Abstract
Purpose
The present study investigated whether dietary methionine supplementation might protect against intrauterine growth retardation (IUGR)-induced damage in the intestine of piglets.
Methods
Thirty normal birth weight (NBW) female piglets and sixty same-sex IUGR piglets were weaned at 21 days of postnatal age and fed the control diet (4.0 g methionine per kg of feed, NBW-CON, and IUGR-CON groups) or the methionine-supplemented diet (5.2 g methionine per kg of feed, IUGR-MET group) for 28 days (n = 6).
Results
Piglets in the IUGR-CON group showed decreased average daily feed intake and average daily gain and an increased feed conversion ratio than those in the NBW-CON group. Compared with NBW-CON piglets, IUGR-CON piglets had decreased villus height (VH) and villus height-to-crypt depth ratio in both the jejunum and ileum. In addition, in comparison with the NBW-CON piglets, IUGR increased the concentration of malondialdehyde (MDA) and the index of apoptosis, while it decreased the concentrations of methionine and reduced glutathione (GSH), the ratio of reduced glutathione/oxidized glutathione (GSH/GSSG), and the protein expression of occludin (OCLN) in both the jejunum and ileum. Dietary methionine supplementation decreased the MDA and protein carbonyl concentrations and the apoptotic index, while it increased the VH level, methionine and GSH concentrations, GSH/GSSG ratio, and the OCLN protein expression in the jejunum of IUGR-MET piglets.
Conclusions
Methionine may have beneficial effects in improving intestinal integrity and oxidative status in IUGR weanling piglets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Uteroplacental insufficiency, the major cause of intrauterine growth retardation (IUGR), limits the availability of critical substrates such as amino acids, glucose, and hormones to the fetus during the gestation period [1, 2]. When a fetus is exposed to a limited nutrient supply, the growth of the brain may be protected at the expense of other organs [3], such as the small intestine. However, the small intestine is an important organ responsible for the digestion, absorption, and metabolism of nutrients, including amino acids [4]. Accumulating evidence shows that IUGR is associated with abnormal intestinal mucosal growth, as indicated by decreased villus height (VH), villus surface area (VSA), and the ratio of villus height to crypt depth (VH/CD) [5, 6]. A previous study found that IUGR offspring exhibited increased levels of heat-shock proteins and glutathione S-transferase omega in the jejunum, which provide a line of evidence for the presence of oxidative stress during postnatal life [7]. In addition, several studies with rodents showed that oxidative stress may contribute to increased intestinal paracellular permeability by impairing intestinal integrity [8, 9]. Therefore, increasing the intestinal antioxidant capacity of IUGR animals is a potential approach to alleviate intestinal damage.
Methionine is a sulfur amino acid (SAA) with numerous biological functions, including protein metabolism, methylation, the synthesis of cysteine, and reduced glutathione (GSH), and as a component of antioxidant systems [10]. A recent report pointed out that a dietarily adequate methionine level improved intestinal integrity in weanling piglets [11]. The previous studies have verified that IUGR piglets have decreased feed intake compared with their heavier littermates [12, 13], and therefore, SAA is provided at insufficient levels. It has been reported that SAA deficiency could impair the growth and development of the small intestine [14]. SAA deficiency also significantly increased intestinal oxidative stress in piglets, as indicated by diminished cellular cysteine and GSH concentrations [15]. Therefore, increasing the methionine level in IUGR infants’ diet may help to maintain normal growth and basic function in their intestines.
However, to our knowledge, data are lacking about the effects of dietary methionine supplementation in the intestine of IUGR infants. Thus, the current study was conducted to investigate the effects of dietary methionine supplementation on intestinal integrity and oxidative status in IUGR weanling piglets. In swine production, piglets usually weaned at 21 days [13, 16, 17]. Weaning is a critical process for piglets due to dramatic changes in diets and environment [18]. IUGR piglets have more severe weaning stress because of decreased feed intake compared with their heavier littermates [13]. Therefore, study on the potential role of methionine supplementation in regulating intestinal homeostasis in the IUGR weanling piglets may be helpful in solving the complications. Considering the biological similarity between humans and pigs [19], this study may provide some guidance to guarantee the appropriate development of IUGR offspring during the early periods after weaning.
Materials and methods
Experimental design, diets, and management
All experiments were approved by the Institutional Animal Care and Use Committee of Nanjing Agricultural University (NJAU-CAST-2015-098). Approximately 90 healthy pregnant sows with similar expected dates of confinement and parity (second or third) were chosen during pregnancy. In each litter, one newborn normal birth weight (NBW) (~ 1.52 kg) and two IUGR (~ 0.87 kg) female piglets were carefully selected based on guidelines laid out in the previous studies [20, 21]. At weaning (21 days of postnatal age), 30 pairs of NBW (6.55 ± 0.14 kg) and IUGR (4.85 ± 0.10 kg) piglets were allocated to receive a control diet (4.0 g methionine per kg of feed, NBW-CON, and IUGR-CON groups), and the remaining IUGR (4.83 ± 0.08 kg) piglets were allocated to receive a methionine-supplemented diet (5.2 g methionine per kg of feed, IUGR-MET group) for 28 days. Thereafter, all piglets were divided into three treatments × six replicates (pens) × five piglets per replicate. The inclusion of methionine in the control diet was determined according to the recommendation of National Research Council (2012) [22], while the inclusion of methionine in the diet for IUGR-MET piglets was confirmed according to an independent study by colleagues. A total of 200 21-day-old NBW female piglets were randomly distributed into five treatments, and each treatment consisted of five replicates with eight piglets per replicate. Piglets were fed a control diet (4.0 g methionine per kg of feed) supplemented with 0, 0.6, 1.2, 1.8, and 2.4 g methionine per kg diet, respectively. In the preliminary study, the optimum effects of methionine on growth performance and plasma antioxidant capacity of piglets were observed when its inclusion was 5.2 g/kg of the diet (unpublished). The dietary supplementation of methionine was achieved by supplementing l-methionine (99%; CJ CheilJedang, Seoul, Korea) into the piglets’ feed, replacing the equivalent weight of l-alanine; the concentrations of other nutrients were maintained at a constant level in all experimental diets. The composition and nutrient levels of the diets are shown in Supplementary Table 1. The average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR) of piglets were recorded carefully.
Sample collection
At 49 days of postnatal age, the piglet whose weight was the nearest to the average weight of each pen was selected (one pig per pen). The piglets were sacrificed by intramuscular injection of sodium pentobarbital (50 mg/kg body weight) at 8 h after the last meal. The entire small intestine starting from the pyloric sphincter to the ileocecal valve was removed from the abdominal cavity and divided into three segments, including the duodenum, jejunum, and ileum. The jejunal and ileal segments were immediately flushed with ice-cold physiological saline to remove luminal contents, and the mesenteric attachments were carefully removed. Sections of approximately 1 cm in length were carefully collected from the mid of each segment, and fixed in 4% (w/v) paraformaldehyde in 100 mmol/L phosphate-buffered saline (PBS), pH 7.4 for 24 h for histological analyses. Jejunal and ileal mucosae were scraped from the rest of the tissue using a glass microscope slide. The intestinal mucosae were then rapidly frozen in liquid nitrogen and stored at −80 °C for further analysis.
Determination of intestinal amino acids
Approximately 150 mg of jejunal and ileal samples were weighed and detected using a Hitachi L-8900 amino acid analyzer (Hitachi, Tokyo, Japan) as described in a previous study [23]. The amount of each amino acid in the samples was calculated with reference to their corresponding standard solution. Intestinal amino acid concentrations included both protein-bound and free amino acids and were expressed as g/100 g wet weight.
Intestinal morphology analysis
The jejunal and ileal samples fixed in 4% paraformaldehyde were dried using a graded series of xylene and ethanol, and embedded in paraffin. The samples (5 μm) were then deparaffinized using xylene and rehydrated with graded dilutions of ethanol. The slides were stained with hematoxylin and eosin. Eight slides for each tissue were prepared, and the images were acquired using an optical binocular microscope with a digital camera (Nikon ECLIPSE 80i, Tokyo, Japan). The morphological measurements of the VH, villus width (VW), crypt depth (CD), and VH/CD ratio were calculated using the Image-Pro Plus software, and the VSA was calculated using the previously reported equations [24]:
Measurement of intestinal enzyme activities
The activities of the sucrase, maltase, lactase, superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and glutathione reductase (GR) and the concentrations of malondialdehyde (MDA), protein carbonyls, GSH, and oxidized glutathione (GSSG) were determined using the commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China). All results were normalized to total protein concentration in each sample for inter-sample comparison. The protein concentrations were quantified using the bicinchoninic acid protein assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China).
TUNEL staining
Apoptosis was evaluated using a terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) assay with the TUNEL BrightGreen Apoptosis Detection Kit (Vazyme Biotech, Nanjing, Jiangsu, China). In brief, the jejunal and ileal specimens were de-waxed and incubated with Proteinase K (20 μg/ml) for 20 min at room temperature. The specimens were then treated with the terminal deoxynucleotidyl transferase (TdT) buffer including BrightGreen Labeling Mix and recombinant TdT enzyme for 60 min at 37 °C followed by a thorough washing with PBS to stop the reaction. Finally, the specimens were stained with 4,6-diamidino-2-phenylindole solution (Beyotime Institute of Biotechnology, Nantong, Jiangsu, China) for 5 min to detect cell nuclei. The number of positive cells was counted using an LSM 700 confocal laser scanning microscope (Carl Zeiss, Oberkochen, Germany). The apoptotic index was defined as the ratio of apoptotic cells to total cells.
Total RNA extraction and mRNA quantification
Total RNA was extracted from frozen intestinal mucosae using the TRIzol Reagent (Invitrogen Life Technologies, Gaithersburg, MD, USA). After the determination of RNA concentration, mRNA was reverse-transcribed into complementary DNA (cDNA) using a reverse transcription kit (TaKaRa Biotechnology, Dalian, Liaoning, China). Real-time PCR was performed using the ABI StepOnePlus™ Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). The reaction mixture was made by adding 0.4 µL of each of forward and reverse primers, 0.4 µL of ROX Reference Dye (TaKaRa Biotechnology, Dalian, Liaoning, China), 10 µL of SYBR Premix Ex Taq™ (TaKaRa Biotechnology, Dalian, Liaoning, China), 6.8 µL of double-distilled H2O, and 2 µL of cDNA template. Each sample was assayed in triplicate. The reaction condition was as follows: 30 s at 95 °C, 40 cycles of 5 s at 95 °C, and 30 s at 60 °C. The relative mRNA expression levels were calculated by the 2−ΔΔCt method after normalization against the reference gene β-actin [25]. The values of NBW-CON group were used as a calibrator. The primer sequences for superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1), occludin (OCLN), claudin-2 (CLDN-2), zonula occludens-1 (ZO-1), B-cell lymphoma/leukaemia 2 (Bcl-2), Bcl-2-associated X protein (Bax), and β-actin are shown in Supplementary Table 2.
Western blot
Proteins were extracted from approximately 40 mg of frozen intestinal mucosae by grinding with RIPA lysis buffer (Beyotime Institute of Biotechnology, Nantong, Jiangsu, China) and phenylmethanesulfonyl fluoride (Beyotime Institute of Biotechnology, Nantong, Jiangsu, China). The protein concentrations were measured using a bicinchoninic acid protein assay kit (Beyotime Institute of Biotechnology, Nantong, Jiangsu, China). Equal amounts of protein (40 μg/lane) were electrophoresed in sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred on to polyvinylidene difluoride membranes. After blocking with Tris-buffered saline Tween-20 buffer (TBST; 0.1% Tween-20, 100 mmol/L Tris–HCl, and 150 mmol/L NaCl, pH 8.0) containing 5% non-fat dry milk for 90 min at room temperature, the membranes were washed in TBST three times and incubated overnight with the primary antibodies. The primary antibodies were OCLN (1:1000; Novus Biologicals, Littleton, CO, USA) and β-actin (1:1000; Cell Signaling Technology, Danvers, MA, USA). The membranes were washed in TBST three times and processed with a secondary antibody (horseradish peroxidase-conjugated anti-rabbit IgG, 1:2000; Cell Signaling Technology, Danvers, MA, USA) for 60 min at room temperature. The blots were developed using an enhanced chemiluminescence reagents (Beyotime Institute of Biotechnology, Nantong, Jiangsu, China) followed by autoradiography. Images were recorded with a Luminescent Image Analyzer LAS-4000 system (Fujifilm, Tokyo, Japan) and quantified by Gel-Pro Analyzer 4.0 software (Media Cybernetics, Silver Spring, MD, USA).
Statistical analysis
Data were analyzed using SPSS 16.0 statistical software (SPSS, Chicago, IL, USA). Statistical differences between different groups were determined via one-way analysis of variance and Tukey’s post hoc test for multiple comparisons. P values less than 0.05 were considered statistically significant. Results are expressed as mean ± SE.
Results
Growth performance
IUGR decreased (P < 0.05) the ADFI and ADG and increased the FCR of piglets in the IUGR-CON group when compared with the NBW-CON group (Table 1). There were no significant differences (P > 0.05) in the ADFI, ADG, and FCR of piglets in the IUGR-MET group when compared with the IUGR-CON group.
Intestinal concentrations of amino acids
In comparison with NBW-CON piglets, IUGR decreased (P < 0.05) the concentrations of methionine, cysteine, and valine in the jejunum of IUGR-CON piglets (Table 2). In addition, IUGR-CON piglets showed a decrease (P < 0.05) in the concentration of methionine in the ileum compared with NBW-CON piglets. Dietary methionine supplementation increased (P < 0.05) the concentration of methionine in the jejunum of IUGR-MET piglets. There were no differences in other parameters among the groups (P > 0.05).
Intestinal mucosal morphology
IUGR induced obvious decreases (P < 0.05) in VH and VH/CD ratio in both the jejunum and ileum of IUGR-CON piglets compared with the NBW-CON data (Table 3). Compared with NBW-CON piglets, IUGR-CON piglets had decreased (P < 0.05) VSA in the jejunum. An increased (P < 0.05) VH in the jejunum was observed in IUGR-MET piglets as compared with their IUGR-CON counterparts. There were no differences in other parameters among the groups (P > 0.05).
Disaccharidases activities
IUGR significantly decreased (P < 0.05) maltase activity in the jejunum of the IUGR-CON group in comparison with the NBW-CON group (Table 4). In addition, a remarkable decrease (P < 0.05) in sucrase activity was observed in the ileum of IUGR-CON piglets. The IUGR-MET group had increased maltase activity (P < 0.05) in the jejunum when compared with the IUGR-CON data. There were no differences in other parameters among the groups (P > 0.05).
Intestinal oxidative status
The IUGR-CON piglets had significantly increased (P < 0.05) MDA and protein carbonyl concentrations and decreased (P < 0.05) GSH and GSSG concentrations in the jejunum in comparison with the NBW-CON piglets (Table 5). In addition, IUGR significantly increased (P < 0.05) the concentration of MDA, whereas it decreased (P < 0.05) the GSH and GSSG concentrations in the ileum of IUGR-CON piglets when compared with the NBW-CON piglets. In contrast, decreased (P < 0.05) MDA and protein carbonyl concentrations and an increased (P < 0.05) GSH concentration and GSH/GSSG ratio were observed in the jejunum of IUGR-MET piglets in comparison with the IUGR-CON piglets. Furthermore, methionine treatment alleviated (P < 0.05) the increased MDA concentration and decreased GSH/GSSG ratio in the ileum of IUGR piglets in comparison with the piglets who received a control diet. There were no differences in other parameters among the groups (P > 0.05).
Apoptotic index
Piglets in the IUGR-CON group showed a greater (P < 0.05) apoptotic percentage in both the jejunum and ileum than the NBW-CON group (Fig. 1). In contrast, piglets treated with methionine had an increased (P < 0.05) apoptotic percentage in both the jejunum and ileum than the IUGR-CON piglets.
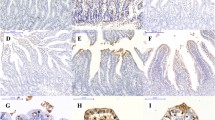
Effects of dietary l-methionine supplementation on apoptotic indices in the jejunum (a) and ileum (b) of intrauterine growth-retarded weanling piglets. NBW-CON normal birth weight group given a control diet, IUGR-CON intrauterine growth retardation group given a control diet, IUGR-MET intrauterine growth retardation group given a methionine-supplemented diet. Data are presented as mean ± SE, n = 6/group. Mean values in columns with unlike superscript letters were significantly different (P < 0.05)
Messenger RNA expressions
IUGR down-regulated (P < 0.05) the mRNA expression levels of OCLN and Bcl-2 in the jejunum of the IUGR-CON group in comparison with the NBW-CON group (Table 6). In addition, administering methionine up-regulated (P < 0.05) the mRNA abundances of OCLN and Bcl-2 in the jejunum of the IUGR-MET group when compared with the IUGR-CON group. However, there were no differences in the mRNA expression levels of SOD1, GPx1, CLDN-2, ZO-1, and Bax among the groups (P > 0.05).
Protein expression
The protein expression of OCLN was decreased (P < 0.05) in the jejunum of the IUGR-CON piglets compared to the NBW-CON piglets (Fig. 2). Dietary methionine supplementation increased (P < 0.05) the protein expression of OCLN in the jejunum of the IUGR-MET piglets. However, there was no difference in the protein expression of OCLN in the ileum among the groups (P > 0.05).
Effects of dietary l-methionine supplementation on OCLN protein contents in the jejunum (a) and ileum (b) of intrauterine growth-retarded weanling piglets. NBW-CON normal birth weight group given a control diet, IUGR-CON intrauterine growth retardation group given a control diet, IUGR-MET intrauterine growth retardation group given a methionine-supplemented diet. Data are presented as mean ± SE, n = 6/group. Mean values in columns with unlike superscript letters were significantly different (P < 0.05)
Discussion
It has been reported that insufficient intake of SAA is detrimental in both humans and animals [15, 26]. Methionine is an essential amino acid not only for whole body (protein biosynthesis, redox balance, and transsulfuration and transmethylation reactions) but also for enterocyte metabolism (GSH and cysteine synthesis) [27, 28]. Its importance as a key amino acid is reflected by the fact that it is usually absorbed from the diet with highest fractional absorption rates among all proteinogenic amino acid [28]. Therefore, dietary methionine supply is important for humans and animals to provide health benefits. A considerable amount of evidence has shown that IUGR delays postnatal growth in infants and is associated with the impaired integrity and antioxidant capacity in the intestine [7, 29, 30]. The present study corroborated these findings and found that methionine treatment increased the GSH content and GSH/GSSG ratio and concomitantly decreased the apoptotic percentage, increased the VH, and up-regulated the protein expression of OCLN in the jejunum of IUGR piglets. These observations might further strengthen the potential effect of methionine to protect against the intestinal damage of weanling piglets induced by IUGR and also provide an applicable rationale for properly targeted methionine supply in IUGR humans.
Intestinal integrity is a key factor that reflects gut health in humans and animals [31]. The present study found that IUGR impaired intestinal morphology in weanling piglets, as indicated by the decreased VH, VSA, and VH/CD ratio. The structural damage of the intestinal villus indicates a decreased ability to absorb nutrients and a fragile mechanical barrier in IUGR piglets [24]. Moreover, the VH/CD ratio is widely considered to be a good indicator of mucosal turnover [32]. In keeping with our findings, Wang et al. [6] found that IUGR offspring had a decreased VH and VH/CD ratio. Abnormal expressions of tight junctions obviously affect paracellular permeability and lead to many pathological states [33]. In the present study, IUGR decreased OCLN at both transcription and translation levels in the jejunum. Wang et al. [30] also found that decreased OCLN expression was correlated with increased paracellular permeability in IUGR piglets. The results obtained in this study may provide an explanation for impaired intestinal integrity in IUGR piglets.
Disaccharidases enzymes have crucial roles in facilitating mucosal maturation and gut digestive function [34]. An in vivo trial showed that IUGR decreased the activities of lactase and maltase in rabbits [35]. Likewise, D’Inca et al. [36] found that preterm IUGR piglets had significantly lower sucrase and maltase activities at birth compared with their normal littermates. In the present study, IUGR obviously reduced the activity of maltase in the jejunum. Similarly, a decreased sucrase activity was also presented in the ileum of IUGR weanling piglets. Thus, the results obtained herein may provide another explanation for the impaired development of the small intestine in IUGR weanling piglets.
Until now, the exact mechanisms underlying the intestinal damage of IUGR piglets remained unknown. However, it is likely associated with the increased level of cell apoptosis induced by IUGR. Although apoptosis is essential for epithelial turnover and tissue homeostasis in the intestine, excessive apoptosis could result in intestinal barrier dysfunction [37]. In the present study, the increased apoptotic indices of both the jejunum and ileum induced by IUGR were observed using a TUNEL assay. Similarly, IUGR increased the apoptotic index with a concomitant decrease in mRNA expression levels of Bcl-2 in rats [38]. The present study showed that the mRNA expression of Bcl-2 was down-regulated by IUGR, which was consistent with the previous findings [13, 38]. Bcl-2 contributes significantly to apoptosis regulation and tissue homeostasis [39]. Hence, this result also indicates that IUGR might affect intestinal development through increasing cell apoptosis in weanling piglets.
Oxidative stress can induce apoptosis and affect cellular homeostasis. As previously mentioned, oxidative stress is a pivotal factor contributing to the intestinal damage of IUGR offspring [7]. In this work, IUGR was found to increase the concentrations of MDA and protein carbonyl in the jejunum, which reflects the degree of lipid peroxidation or protein oxidation, respectively. Likewise, Zhang et al. [40] reported that IUGR increased plasma MDA and protein carbonyl concentrations in piglets. As a scavenger of reactive oxygen species (ROS), GSH plays an important role in antioxidant defense [41]. Oxidative stress shifts the GSH/GSSG ratio towards lower GSH content and higher GSSG content [42]. A previous study observed decreased GSH content in IUGR piglets compared with their heavier counterparts [43]. In this study, significant differences were found for GSH content and the GSH/GSSG ratio in both the jejunum and ileum between NBW-CON and IUGR-CON piglets. These investigations illustrate that IUGR infants have an impaired antioxidant capacity and reveal widespread oxidative damages.
It has been demonstrated that SAA deficiency decreases the total GSH content in the proximal jejunum of piglets [14]. The present study observed decreased methionine and cysteine concentrations in the jejunum of IUGR piglets, which is consistent with the previous results [44, 45]. Cysteine is required for the synthesis of GSH and taurine, which are essential compounds for host defense against oxidative stress [27]. However, in a previous study, cysteine administration did not accelerate the GSH synthesis rate and its concentration in preterm infants in the early life [46], possibly because cysteine undergoes rapid spontaneous oxidation at neutral pH to form cystine and hydrogen peroxide, and the production of large amounts of hydrogen peroxide at certain extracellular and intracellular sites has negative consequences [47]. Thus, there seems to be a particular need for the nutritional and functional role of methionine in the intestinal development of IUGR infants. In the current study, dietary methionine supplementation increased the methionine concentration with a concomitant increase of GSH content and the GSH/GSSG ratio in the jejunum of IUGR piglets. Likewise, higher GSH and lower GSSG concentrations were also observed in the duodenum and jejunum of methionine-supplemented piglets [11]. The underlying mechanisms may involve at least two possible processes. First, a variety of ROS react readily with methionine residues in proteins to generate methionine sulfoxide [48], and, therefore, decrease the consumption of GSH. Second, methionine provides sulfur for cysteine synthesis and may increase the efficiency of the GSH redox cycle [49]. Moreover, IUGR piglets given a methionine-supplemented diet had decreased concentrations of MDA and protein carbonyl in the jejunum. The decreased percentage of apoptosis and the increased Bcl-2 mRNA expression were also observed in the jejunum of methionine-supplemented piglets. The enhanced efficiency of methionine metabolism, and, consequently, an improved GSH redox cycle in the intestine, may explain the attenuated oxidative stress and cell apoptosis in methionine-supplemented piglets [41].
In this study, methionine intervention improved VH in the jejunum of IUGR piglets. Similarly, a higher VH in the jejunum and a lower CD in the duodenum were also noted in methionine-supplemented weanling piglets [11]. A previous report demonstrated that SAA deficiency suppressed intestinal mucosal growth, correlated with villus atrophy, and reduced epithelial cell proliferation in piglets [15]. The effectiveness of methionine as an antioxidant and on the GSH redox cycle may explain the improved villus development in the intestine [15, 50]. Here, the up-regulated expression of OCLN in the jejunum induced by methionine was also observed at both mRNA and protein levels, which was consistent with a previous finding [11]. Hou et al. [51] revealed that the dietary supplementation of N-acetylcysteine, a precursor of cysteine, could increase the OCLN protein expression in the ileum of piglets challenged with lipopolysaccharide, which indirectly supports the present results. Therefore, dietary methionine supplementation may contribute to the epithelial cell metabolism and gut function through the regulation of cysteine synthesis. In the current study, piglets in the IUGR-MET group showed an increased activity of maltase in the jejunum. Fang et al. [52] reported that SAA are critical for the gut to maintain its functions including the digestion, absorption, and metabolism of nutrients. Thus, increasing the methionine level in their diet may be favorable to maintain the gut health of IUGR piglets.
In conclusion, methionine treatment has therapeutic potential for improving intestinal integrity and oxidative status in IUGR weanling piglets. The results of this study demonstrate the nutritional and functional importance of intestinal methionine metabolism, and may be helpful in the development of new nutritional strategies for IUGR offspring to attenuate intestinal damage during the early life.
References
Xita N, Tsatsoulis A (2010) Fetal origins of the metabolic syndrome. Ann N Y Acad Sci 1205:148–155. doi:10.1111/j.1749-6632.2010.05658.x
Ogata ES, Bussey ME, Finley S (1986) Altered gas exchange, limited glucose and branched chain amino acids, and hypoinsulinism retard fetal growth in the rat. Metabolism 35:970–977
Hales CN, Barker DJP (1992) Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 35:595–601
Wu G (1998) Intestinal mucosal amino acid catabolism. J Nutr 128:1249–1252
Dong L, Zhong X, Ahmad H, Li W, Wang Y, Zhang L, Wang T (2014) Intrauterine growth restriction impairs small intestinal mucosal immunity in neonatal piglets. J Histochem Cytochem 62:510–518. doi:10.1369/0022155414532655
Wang Y, Zhang L, Zhou G, Liao Z, Ahmad H, Liu W, Wang T (2012) Dietary l-arginine supplementation improves the intestinal development through increasing mucosal Akt and mammalian target of rapamycin signals in intra-uterine growth retarded piglets. Br J Nutr 108:1371–1381. doi:10.1017/S0007114511006763
Wang X, Wu W, Lin G, Li D, Wu G, Wang J (2010) Temporal proteomic analysis reveals continuous impairment of intestinal development in neonatal piglets with intrauterine growth restriction. J Proteome Res 9:924–935. doi:10.1021/pr900747d
Maeda T, Miyazono Y, Ito K, Hamada K, Sekine S, Horie T (2010) Oxidative stress and enhanced paracellular permeability in the small intestine of methotrexate-treated rats. Cancer Chemother Pharmacol 65:1117–1123. doi:10.1007/s00280-009-1119-1
Sheth P, Basuroy S, Li C, Naren AP, Rao RK (2003) Role of phosphatidylinositol 3-kinase in oxidative stress-induced disruption of tight junctions. J Biol Chem 278:49239–49245. doi:10.1074/jbc.M305654200
Tesseraud S, Métayer Coustard S, Collin A, Seiliez I (2009) Role of sulfur amino acids in controlling nutrient metabolism and cell functions: implications for nutrition. Br J Nutr 101:1132–1139. doi:10.1017/S0007114508159025
Chen Y, Li D, Dai Z, Piao X, Wu Z, Wang B, Zhu Y, Zeng Z (2014) l-Methionine supplementation maintains the integrity and barrier function of the small-intestinal mucosa in post-weaning piglets. Amino Acids 46:1131–1142. doi:10.1007/s00726-014-1675-5
Xu W, Bai K, He J, Su W, Dong L, Zhang L, Wang T (2016) Leucine improves growth performance of intrauterine growth retardation piglets by modifying gene and protein expression related to protein synthesis. Nutrition 32:114–121. doi:10.1016/j.nut.2015.07.003
Zhang H, Chen Y, Li Y, Yang L, Wang J, Wang T (2014) Medium-chain TAG attenuate hepatic oxidative damage in intra-uterine growth-retarded weanling piglets by improving the metabolic efficiency of the glutathione redox cycle. Br J Nutr 112:876–885. doi:10.1017/S000711451400155X
Conde-Aguilera JA, Le Floc’h N, Le Huërou-Luron I, Mercier Y, Tesseraud S, Lefaucheur L, van Milgen J (2016) Splanchnic tissues respond differently when piglets are offered a diet 30% deficient in total sulfur amino acid for 10 days. Eur J Nutr 55:2209–2219. doi:10.1007/s00394-015-1031-x
Bauchart-Thevret C, Stoll B, Chacko S, Burrin DG (2009) Sulfur amino acid deficiency upregulates intestinal methionine cycle activity and suppresses epithelial growth in neonatal pigs. Am J Physiol Endocrinol Metab 296:E1239–E1250. doi:10.1152/ajpendo.91021.2008
Deng QH, Jia G, Zhao H, Chen ZL, Chen XL, Liu GM, Wang KN (2016) The prolonged effect of glucagon-like peptide 2 pretreatment on growth performance and intestinal development of weaned piglets. J Anim Sci Biotechnol 7:28. doi:10.1186/s40104-016-0087-7
Tang Z, Yin Y, Zhang Y, Huang R, Sun Z, Li T, Chu W, Kong X, Li L, Geng M, Tu Q (2009) Effects of dietary supplementation with an expressed fusion peptide bovine lactoferricin–lactoferrampin on performance, immune function and intestinal mucosal morphology in piglets weaned at age 21 d. Br J Nutr 101:998–1005. doi:10.1017/S0007114508055633
Zhu L, Cai X, Guo Q, Chen X, Zhu S, Xu J (2013) Effect of N-acetyl cysteine on enterocyte apoptosis and intracellular signalling pathways’ response to oxidative stress in weaned piglets. Br J Nutr 110:1938–1947. doi:10.1017/S0007114513001608
Ferenc K, Pietrzak P, Godlewski MM, Piwowarski J, Kiliańczyk R, Guilloteau P, Zabielski R (2014) Intrauterine growth retarded piglet as a model for humans—studies on the perinatal development of the gut structure and function. Reprod Biol 14:51–60. doi:10.1016/j.repbio.2014.01.005
Wang T, Huo YJ, Shi F, Xu RJ, Hutz RJ (2005) Effects of intrauterine growth retardation on development of the gastrointestinal tract in neonatal pigs. Biol Neonate 88:66–72. doi:10.1159/000084645
D’Inca R, Kloareg M, Gras-Le Guen C, Le Huërou-Luron I (2010) Intrauterine growth restriction modifies the developmental pattern of intestinal structure, transcriptomic profile, and bacterial colonization in neonatal pigs. J Nutr 140:925–931. doi:10.3945/jn.109.116822
National Research Council (2012) Nutrient requirements of swine, 11th edn. National Academy Press, Washington, DC
Moore S, Stein WH (1963) [117] Chromatographic determination of amino acids by the use of automatic recording equipment. Methods Enzymol 6:819–831
Dong L, Zhong X, He J, Zhang L, Bai K, Xu W, Wang T, Huang X (2016) Supplementation of tributyrin improves the growth and intestinal digestive and barrier functions in intrauterine growth-restricted piglets. Clin Nutr 35:399–407. doi:10.1016/j.clnu.2015.03.002
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
van de Poll MC, Dejong CH, Soeters PB (2006) Adequate range for sulfur-containing amino acids and biomarkers for their excess: lessons from enteral and parenteral nutrition. J Nutr 136:1694S–1700S
Métayer S, Seiliez I, Collin A, Duchêne S, Mercier Y, Geraert PA, Tesseraud S (2008) Mechanisms through which sulfur amino acids control protein metabolism and oxidative status. J Nutr Biochem 19:207–215. doi:10.1016/j.jnutbio.2007.05.006
Mastrototaro L, Sponder G, Saremi B, Aschenbach JR (2016) Gastrointestinal methionine shuttle: priority handling of precious goods. IUBMB Life 68:924–934. doi:10.1002/iub.1571
Han F, Hu L, Xuan Y, Ding X, Luo Y, Bai S, He S, Zhang K, Che L (2013) Effects of high nutrient intake on the growth performance, intestinal morphology and immune function of neonatal intra-uterine growth-retarded pigs. Br J Nutr 110:1819–1827. doi:10.1017/S0007114513001232
Wang W, Degroote J, Van Ginneken C, Van Poucke M, Vergauwen H, Dam TM, Vanrompay D, Peelman LJ, De Smet S, Michiels J (2016) Intrauterine growth restriction in neonatal piglets affects small intestinal mucosal permeability and mRNA expression of redox-sensitive genes. FASEB J 30:863–873. doi:10.1096/fj.15-274779
Wang WW, Qiao SY, Li DF (2009) Amino acids and gut function. Amino Acids 37:105–110. doi:10.1007/s00726-008-0152-4
Montagne L, Pluske JR, Hampson DJ (2003) A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim Feed Sci Technol 108:95–117. doi:10.1016/S0377-8401(03)00163-9
Walsh SV, Hopkins AM, Nusrat A (2000) Modulation of tight junction structure and function by cytokines. Adv Drug Deliv Rev 41:303–313
Cummins AG, Steele TW, Labrooy JT, Shearman DJ (1988) Maturation of the rat small intestine at weaning: changes in epithelial cell kinetics, bacterial flora, and mucosal immune activity. Gut 29:1672–1679
Buchmiller-Crair TL, Gregg JP, Rivera FA Jr, Choi RS, Diamond JM, Fonkalsrud EW (2001) Delayed disaccharidase development in a rabbit model of intrauterine growth retardation. Pediatr Res 50:520–524. doi:10.1203/00006450-200110000-00016
D’Inca R, Gras-Le Guen C, Che L, Sangild PT, Le Huërou-Luron I (2010) Intrauterine growth restriction delays feeding-induced gut adaptation in term newborn pigs. Neonatology 99:208–216. doi:10.1159/000314919
Günther C, Neumann H, Neurath MF, Becker C (2013) Apoptosis, necrosis and necroptosis: cell death regulation in the intestinal epithelium. Gut 62:1062–1071. doi:10.1136/gutjnl-2011-301364
Baserga M, Bertolotto C, Maclennan NK, Hsu JL, Pham T, Laksana GS, Lane RH (2004) Uteroplacental insufficiency decreases small intestine growth and alters apoptotic homeostasis in term intrauterine growth retarded rats. Early Hum Dev 79:93–105. doi:10.1016/j.earlhumdev.2004.04.015
Adams JM, Cory S (1998) The Bcl-2 protein family: arbiters of cell survival. Science 281:1322–1326
Zhang H, Li Y, Wang T (2015) Antioxidant capacity and concentration of redox-active trace mineral in fully weaned intra-uterine growth retardation piglets. J Anim Sci Biotechnol 6:48. doi:10.1186/s40104-015-0047-7
Wu G, Fang YZ, Yang S, Lupton JR, Turner ND (2004) Glutathione metabolism and its implications for health. J Nutr 134:489–492
Shan X, Aw TY, Jones DP (1990) Glutathione-dependent projection against oxidative injury. Pharmacol Ther 47:61–71
He Q, Ren P, Kong X, Xu W, Tang H, Yin Y, Wang Y (2011) Intrauterine growth restriction alters the metabonome of the serum and jejunum in piglets. Mol BioSyst 7:2147–2155. doi:10.1039/c1mb05024a
Alexandre-Gouabau M, Courant F, Le Gall G, Moyon T, Darmaun D, Parnet P, Coupé B, Antignac JP (2011) Offspring metabolomic response to maternal protein restriction in a rat model of intrauterine growth restriction (IUGR). J Proteome Res 10:3292–3302. doi:10.1021/pr2003193
MacLennan NK, James SJ, Melnyk S, Piroozi A, Jernigan S, Hsu JL, Janke SM, Pham TD, Lane RH (2004) Uteroplacental insufficiency alters DNA methylation, one-carbon metabolism, and histone acetylation in IUGR rats. Physiol Genomics 18:43–50. doi:10.1152/physiolgenomics.00042.2004
te Braake FW, Schierbeek H, Vermes A, Huijmans JG, van Goudoever JB (2009) High-dose cysteine administration does not increase synthesis of the antioxidant glutathione preterm infants. Pediatrics 124:e978–e984. doi:10.1542/peds.2008-2477
Anderson ME, Meister A (1987) Intracellular delivery of cysteine. Methods Enzymol 143:313–325
Levine RL, Mosoni L, Berlett BS, Stadtman ER (1996) Methionine residues as endogenous antioxidants in proteins. Proc Natl Acad Sci USA 93:15036–15040
Stipanuk MH (2004) Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr 24:539–577. doi:10.1146/annurev.nutr.24.012003.132418
Shen YB, Weaver AC, Kim SW (2014) Effect of feed grade l-methionine on growth performance and gut health in nursery pigs compared with conventional dl-methionine. J Anim Sci 92:5530–5539. doi:10.2527/jas.2014-7830
Hou Y, Wang L, Zhang W, Yang Z, Ding B, Zhu H, Liu Y, Qiu Y, Yin Y, Wu G (2012) Protective effects of N-acetylcysteine on intestinal functions of piglets challenged with lipopolysaccharide. Amino Acids 43:1233–1242. doi:10.1007/s00726-011-1191-9
Fang Z, Yao K, Zhang X, Zhao S, Sun Z, Tian G, Yu B, Lin Y, Zhu B, Jia G, Zhang K, Chen D, Wu D (2010) Nutrition and health relevant regulation of intestinal sulfur amino acid metabolism. Amino Acids 39:633–640. doi:10.1007/s00726-010-0502-x
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant number 31572418) and the Phase II Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
The use of animals for this research was approved by the Institutional Animal Care and Use Committee of Nanjing Agricultural University.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Su, W., Zhang, H., Ying, Z. et al. Effects of dietary l-methionine supplementation on intestinal integrity and oxidative status in intrauterine growth-retarded weanling piglets. Eur J Nutr 57, 2735–2745 (2018). https://doi.org/10.1007/s00394-017-1539-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-017-1539-3