Abstract
1,3,5-Benzenetricarboxylic acid tris(phenylamide) (hereinafter called as “BTA-93”) was synthesized and proved to be a highly efficient α-crystalline nucleating agent through investigation of crystallization and melting behaviors of nucleated isotactic polypropylene (iPP) by differential scanning calorimetry. The optimum addition amount of BTA-93 in iPP was 0.07 wt%. At this low addition amount, crystallization peak temperature of iPP was increased from 119.3 °C of neat iPP to 126 °C and the nucleation effect was comparable to that of a highly effective commercial nucleating agent disodium bicyclo [2.2.1] heptane dicarboxylate (HPN-68) at addition amount of 0.2 wt%. Furthermore, the crystal structure of BTA-93 was obtained by single crystal X-ray diffraction. The crystal data indicated that the b-direction of BTA-93 crystal was about four times of 0.655 nm which was the cell edge of (010)iPP plane, and the lattice mismatch degree was 2.81%. The outstanding nucleation efficiency of BTA-93 in iPP could relate to the lattice matching between BTA-93 and α-iPP crystals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Isotactic polypropylene (iPP) is one of the most commonly used thermoplastics, which has various advantages, such as low production cost, feasible processing, and good mechanical properties [1–6]. As a semicrystalline polymer, iPP can crystallize in monoclinic (α), trigonal (β), orthorhombic (γ), and other forms [7, 8]. However, application of iPP in many fields was limited because of its bad transparency and low impact strength, especially under low temperature. Therefore, it is important to find effective methods that can improve mechanical and optical properties of iPP. Generally, spherulite size and crystallization behaviors of iPP play an important role in improving mechanical and optical properties, while spherulite size depends on nucleation rate and spherulites growth rate [9–13]. Incorporation of nucleating agents into iPP is one of the most important methods to increase the nucleation density and the nucleation rate [14–16]. The addition of nucleating agents is contributed to increase crystallization peak temperature, increase nucleation and crystallization rate of iPP, and therefore reduce spherulite size, leading to better mechanical and optical properties.
At present, organic carboxylates, aromatic heterocyclic phosphate salts, and sorbitol derivatives are regarded as efficient nucleating agents for iPP. Many researches focused on properties and crystallization behaviors of iPP nucleated with abovementioned nucleating agents [14, 17–24]. Although they are highly effective nucleating agents, they also have some disadvantages. Dispersion of organic carboxylates and aromatic heterocyclic phosphate salts in iPP is not well, so that their nucleation effects are compromised. Sorbitol derivatives have drawbacks such as limited applicability, relatively poor thermal stability, and uncomfortable odor during thermal processing [10].
In recent years, amide derivatives of 1,3,5-benzenetricarboxylic acid are promising nucleating agents to overcome drawbacks of abovementioned conventional nucleating agents. A series of amide derivatives of 1,3,5-benzenetricarboxylic acid was synthesized, and their potential uses as nucleating agents for iPP were studied as yet [25–27]. Many 1,3,5-benzenetricarboxylic acid amide derivatives were highly efficient nucleating agents for iPP in these researches. They were capable of significantly improving mechanical and optical properties of iPP. Blomenhofer et al. reported amide derivatives of 1,3,5-benzenetricarboxylic acid were novel nucleating agents for polypropylene [26]. However, nucleating agents which have similar chemical structures based on 1,3,5-benzenetricarboxylic acid amide and the only difference is the N-substituent group have obviously different nucleation effects in iPP. The optical properties of a 1.1-mm thick sample of iPP nucleated with 1,3,5-benzenetricarboxylic acid tris(3-methylbutylamide) were improved from 79% of clarity to 98.7% and from a haze value of 64% to 27.4%. The optical properties of iPP nucleated with 1,3,5-benzenetricarboxylic acid tris(3-dimethylcyclohexylamide), however, were reduced from 79% of clarity to 72.3% and from a haze value of 64% to 98.2%. On the aspect of crystallization peak temperature, different nucleating agents also have different nucleation effects. The crystallization peak temperature of iPP nucleated with 1,3,5-benzenetricarboxylic acid tris(2-adamantylamide) was increased by about 11 °C, from 110 °C of neat iPP to 130.9 °C. However, that of iPP nucleated with 1,3,5-benzenetricarboxylic acid tris(n-propylamide) was increased from 110 °C of neat iPP only to 114.8 °C. These data indicated that the nucleation efficiency of nucleating agents was affected by N-substituent group of 1,3,5-benzenetricarboxylic acid amide derivatives. All of these implied that the nucleation efficiency might depend on subtle details of the substituent groups, such as crystal structure of nucleating agents considering that these nucleating agents have very similar chemical structures.
A lot of studies have shown that epitaxial interaction between nucleating agents and iPP was a popular theory to explain how nucleating agent increases nucleation rate [28–35]. But only few studies reported crystallographic structures of 1,3,5-benzenetricarboxylic acid derivatives (BTAs). Lightfoot et al. reported that crystal of 1,3,5-benzenetricarboxylic acid tris(2-methoxyethyl) was a columnar structure which was monoclinic [28]. Jimenez et al. reported the crystal structure of n-alkyl substituted BTAs, which was orthorhombic [29]. Kristiansen et al. reported the crystal structure of a tert-butyl substituted BTA (all based on 1,3,5-benzenetricarboxylic acid), and the c-direction of crystal lattice was about one time of 0.655 nm which was the cell edge of (010)iPP plane [30].
In this work, the nucleation efficiency of 1,3,5-benzenetricarboxylic acid tris(phenylamide) (BTA-93), one of the amide derivatives of 1,3,5-benzenetricarboxylic acid, was investigated through crystallization behaviors of nucleated iPP, and it was found that BTA-93 was an efficient α-crystalline nucleating agent for iPP at very low addition amount. In addition, the crystal structure of BTA-93 was obtained by single crystal X-ray diffraction to further seek the reason for such excellent nucleation effects. From the geometric analysis, the B cell dimension of BTA-93 was about four times of the cell edge of (010)iPP and good lattice matching exists between BTA-93 and α-iPP, leading to good nucleation effects of BTA-93.
Experimental
Materials
The iPP powders (MFR = 2.5 g/10 min) used in this experiment were supplied by SINOPEC BaLing Company (China). Nucleating agent, BTA-93, with the melting temperature of 307 °C, whose structure is shown in Scheme 1, was synthesized according to literature [30]. Antioxidants 1010 and 168 were obtained from Ciba Specialty Chemicals (Switzerland). All the other chemicals of reagent grade and analytical grade were purchased from Sinopharm Chemical Reagent Company, used without further purification.
Sample preparation
The iPP powders and different amounts of nucleating agent BTA-93 powders in combination with antioxidants 1010 and 168 (addition concentration of both 1010 and 168 in iPP was 0.1 wt%) were firstly mixed in a SHR 100 high-speed mixer (Zhangjiagang HaiChuan Machinery Co., Ltd., China) for 5 min. Then, the obtained mixture was extruded in a SHJ-20B twin-screw extruder (Nanjing GIANT Machinery Co., Ltd., China) and pelletized. The pellets were used for subsequent crystallization and melting behavior investigation.
Crystallization and melting behaviors
Using a TA Q2000 differential scanning calorimetry (DSC) (TA Instruments Company, USA), crystallization and melting behaviors of neat iPP and iPP nucleated with BTA-93 were investigated. The samples were firstly heated to 200 °C at a heating rate of 100 °C/min and maintained for 5 min to erase effects of the previous thermal history. The samples were then cooled to 60 °C at a cooling rate of 10 °C/min. After crystallization is finished, a heating scan at 10 °C/min was run again from 60 to 200 °C to obtain the melting curves of the samples.
Preparation of single crystal of BTA-93
BTA-93 (43.5 mg, 0.1 mmol) was weighted and dissolved into 10 ml of methanol. Precipitation was filtrated and recrystallized in methanol at ambient temperature. The single crystals of BTA-93 were grown by vapor diffusion of ether into methanol. After slow evaporation, crystals were harvested and suitable crystals were selected for single crystal X-ray diffraction.
Powder X-ray diffraction (PXRD) of nucleating agent BTA-93
Powder X-ray diffraction patterns were collected on a Bruker D8 Advance X-ray diffractometer (Cu Kα radiation). The generator was operated at 40 kV and 40 mA. In the 2θ range of 3°–40°, data were collected with a scan rate of 5°/min and were then peak-analyzed with Jade 6.0 from Rigaku after being integrated with RINT Rapid.
Single crystal X-ray diffraction (SCXRD)
X-ray diffractions of all single crystals were collected at 100(2) K on a Bruker Apex II CCD diffractometer using Mo Kα radiation (λ = 0.71073 Å). The SAINT programs were used to perform the integration and scaling of intensity data. The SADABS was used to correct the effects of absorption of data. Using SHELX-97 software with the full-matrix least-squares technique, the crystal structures were solved by direct methods and refined. Non-hydrogen atoms were refined, and H atoms were refined and placed in suitable positions. Crystallographic data in cif format have been uploaded in the Cambridge Crystallographic Data Center, CCDC no. 1516748.
Wide-angle X-ray diffraction (WARD) of iPP
The extruded samples were placed between two cover glasses on a hot stage at 200 °C. The melted specimen was then quickly placed onto another hot stage set to the desired temperature in the range 90–140 °C. The samples were held isothermally until the crystallization process was completed. Then, the crystal structure of the samples was investigated by wide-angle X-ray diffraction. The WARD patterns were recorded in transmission with a Rigaku D/max-2550VB/PC apparatus (Japan). Using Cu Kα (λ = 1.54 Å) radiation, the spectra were recorded in the 2θ range of 5°–35° (8°/min).
Results and discussion
Crystallization and melting behaviors of iPP nucleated with BTA-93
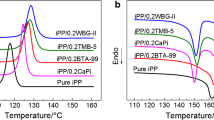
The crystallization and melting behaviors of iPP nucleated with BTA-93 at different addition amount were investigated by comparing with those of iPP nucleated with HPN-68 at addition amount of 0.2 wt%. HPN-68 was a common and highly efficient commercial organic dicarboxylate nucleating agent. In general, the optimal addition amount of common nucleating agents was approximately 0.2 wt%, so the addition range of nucleating agent BTA-93 in iPP was firstly determined as 0.1∼0.5 wt%. In different addition amounts of nucleating agent BTA-93, Fig. 1 shows the crystallization behaviors and crystallization peak temperature (T p) shift of nucleated iPP.
T p of neat iPP was about 119.3 °C. With increasing the addition amount of BTA-93 in iPP, T p shifted to higher temperature. Compared with that of neat iPP, the T p of iPP nucleated with BTA-93 at addition amount of 0.1 wt% was increased to 125.5 °C, increased by 6.2 °C. A remarkable phenomenon can be noticed in Fig. 1. The T p value of nucleated iPP was kept at about 126 °C when the addition amount of BTA-93 exceeds 0.1 wt% while that of iPP nucleated with HPN-68 at addition amount of 0.2 wt% was 126.6 °C, indicating that nucleation efficiency of BTA-93 at low addition amount was comparable to that of highly effective commercial nucleating agent HPN-68 and BTA-93 was also a highly effective nucleating agent for iPP. In order to determine the optimal addition amount of BTA-93 in iPP, it is necessary to investigate the crystallization behaviors of nucleated iPP at lower addition amount. Figure 2 shows the crystallization behaviors and crystallization peak temperature shift of nucleated iPP at lower addition amount of BTA-93.
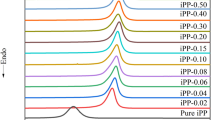
It can be seen from Fig. 2 that BTA-93 still has obvious nucleation effects and can increase T p of iPP significantly even at very low addition amount. The T p value of nucleated iPP reached a stable value (125 °C) at addition amount of 0.07 wt% and was increased by 5.7 °C when compared with that of neat iPP, which is similar to that of iPP nucleated with HPN-68, indicating that BTA-93 increases T p of iPP obviously and has excellent nucleation efficiency at very low addition amount, which is difficult to achieve for any other highly efficient commercial nucleating agents for iPP.
Figure 3 shows the DSC melting curves of iPP nucleated with BTA-93 at different addition amounts of BTA-93. It can be seen that only one melting peak at about 165 °C [36–38] which corresponds to the melting temperature of α-iPP appears for both neat iPP and nucleated iPP, indicating that BTA-93 is a typical α-nucleating agent for iPP, which can be further proven by X-ray diffraction patterns of iPP nucleated with BTA-93 (Fig. 4).
Figure 4 is the X-ray diffraction patterns of neat iPP and iPP nucleated with BTA-93. The main diffraction peaks appear at 2θ = 14.0°, 16.8°, 18.5°, 20.9°, and 21.4°, respectively. In these diffraction peaks, 2θ at 14.0°, 16.8°, and 18.5° correspond to strong diffraction peaks of α-iPP at (110), (040), and (130) planes respectively, while 20.9° and 21.4° correspond to normal diffraction peaks of α-iPP at (111) and (131) planes [39, 40]. At the same time, the characteristic diffraction peak of β-iPP at 2θ = 15.8° does not appear. These results show that BTA-93 only induces iPP to generate α-spherulites and it is a typical α nucleating agent for iPP.
Crystal structure of BTA-93
Suitable single crystals were selected and their structures were determined by single crystal X-ray diffraction analysis. Crystal data and structure refinement are given in Table 1, and selected bonds and angles are listed in Table 2. Hydrogen bonds are listed in Table 3. Molecular structure of BTA-93 is shown in Scheme 1 and Fig. 5.
The crystallographic structure of BTA-93 was investigated. The crystals data revealed that its structure consists of monoclinic lattice, and the space group is P21/n. Comparison between the predicted X-ray powder diffraction pattern and the experimental pattern for the refined structure is shown in Fig. 6. The simulated XRD pattern and the experimental XRD patterns almost have the same diffraction peaks. The R1 and wR2 of the Rietveld refinement were 7.17 and 2.19%, respectively, which indicated the reliability of the refinement.
The N—H…O hydrogen bonds connect two molecules together to form a supramolecular structure. Meanwhile, the benzene rings of BTA-93 are parallel with each other, which were arranged in 3D supramolecular by π-π interactions, as shown in Fig. 7. All the hydrogen bonds and π-π stacking interactions have an effect on the stabilization of 3D supramolecular. But the aggregation behavior of molecules might be dictated by π-π interactions rather than directional hydrogen bonds. Each BTA-93 molecule has four benzene rings, and each benzene ring can interact with the other. So, the 3D supramolecular of BTA-93 was similar to a sheet structure, not a columnar structure in which the feature was helicity.
The crystal data reveal that BTA-93 had a monoclinic with parameters a = 0.5015(3) nm, b = 2.6937(19) nm, and c = 1.5801 nm while α-iPP also had a monoclinic with parameters a = 0.666 nm, b = 2.078 nm, c = 0.6459 nm [34, 35, 41]. It is obvious that the b-direction of BTA-93 was about four times of 0.655 nm which was the cell edge of (010)iPP plane, indicating good lattice matching exists between BTA-93 and iPP. The schematic diagram is shown in Fig. 8. Epitaxial crystallization could occur due to such good lattice matching between BTA-93 and α-iPP, and crystals of BTA-93 could stabilize helix of α-iPP crystals during crystallization, leading to higher crystallization rate. Epitaxial crystallization, as Willmann et al. believe, was the growth of one phase on the surface of a crystal of another phase in one or more strictly defined dimension in terms of purely geometric lattice matching, and 10–15% disregistry between the matching lattice spacing of host and guest crystal was considered as an upper limit [32, 33]. The epitaxial crystallization was based on one- or two-dimensional lattice matching, so the lattice matching between iPP and nucleating agent was a major factor. According to existing researches, the lattice mismatch between iPP and a lot of highly effective commercial nucleating agents was very low, which may be the reason of excellent nucleation effects of these nucleating agents in iPP. The lattice mismatch between the crystal of benzoic acid and the iPP was 2.18% and that between a highly effective aromatic heterocyclic phosphate salt nucleating agent NA03 and (010)iPP plane was 2.89% [34]. In our study, the lattice mismatch between the crystals of BTA-93 and α-iPP was only 2.81%, which was far lower than the upper limit for epitaxial crystallization. Therefore, in these features, the increased nucleation rate of iPP, as shown by an increase in peak crystallization temperature of iPP after addition of BTA-93, can be easily understood.
Conclusions
In this work, the crystallization and melting behaviors of iPP nucleated with 1,3,5-benzenetricarboxylic acid tris(phenylamide) (BTA-93) was investigated. The crystallization peak temperature of iPP was increased from 119.3 °C of neat iPP to 125 °C when the addition amount of nucleating agent BTA-93 in iPP was very low (i.e., 0.07 wt%), and the nucleation efficiency of BTA-93 at this lower amount was comparable to that of highly effective conventional nucleating agent HPN-68 at addition amount of 0.2 wt%. In addition, the crystal structure of BTA-93 was obtained by single crystal X-ray diffraction. The results implied that the molecules of BTA-93 were bonded by hydrogen bonds and π-π stacking. From the geometric analysis, the b cell dimension of BTA-93 crystals was about four times of the cell edge of (010)iPP plane, indicating that good lattice matching could be performed between BTA-93 and α-iPP crystals. The lattice mismatch degree between the crystals of BTA-93 and α-iPP was only 2.81%. The excellent nucleation efficiency of BTA-93 at very low addition amount can be attributed to such crystal lattice matching.
References
Zhang YF, Chen H (2014) Effects of nucleating agent 1,3,5-benzenetricarboxylic acid tris (cyclohexylamide) on properties and crystallization behaviors of isotactic polypropylene. Colloid Polym Sci 292:493–498
Zhang N, Zhang Q, Wang K, Deng H, Fu Q (2012) Combined effect of β-nucleating agent and multi-walled carbon nanotubes on polymorphic composition and morphology of isotactic polypropylene. J Therm Ana Calorim 107:733–743
Housmans JW, Gahleitner M, Peters GWM, Meijer HEH (2009) Structure–property relations in molded, nucleated isotactic polypropylene. Polymer 50:2304–2319
Zhang YF, Luo XZ, Yang XJ, Chang Y (2012) Effects of α/β compound nucleating agents on mechanical properties and crystallization behaviors of isotactic polypropylene. J. Macromol. Sci. Part B: Phys. 51:2352–2360
Varga J (1992) Supermolecular structure of isotactic polypropylene—review. J Mater Sci 27:2557–2579
Zhao SC, Xin Z (2010) Nucleation characteristics of the α/β compounded nucleating agents and their influences on crystallization behavior and mechanical properties of isotactic polypropylene. J Polym Sci B Polym Phys 48(6):653–665
Lotz B, Wittmann JC, Lovinger AJ (1996) Structure and morphology of poly(propylenes): a molecular analysis. Polymer 37:4979–4992
Varga J, Karger-Kocsis J (1995) Interfacial morphologies in carbon fibre-reinforced polypropylene microcomposites. Polymer 36(25):4877–4881
Fanegas N, Gómez MA, Marco C, Jiménez I, Ellis G (2007) Influence of a nucleating agent on the crystallization behaviour of isotactic polypropylene and elastomer blends. Polymer 48:5324–5331
Lv ZP, Yang YF, Wu R, et al. (2012) Design and properties of a novel nucleating agent for isotactic polypropylene. Mater Des 37(5):73–78
Zhang YF, Chen H, Liu BB, Gu YH, Li XF (2014) Isothermal and non-isothermal crystallization of isotactic polypropylene nucleated with 1,3,5-benzenetricarboxylic acid tris (cyclohexylamide). Thermochim Acta 590:226–231
Libster D, Aserin A, Garti N (2007) Advanced nucleating agents for polypropylene. Polym Adv Technol 18:685–695
Meer DWVD, Milazzo D, Sanguineti A, Vancso GJ (2005) Oriented crystallization and mechanical properties of polypropylene nucleated on fibrillated polytetrafluoroethylene scaffolds. Polym Eng Sci 45(4):458–468
Gui Q, Xin Z, Zhu WP, Dai GC (2003) Effects of an organic phosphorus nucleating agent on crystallization behaviors and mechanical properties of poly(propylene). J Appl Polym Sci 88:297–301
Li C, Isshiki N, Saito H, Ogata K, Toyota A (2009) Nucleation effect of cyclodextrin inclusion compounds on the crystallization of polypropylene. J Polym Sci B Polym Phys 47:130–137
Li C, Isshiki N, Saito H, Ogata K, Toyota A (2010) Nucleation effect of inclusion complexes with different polyolefin as guest molecules on the crystallization of polypropylene. J Appl Polym Sci 115:1098–1104
Zhang YF (2008) Crystallization and melting behaviors of isotactic polypropylene nucleated with compound nucleating agents. J Polym Sci B Polym Phys 46(9):911–916
Menyhárd A, Varga J, Molnár G (2006) Comparison of different β-nucleators for isotactic polypropylene, characterization by DSC and temperature-modulated DSC (TMDSC) measurements. J Therm Anal Calorim 83:625–630
Zhang YF, Xin Z (2006) Effects of substituted aromatic heterocyclic phosphate salts on properties, crystallization and melting behaviors of isotactic polypropylene. J Appl Polym Sci 100:4868–4874
Zhang YF (2008) Isothermal crystallization behaviors of isotactic polypropylene nucleated with the third generation sorbitol derivative nucleating agents. J Macromol Sci Part B: Phys 47(5):891–899
Marco C, Ellis G, Gomez MA, Arribas JM (2002) Comparative study of the nucleation activity of third-generation sorbitol-based nucleating agents for isotactic polypropylene. J Appl Polym Sci 84:2440–2450
Marco C, Ellis G, Gomez MA, Arribas JM (2003) Analysis of the isothermal crystallization of isotactic polypropylene nucleated with sorbitol derivatives. J Appl Polym Sci 88:2261–2274
Shi YH, Dou Q (2013) Non-isothermal crystallization kinetics of β-nucleated isotactic polypropylene. J Therm Anal Calorim 112:901–911
Zhang YF, Xin Z (2006) Isothermal and nonisothermal crystallization kinetics of isotactic polypropylene nucleated with substituted aromatic heterocyclic phosphate salts. J Appl Polym Sci 101:3307–3316
Schmidt HW, Smith P, Blomenhofer M (2007) Polypropylene resin compositions. US Patent US7235191
Blomenhofer M, Ganzleben S, Hanft D, Schmidt HW, Kristiansen M, Smith P, Stoll K, Mader D, Hoffmann K (2005) “Designer” nucleating agent for polypropylene. Macromolecules 38(9):3688–3695
Kristiansen M, Gress A, Smith P, Hanft D, Schmidt HW (2006) Phase behavior, nucleation and optical properties of the binary system isotactic polypropylene/N,N′N″-tris-isopentyl-1,3,5-benzene-tricarboxamide. Polymer 47:249–253
Lightfoot MP, Mair FS, Pritchard RG, Warren JE (1999) New supramolecular packing motifs: π-stacked rods encased in triply-helical hydrogen bonded amide strands. Chem Commun 19:1945–1946
Jimenez CA, Belmar JB, Ortiz L, Hidalgo P, Fabelo O, Pasan J, Ruiz-Perez C (2009) Influence of the aliphatic wrapping in the crystal structure of benzene tricarboxamide supramolecular polymers. Cryst Growth Des 9:4987–4989
Kristiansen M, Smith P, Chanzy H, Baerlocher C, Gramlich V, McCusker L, Weber T, Pattison P, Blomenhofer M, Schmidt HW (2009) Structural aspects of 1,3,5-benzenetrisamides—a new family of nucleating agents. Cryst Growth Des 9:2556–2558
Abraham F, Ganzleben S, Hanft D, Smith P, Schmidt HW (2010) Synthesis and structure–efficiency relations of 1,3,5-benzenetrisamides as nucleating agents and clarifiers for isotactic poly(propylene). Macromol Chem Phys 211:171–181
Wittmann JC, Lotz B (1981) Epitaxial crystallization of polyethylene on organic substrates: a reappraisal of the mode of action of selected nucleating agents. J Polym Sci B Polym Phys 19:1837–1851
Wittmann JC, Lotz B (1990) Epitaxial crystallization of polymers on organic and polymeric substrates. Prog Polym Sci 15:909–948
Shi YQ, Xin Z (2012) The correlation between crystal structure and nucleation efficiency of a lithium (I) complex on isotactic polypropylene. J Appl Polym Sci 125:2963–2969
Yoshimoto S, Ueda T, Yamanaka K, Kawaguchia A, Tobitab E, Haruna T (2001) Epitaxial act of 2,2′-methylene-bis (4,6-di-tert-butylphenyl) phosphate on isotactic polypropylene. Polymer 42(23):9627–9631
Fillon B, Wittmann JC, Lotz B, Thierry A (1993) Self-nucleation and recrystallization of isotactic polypropylene (α phase) investigated by differential scanning calorimetry. J Polym Sci B Polym Phys 31:1383–1393
Fillon B, Lotz B, Thierry A, Wittmann JC (1993) Self-nucleation and enhanced nucleation of polymers. Definition of a convenient calorimetric “efficiency scale” and evaluation of nucleating additives in isotactic polypropylene (α phase). J Polym Sci B Polym Phys 31:1395–1405
Fillon B, Thierry A, Wittmann JC, Lotz B (1993) Self-nucleation and recrystallization of polymers. Isotactic polypropylene, β phase: β-α conversion and β-α growth transitions. J Polym Sci B Polym Phys 31:1407–1424
Turner-Jones A, Aizlewood JM, Beckett DR (1964) Crystalline forms of isotactic polypropylene. Makromol Chem 75:134–158
Wang JB, Dou Q, Chen XY, Li D (2008) Crystal structure and morphologies of polypropylene homopolymer and propylene-ethylene random copolymer: effect of the substituted 1,3,5-benzenetrisamides. J Polym Sci B Polym Phys 46(11):1067–1078
Awaya H (1988) Morphology of different types of isotactic polypropylene spherulites crystallized from the melt. Polymer 26:591–596
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (Grant No. 21376031) and Scientific Research Fund of Hunan Provincial Education Department (Grant No. 16A004).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhang, YF., Zhou, PZ., Guo, LH. et al. The relationship between crystal structure and nucleation effect of 1,3,5-benzenetricarboxylic acid tris(phenylamide) in isotactic polypropylene. Colloid Polym Sci 295, 619–626 (2017). https://doi.org/10.1007/s00396-017-4030-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-017-4030-z