the cloud point of the Tergitol, due to the greater impact that these folded structures have in the intermolecular interactions with Tergitol and water, which increases the mixed micelles solubility. In a similar way, the IL concentration effect also is greater for the more hydrophobic ILs. The results showed that the hydrodynamic diameter of the mixed micelles does not depend on the length of the IL’s alkyl spacer chain. The [BisDec(MIM)2][2Br] IL had less pronounced growth in micellar hydrodynamic diameter than the other ILs (when comparing with Tergitol micelles), which can be rationalized by considering that the aggregation number is lower than for [BisAlk(MIM)2][2Br], in which Alk = But, Hex, and Oct. Greater surface activity was observed for mixed micelles than for the Tergitol micelles. These new mixed micelles open applications possibilities in several fields including pharmaceutical sciences.
References
Lindman B, Medronho B, Karlström G (2016) Clouding of nonionic surfactants. Curr Opin Colloid Interface Sci 22:23–29. https://doi.org/10.1016/j.cocis.2016.01.005
Khan TA, Mahler H, Kishore RSK (2015) Key interactions of surfactants in therapeutic protein formulations: a review. Eur J Pharm Biopharm 97:60–67. https://doi.org/10.1016/j.ejpb.2015.09.016
Bordat A, Boissenot T, Nicolas J, Tsapis N (2019) Thermoresponsive polymer nanocarriers for biomedical applications. Adv Drug Deliv Rev 138:167–192. https://doi.org/10.1016/j.addr.2018.10.005
Chen S, Hanning S, Falconer J et al (2019) Recent advances in non-ionic surfactant vesicles (niosomes): fabrication, characterization, pharmaceutical and cosmetic applications. Eur J Pharm Biopharm 144:18–39. https://doi.org/10.1016/j.ejpb.2019.08.015
Molino JVD, Viana Marques DDA, Júnior AP et al (2013) Different types of aqueous two-phase systems for biomolecule and bioparticle extraction and purification. Biotechnol Prog 29:1343–1353. https://doi.org/10.1002/btpr.1792
Martínez-Aragón M, Burghoff S, EL Goetheer V, de Haan AB (2009) Guidelines for solvent selection for carrier mediated extraction of proteins. Sep Purif Technol 65:65–72. https://doi.org/10.1016/j.seppur.2008.01.028
Vicente FA, Malpiedi LP, eSilva FA et al (2014) Design of novel aqueous micellar two-phase systems using ionic liquids as co-surfactants for the selective extraction of (bio)molecules. Sep Purif Technol 135:259–267. https://doi.org/10.1016/j.seppur.2014.06.045
Vicente FA, Cardoso IS, Martins M et al (2019) R-phycoerythrin extraction and purification from fresh Gracilaria sp. using thermo-responsive systems. Green Chem 21:3816–3826. https://doi.org/10.1039/C9GC00104B
Vicente FA, Cardoso IS, Sintra TE et al (2017) Impact of surface active ionic liquids on the cloud points of nonionic surfactants and the formation of aqueous micellar two-phase systems. J Phys Chem B 121:8742–8755. https://doi.org/10.1021/acs.jpcb.7b02972
Li J, Chen B (2003) Equilibrium partition of polycyclic aromatic hydrocarbons in a cloud-point extraction process. J Colloid Interface Sci 263:625–632. https://doi.org/10.1016/S0021-9797(03)00403-X
Tani H, Kamidate T, Watanabe H (1998) Aqueous micellar two-phase systems for protein separation. Anal Sci 14:875–888. https://doi.org/10.2116/analsci.14.875
Galaev IY, Mattiasson B (1993) Thermoreactive water-soluble polymers, nonionic surfactants, and hydrogels as reagents in biotechnology. 15:
Liu CL, Nikas YJ, Blankschtein D (1996) Novel bioseparations using two-phase aqueous micellar systems. Biotechnol Bioeng 52:185–192. https://doi.org/10.1002/(SICI)1097-0290(19961020)52:2%3c185::AID-BIT1%3e3.0.CO;2-M
Vicente FA, Lario LD, Pessoa A, Ventura SPM (2016) Recovery of bromelain from pineapple stem residues using aqueous micellar two-phase systems with ionic liquids as co-surfactants. Process Biochem 51:528–534. https://doi.org/10.1016/j.procbio.2016.01.004
Li J, Bai D, Chen B (2009) Effects of additives on the cloud points of selected nonionic linear ethoxylated alcohol surfactants. Colloids Surfaces A Physicochem Eng Asp 346:237–243. https://doi.org/10.1016/j.colsurfa.2009.06.020
Huibers PDT, Shah DO, Katritzky AR (1997) Predicting surfactant cloud point from molecular structure. J Colloid Interface Sci 193:132–136. https://doi.org/10.1006/jcis.1997.5053
Shigeta K, Olsson U, Kunieda H (2001) Correlation between micellar structure and cloud point in long poly(oxyethylene)n oleyl ether systems. Langmuir 17:4717–4723. https://doi.org/10.1021/la001260x
Mukherjee P, Padhan SK, Dash S et al (2011) Clouding behaviour in surfactant systems. Adv Colloid Interface Sci 162:59–79. https://doi.org/10.1016/j.cis.2010.12.005
Anufrikov YA, Kondrakhina PS, Koneva AS, Safonova EA (2019) Impact of Bioorganic Additives of Different Nature on Aggregation Behavior and on Cloud Point Temperatures of Nonionic Surfactants Tergitol NP-7 and Triton X-114 in Buffer Solutions. Colloid J 81:683–689. https://doi.org/10.1134/S1061933X19060024
Li P, Tan S, Wu Y et al (2020) Azobenzene-based ionic liquid switches phase separation of poly(N-isopropylacrylamide) aqueous solutions as a molecular trigger, leading to UV shutdown of Ionic transport. ACS Macro Lett. https://doi.org/10.1021/acsmacrolett.0c00170
Akl ZF, Hegazy M, a. (2020) Selective cloud point extraction of thorium (IV) using tetraazonium based ionic liquid. J Environ Chem Eng 8:104185. https://doi.org/10.1016/j.jece.2020.104185
Frizzo CP, Bender CR, Gindri de Mello I et al (2016) Elucidating anion effect on nanostructural organization of dicationic imidazolium-based ionic liquids. J Phys Chem C 120:14402–14409. https://doi.org/10.1021/acs.jpcc.6b04262
Frizzo CP, Bender CR, Gindri IM et al (2015) Anion effect on the aggregation behavior of the long-chain spacers dicationic imidazolium-based ionic liquids. Colloid Polym Sci 293:2901–2910. https://doi.org/10.1007/s00396-015-3680-y
Frizzo CP, Gindri IM, Bender CR et al (2015) Effect on aggregation behavior of long-chain spacers of dicationic imidazolium-based ionic liquids in aqueous solution. Colloids Surfaces A Physicochem Eng Asp 468:285–294. https://doi.org/10.1016/j.colsurfa.2014.12.029
Frizzo CP, Bender CR, Salbego PRS et al (2016) Thermodynamic properties of the aggregation behavior of a dicationic ionic liquid determined by different methods. Colloids Surfaces A Physicochem Eng Asp 494:1–8. https://doi.org/10.1016/j.colsurfa.2016.01.015
Mortada WI (2020) Recent developments and applications of cloud point extraction: a critical review. Microchem J 157:105055. https://doi.org/10.1016/j.microc.2020.105055
Moosavi M, Khashei F, Sharifi A, Mirzaei M (2017) The effects of temperature and alkyl chain length on the density and surface tension of the imidazolium-based geminal dicationic ionic liquids. J Chem Thermodyn 107:1–7. https://doi.org/10.1016/j.jct.2016.12.009
Plechkova NV, Seddon KR (2008) Applications of ionic liquids in the chemical industry. Chem Soc Rev 37:123–150. https://doi.org/10.1039/b006677j
Hallett JP, Welton T (2011) Room-temperature ionic liquids: solvents for synthesis and catalysis. Chem Rev 111:3508–3576. https://doi.org/10.1021/cr1003248
De Freitas DV, Kuhn BL, Bender CR et al (2020) Thermodynamics of the aggregation of imidazolium ionic liquids with sodium alginate or hydroxamic alginate in aqueous solution. J Mol Liq 297:111734. https://doi.org/10.1016/j.molliq.2019.111734
Frizzo CP, Bender CR, Salbego PRS et al (2018) Heteroassembly ability of dicationic ionic liquids and neutral active pharmaceutical ingredients. ACS Omega 3:2282–2291. https://doi.org/10.1021/acsomega.7b02097
Shirota H, Mandai T, Fukazawa H, Kato T (2011) Comparison between dicationic and monocationic ionic liquids: liquid density, thermal properties, surface tension, and shear viscosity. J Chem Eng Data 56:2453–2459. https://doi.org/10.1021/je2000183
Blankschtein D, Thurston GM, Benedek GB (1986) Phenomenological theory of equilibrium thermodynamic properties and phase separation of micellar solutions and phase separation of micellar solutions. J Chem Phys 85:7268–7288. https://doi.org/10.1063/1.451365
Florence AT (1969) Micellar size, shape, and hydration of nonionic surfactants. J Colloid Interface Sci 31:580–583. https://doi.org/10.1016/0021-9797(69)90064-2
Carlström G, Halle B (1989) The state of water in non-ionic surfactant solutions and lyotropic phases Oxygen-17 magnetic relaxation study. J Chem Soc Faraday Trans 1 Phys Chem Condens Phases 85:1049–1063 https://doi.org/10.1039/F19898501049
Hennemann BL, Bender CR, Salbego PRS et al (2018) Models for understanding the structural effects on the cation-anion interaction strength of dicationic ionic liquids. J Mol Liq 252:184–193. https://doi.org/10.1016/j.molliq.2017.12.110
Bera A, Mandal A, Belhaj H, Kumar T (2017) Enhanced oil recovery by nonionic surfactants considering micellization, surface, and foaming properties. Pet Sci 14:362–371. https://doi.org/10.1007/s12182-017-0156-3
Thakkar K, Bharatiya B, Ray D et al (2016) Molecular interactions involving aqueous triton X-100 micelles and anionic surfactants: investigations on surface activity and morphological transitions. J Mol Liq 223:611–620. https://doi.org/10.1016/j.molliq.2016.08.086
Phillips BYJN (1955) The energetics of micelle formation. Trans Faraday Soc 51:561–569
Jungnickel C, Łuczak J, Ranke J et al (2008) Micelle formation of imidazolium ionic liquids in aqueous solution. Colloids Surfaces A Physicochem Engi Aspects 316:278–284. https://doi.org/10.1016/j.colsurfa.2007.09.020
Vicente FA, Bairos J, Roque M et al (2019) Use of ionic liquids as cosurfactants in mixed aqueous micellar two-phase systems to improve the simultaneous separation of immunoglobulin g and human serum albumin from expired human plasma. ACS Sustain Chem Eng 7:15102–15113. https://doi.org/10.1021/acssuschemeng.9b03841
Nandwani SK, Malek NI, Chakraborty M, Gupta S (2019) Potential of a novel surfactant slug in recovering additional oil from highly saline calcite cores during the EOR process: synergistic blend of surface active ionic liquid and nonionic surfactant. Energy Fuels 33:541–550. https://doi.org/10.1021/acs.energyfuels.8b03419
Eastoe J, Nave S, Downer A et al (2000) Adsorption of Ionic Surfactants at the Air - Solution Interface. Langmuir 25(4511):4518
Acknowledgements
The authors acknowledge the financial support from CNPq, CAPES, and FAPERGS (edital 04/2019 - Auxílio Recém Doutor (ARD); grant nº 19/2551-0001242-0), as well as the research fellowships from CNPq (C. P. F. and M. A. V.) and CAPES (B.L.K). This work was also developed within the scope of the CICECO-Aveiro Institute of Materials project (UIDB/50011/2020 and UIDP/50011/2020), financed by national funds from Portugal’s Foundation for Science and Technology (Fundação para a Ciência e a Tecnologia — FCT) of the Ministry of Science, Technology, and Higher Education (Ministério da Ciência, Tecnologia e Ensino Superior — MCTES). The authors are also grateful for the national funds from the FCT for the doctoral grant (SFRH/BD/101683/2014) of F.A.V. Also, P.J.C. acknowledges the FCT/MCTES for research contract IF/00758/2015 as part of the “Investigador FCT” program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Annela M. Seddon.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bender, C.R., Vicente, F.A., Kuhn, B.L. et al. Effect of dicationic ionic liquids on cloud points of tergitol surfactant and the formation of aqueous micellar two-phase systems. J Mater Sci 56, 12171–12182 (2021). https://doi.org/10.1007/s10853-021-06055-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-021-06055-1