Abstract
Purpose
This study systematically reviewed our team’s research on the mechanism and assessment of liver fibrosis in BA, summarized our experience, and discussed the future development direction.
Methods
In this study, Pubmed and Wanfang databases were searched to collect the literature published by our team on the mechanisms of liver fibrosis in BA and the assessment of liver fibrosis in BA, and the above research results were systematically reviewed.
Results
A total of 58 articles were retrieved. Among the included articles, 25 articles related to the mechanism of liver fibrosis in BA, and five articles evaluated liver fibrosis in BA. This article introduces the key pathways and molecules of liver fibrosis in BA and proposes a new grading system for liver fibrosis in BA.
Conclusions
The new BA liver fibrosis grading method is expected to assess children’s conditions, guide treatment, and improve prognosis more accurately. In addition, we believe that the TGF-β1 signaling pathway is the most important in the study of liver fibrosis in BA, and at the same time, the study of EMT occurrence in BA should also be deepened to resolve the controversy on this issue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
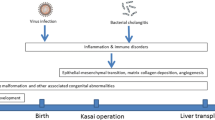
Biliary atresia (BA) is a disease involving progressive fibrosis and inflammatory destruction of intrahepatic and extrahepatic bile ducts that, without intervention, leads to biliary cirrhosis and liver failure [1]. In the absence of a single recognized cause, BA is most likely secondary to viral infection, environmental toxins, genetic mutations, and morphogenetic defects [2]. In the treatment of BA children, when there is no obvious contraindication to Kasai surgery, the sequential treatment of Kasai surgery–liver transplantation should be given priority [3].
Unlike liver fibrosis in adults, most children with BA progress rapidly. The severity of liver fibrosis at the time of the Kasai operation can affect the long-term prognosis of children with BA [4]. In addition, about half of BA children after the Kasai operation require liver transplantation due to progression of cirrhosis [5]. Therefore, preventing the progression of liver fibrosis in children with BA is the greatest challenge faced by pediatric surgeons.
To explore the specific mechanism of liver fibrosis in children with BA, our team has done a lot of work in this field. In this study, we systematically reviewed the literature on the mechanism and grading of liver fibrosis in the BA by our group. This may be important for understanding the pathogenesis of BA and providing new targets and strategies for the treatment of BA.
Methods
The study followed the protocol of registration with the International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY). INPLASY ID: INPLASY202450056.
Search strategy
Pubmed and Wanfang databases were searched from the establishment of the database to January 1, 2024. The MeSH keywords covered “Biliary Atresia”, “Liver Cirrhosis” and related free words, and Jianghua Zhan was included in the author. We use the Boolean operator “OR” to connect subject words with free words to extend the search criteria. Then, we connect individual subject words via the Boolean operator “and” to determine the search scope. The database search strategy is described in Online Resource 1. There are no restrictions on the language and publication status of this paper.
Literature searches
Inclusion criteria included: articles related to the mechanism of liver fibrosis in BA and articles related to the assessment of liver fibrosis in BA. Meanwhile, the criteria used to exclude studies were as follows: case reports, guidelines, reviews, expert commentary, and articles with inconsistent content.
Study selection and definitions
All authors independently screened the titles and abstracts of the search results from both databases for relevance. Finally, two authors independently evaluated the full text of the remaining results according to prespecified criteria, and discrepancies were resolved by the third author. The final list of included articles was determined through careful discussion among the authors.
Data extraction
Information extracted from the included studies was as follows:
(1) Molecules associated with the degree of liver fibrosis and their corresponding P and r values.
(2) Grading criteria for liver fibrosis in biliary atresia.
(3) The potential diagnostic molecules of BA liver fibrosis and the following data were extracted: detection method, sample source, cutoff value, sensitivity, specificity, and area under the curve (AUC) value.
Results
Search process
The literature screening flow chart is shown in Fig. 1. First, the first step identified 58 articles by database search. In the second step, we removed 22 articles based on reading the title and abstract of the articles, leaving only 36 articles. In the third step, we conducted a close reading of the full text of 36 articles and removed six of them. Finally, we identified 25 articles related to the mechanism of liver fibrosis in BA [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30]. At the same time, there were five articles related to the assessment of liver fibrosis in BA [4, 31,32,33,34].
Literature data statistics
In recent years, our group has made some progress in the study of the mechanism of liver fibrosis in BA, which involves multiple signaling pathways and molecules (Table 1).
Molecules related to the degree of liver fibrosis
The expression levels of FN1, CCR9, LECT2, M2BPGi, Leptin, GPC3, Hes-1, CD163, IL-6, PDGF-AA, MMP7, and HIF-1α in BA liver tissues were positively correlated with the degree of liver fibrosis (Table 2).
Molecules for differential diagnosis of BA
Our group found that LECT2, HDAC2, CCL25, CD163, M2BPGi, and GPC3 were associated with liver fibrosis in BA and showed good predictive accuracy in differentiating BA from other cholestasis diseases in children (Table 3).
Grading of liver fibrosis in BA
Liver biopsy is the gold standard for the assessment of liver fibrosis. Clinically, Ishark, Metavir, and Scheuer scoring systems are commonly used to grade the progression of liver fibrosis in BA. However, these scoring systems are based on the characteristics of liver fibrosis in adults with chronic hepatitis and are not related to histopathological features related to BA, so there is a large bias in evaluating liver fibrosis in BA. Based on the main pathological features of BA (fibrosis degree and bile duct reaction), our team established the grading criteria for liver fibrosis in BA in 2015 [32] and optimized it in 2023 (Table 4) [4]. This grading standard can not only better reflect the actual status of liver fibrosis in children with BA, but also indicate the tendency of cirrhosis and poor prognosis. It can also make up for the gap in judging the degree of liver fibrosis during operation by observing the portal area, P–P area, and boundary plate under the frozen section during operation. In addition, by comparing the BA-specific grading system with Ishak and Metavir scoring systems with the prognosis of children with BA after KP, our team found that the BA-specific grading system not only reflects the situation of liver fibrosis but also helps to better assess the prognosis of children with BA when combined with infant BA liver fibrosis (iBALF) and severe bile duct proliferation (BDP) [4].
Systematic review
TGF-β signaling pathway
TGF-β regulates extracellular matrix (ECM) formation, degradation, and remodeling and has been shown to play a key role in other chronic liver diseases, while it is also dysregulated in BA [35]. TGF-β1, a member of the TGF-β superfamily. Our group mainly explored the regulatory role of the TGF-β1/SMAD signaling pathway and other molecules involved in the TGF-β1 pathway in BA children.
TGF-β1/SMAD signaling pathway
We studied the expression of TGF-β1, SMAD2, SMAD3, SMAD4, P-SMAD2, and P-SMAD3 in the liver tissue of children with BA and their roles in liver fibrosis [16, 17]. The results showed that SMAD3, P-SMAD2, P-SMAD3, and SMAD4 were closely related to the pro-fibrotic effect of TGF-β1 pathway in BA liver, and their expressions were first increased and then decreased during the progression of BA fibrosis. The decrease of various proteins in the late stage of liver fibrosis may be due to the decrease of cell number and protein source caused by liver decompensation. However, the expression of SMAD2 did not seem to be affected, showing a wavy change without obvious regularity. Therefore, we further investigated the mRNA expression level of SMAD2, and the results showed that its expression also increased first and then decreased with the progression of BA fibrosis.
In the early stage of liver fibrosis, P-Smad3 is positively correlated with the grade of liver fibrosis, and the changes of PAI-1 and P-Smad3 are consistent, suggesting that the secretion of PAI-1 may be affected by the content of P-Smad3 [16]. Based on the above experimental results, our group continued to explore and found that the expression of PAI-1 in the hepatic lobule was stronger than that in the portal area, and PAI-1 was a product of the TGFβ-1 pro-fibrotic pathway, reflecting the direction of the pro-fibrotic pathway, suggesting that the pro-fibrotic effect of TGFβ-1 pathway is more likely to be manifested by damage to the structure of the hepatic lobule in BA [20].
Other molecules involved in the TGF-β1 pathway
In addition to SMAD-dependent pathways, TGF-β1 activates SMAD-independent pathways, such as MAPK, NF-kB, and PI3K pathways [36]. JNK2, p38, and ERK1/2 are different subtypes of MAPK signaling pathway, which promote the process of liver fibrosis by participating in the phosphorylation and nuclear translocation of TGF-β1 signaling pathway-related proteins [37]. Our previous studies have shown that the expression levels of JNK2, p38, and ERK1/2 are increased in the cytoplasm of hepatocytes, bile duct epithelial cells, and vascular endothelial cells in the liver of BA children, suggesting that they may play an important role in the progression of liver fibrosis in BA [18, 19].
αvβ8 acts as a cell adhesion molecule by binding to the latency-associated peptide-1 (LAP-1) region of TGF-β1, it then promotes membrane-type 1 matrix metalloproteinase (MT1-MMP) binding to LAP-1 on TGF-β1, Furthermore, MT1-MMP can form a complex with αvβ8-TGF-β1 and activate the TGF-β1 signaling pathway through their interaction [38]. Our study found that αvβ8 was strongly positive in liver tissues of BA children, indicating that αvβ8 may be involved in the process of liver fibrosis in BA children [18]. In addition, TGF-β1 can inhibit ECM degradation by inhibiting MMP and promoting the natural inhibitor TIMP [36]. Increased expression of TIMP-1 was also found in BA liver tissues in our study [19].
BMP-9 is a member of the TGF-β superfamily, which is like the ligand–receptor binding form in the TGF-β signaling pathway. After binding to the receptor, BMP-9 leads to the phosphorylation of SMAD1/5/8. Phosphorylated SMAD1/5/8 binds to SMAD4 and migrates to the nucleus to regulate gene expression [39]. Studies have confirmed that BMP-9 can promote liver fibrosis, but its expression in BA liver tissue is not clear [40]. Our study found that the expression of BMP-9 increased with the aggravation of liver fibrosis in BA children, and BMP-9 could induce the expression of ID1 in the hepatic stellate cell nucleus by phosphorylating SMAD1/5, leading to the increase of the expression of extracellular matrix α-SMA, and promote the process of fibrosis [21].
Epithelial–mesenchymal transition (EMT)
Intrahepatic cells (including hepatocytes, HSCs, and cholangiocytes) can transform into myofibroblasts through EMT, and play an important role in developing liver fibrosis by involving various pathways [41]. BA-related fibrosis is closely related to the occurrence of EMT in the human normal intrahepatic biliary epithelial cell line [42].
Association of Hedgehog signaling pathway with EMT
In BA, the Hedgehog signaling pathway affects the occurrence of liver fibrosis from many aspects, mainly by activating HSC, OPN regulation, EMT, vascular remodeling, and other biological processes [24]. In addition, several genes (e.g., add3, gpc1) that regulate the Hedgehog pathway have been reported to be associated with BA susceptibility [43, 44]. The previous findings of our group showed that the mRNA and protein expression of SHH and GLI2 in the liver of BA children were significantly higher than those of the control group, and the EMT marker N-cadherin and CK19 were co-expressed in BA biliary epithelial cells. In addition, activation of the Hedgehog signaling effector transcription factor GLI2 with r-SHH treatment promoted EMT (inhibited E-cadherin and enhanced N-cadherin), which was blocked by blocking this pathway [24].
Association of EGF with EMT
EGF is a member of the growth factor family and has been shown to play an important role in EMT, but there is no relevant study in BA [45]. Previous studies from our group identified the role of EGF in liver fibrosis in BA patients. The main findings were as follows: (1) EGF was elevated in BA and correlated with liver fibrosis; (2) EGF promoted EMT and proliferation of BA hepatobiliary epithelial cells through the EGF/EGFR–ERK1/2 signaling pathway; (3) EGF promoted the expression of IL-8 in hepatocytes through the ERK1/2 pathway and activated HSCs in vitro; (4) Neutralizing antibody to EGF attenuated liver fibrosis in BDL mice [26].
Proliferation of blood vessels
In the development of liver diseases, activated hepatic stellate cells can secrete many pro-angiogenic factors to promote the formation and development of new blood vessels. At the same time, new blood vessels stimulate HSCs through activated TGF-β to accelerate the process of liver fibrosis [46]. The hepatic vascular system of BA children is abnormal, and there is a characteristic subcapsular spider telangiectasia [47]. Our previous study found that the process of liver fibrosis in BA was accompanied by vascular proliferation in the portal area [17, 32]. We further found that HIF1-α and VEGF may induce angiogenesis and promote liver fibrosis in BA [27].
LECT2 is a chemokine synthesized and secreted by hepatocytes. After the liver injury, the secretion of LECT2 increases around the portal area and at the injury boundary, which can aggravate liver fibrosis by promoting the capillarization of hepatic sinusoidal endothelial cells [48]. Οur group found that LECT2 was highly expressed in BA liver tissue and serum, and its expression level in liver tissue was significantly positively correlated with the degree of liver fibrosis and the number of neovascularization in the portal area [28]. In addition, macrophages can regulate LECT2 associated with liver fibrosis in BA by secreting TGF-β1 [22].
Discussion
Liver fibrosis in BA has always been a difficult problem for pediatric surgeons. Our team has been committed to the study of liver fibrosis in BA in recent years and has made some progress. We summarize the molecules that have been previously associated with the grade of liver fibrosis in BA and those that are helpful in the differential diagnosis of BA. LECT2 has the highest diagnostic efficiency in differentiating BA from other cholestatic diseases, which can be further studied. In addition, we propose a new grading criterion for liver fibrosis in BA and summarize the mechanisms associated with liver fibrosis in BA.
TGF-β signaling pathway
Figure 2 shows a possible mechanistic diagram of the TGF-β pathway regulating liver fibrosis in BA based on our systematic review described above. The results of our team are consistent with five other studies, which suggest that the TGF-β1 pathway plays an important role in liver fibrosis in BA [49,50,51,52,53]. However, Lee et al. [54] found that TGF-β2 was more actively transcribed TGF-β gene compared to TGF-β1 during the progression of liver fibrosis in BA. We have not previously explored TGF-β2 expression in BA liver fibrosis, and this finding will be further explored in the future. In addition, the results of SMAD2 in our study were peculiar. It has been suggested that SMAD2 plays a protective role during fibrosis [36]. Therefore, we speculate that the inconsistent expression levels of SMAD2 protein and mRNA may be related to the protective effect of liver fibrosis, but this needs to be confirmed by further studies.
EMT
Consistent with the results of the present study, four other studies similarly suggested that EMT may be present in biliary epithelial cells of BA [55,56,57,58]. However, whether EMT occurs in the process of liver fibrosis remains controversial. Lineage tracing studies demonstrated that EMT did not occur in biliary epithelial cells of mice with liver fibrosis [59,60,61]. The possible reasons for this contradiction are as follows: (1) EMT of biliary epithelial cells may be an initial event [62]. (2) Lineage tracing technology has its limitations, and EMT in liver fibrosis still needs to be further explored.
In conclusion, our proposed method for grading liver fibrosis in BA is expected to assess the condition of children more accurately with BA, guide treatment, and improve prognosis. In addition, BA liver fibrosis is a complex pathological mechanism, and its specific pathogenesis cannot be explained by one certain pathway. Multiple signaling pathways mediated by TGF-β1 may be involved in the progression of liver fibrosis in BA, and it is the most important signaling pathway in the process of liver fibrosis in BA. Furthermore, as one of the most controversial processes in BA, EMT still needs to be further explored.
Data availability
No datasets were generated or analyzed during the current study.
References
Sutton H, Karpen SJ, Kamath BM (2024) Pediatric cholestatic diseases: common and unique pathogenic mechanisms. Annu Rev Pathol 19:319–344. https://doi.org/10.1146/annurev-pathmechdis-031521-025623
Quelhas P, Cerski C, Dos Santos JL (2022) Update on etiology and pathogenesis of biliary atresia. Curr Pediatr Rev 19(1):48–67. https://doi.org/10.2174/1573396318666220510130259
Liu S, Yang Q, Ji Q, Wang Z, Sun R, Zhan J (2024) Effect of Kasai procedure on liver transplantation in children with biliary atresia: a systematic review and updated meta-analysis. Transl Pediatr 13(1):10–25. https://doi.org/10.21037/tp-23-504
Xu X, Wang X, Ding M et al (2023) Development and post-Kasai procedure prognostic relevance of histological features for biliary atresia. BMC Pediatr 23(1):589. https://doi.org/10.1186/s12887-023-04413-3
Wang Z, Chen Y, Peng C et al (2019) Five-year native liver survival analysis in biliary atresia from a single large Chinese center: the death/liver transplantation hazard change and the importance of rapid early clearance of jaundice. J Pediatr Surg 54(8):1680–1685. https://doi.org/10.1016/j.jpedsurg.2018.09.025
Song T, Zhan Ji, Gao W, Liu D, Zhang H (2015) Expression of CD14, CD34 and transforming growth factor-1 in liver biopsy of biliary atresia. Chin J Pediatr Surg 36(1):63–67. https://doi.org/10.3760/cma.j.issn.0253-3006.2015.01.015
Yu C, Xiong X, Zhan J, Hu Xi, Gao W (2019) Expression and clinical significance of MMP-7 in hepatic fibrosis of biliary atresia. Tianjin Med J 47(1):38–42. https://doi.org/10.11958/20181302
Wang H, Zhao J, Gou Q et al (2021) Expression of SOX9 in biliary atresia and its relationship with hepatic fibrosis. Chin J Pediatr Surg 42(6):512–518. https://doi.org/10.3760/cma.j.cn421158-20201119-00708
Chen Li, Zhan J, Zhao J et al (2022) The diagnostic value of glypican 3 in children with biliary atresia and its relationship with liver fibrosis. Tianjin Med J 50(1):15–19. https://doi.org/10.11958/20211114
Wang Q, Zheng Q, Zhang C et al (2022) Clinical significance of expression of leptin in patients with biliary atresia and hepatic fibrosis. Chin J Hepatobiliary Surg 28(4):275–279. https://doi.org/10.3760/cma.j.cn113884-20211119-00377
Zhang C, Zhao J, Zheng Q et al (2022) Clinical implications of M2BP and M2BPGi in hepatic fibrosis of children with biliary atresia. Chin J Pediatr Surg 43(3):214–220. https://doi.org/10.3760/cma.j.cn421158-20210110-00016
Liu Z, Zheng Q, Xu X et al (2023) Expression and clinical significance of CD163 in hepatic fibrosis with biliary atresia. Tianjin Med J 51(4):400–403. https://doi.org/10.11958/20221539
Wang X, Xu X, Li M et al (2023) Expression and clinical significance of CCL25/CCR9 in liver fibrosis of biliary atresia. Chin J Pediatr Surg 44(10):897–903. https://doi.org/10.3760/cma.j.cn421158-20230601-00266
Xu X, Wang X, Liu S et al (2023) Relationship between HDAC2 expression score and progression and prognosis of liver fibrosis in biliary atresia. Chin J Pediatr Surg 44(10):874–881. https://doi.org/10.3760/cma.j.cn421158-20230601-00265
Yang R, Zhang C, Chen L et al (2023) Mechanism of FN1 promoting hepatic stellate cell activation via PI3K/Akt signaling pathway in hepatic fibrosis of biliary atresia. Chin J Pediatr Surg 44(2):114–124. https://doi.org/10.3760/cma.j.cn421158-20211116-00563
Ding M, Gao T, Wei Y, Zhao L, Zhan J (2016) The study on mechanism of p-smad3 in hepatic fibrosis of biliary atresia. J Clin Ped Sur 1:29–33. https://doi.org/10.3969/j.issn.1671-6353.2016.01.009
Ding M, Zhan J, Zhao L, Zhao L, Zhang A (2016) The effects of TGF-β1 and Smad2 on liver fibrosis of biliary atresia. Tianjin Med J 44(7):810–813. https://doi.org/10.11958/20150242
Gao T, Zhan J, Ding M, Wei Y (2016) The expression and significance of integrinαvβ8, p38 and ERK1/2 in the liver of children with biliary atresia. Tianjin Med J. https://doi.org/10.11958/20160362
Gao T, Zhan J, Chen Y, Zhang A, Wei Y (2017) Effects of JNK2, TIMP-1 and collagen III on liver fibrosis in patients with biliary atresia. J Clin Ped Sur 16(2):127–132. https://doi.org/10.3969/j.issn.1671-6353.2017.02.006
Yan P, Zheng Y, Chen H et al (2018) Mechanism of plasminogen activator inhibitor-1 promoting liver fibrosis in biliary atresia. J Clin Ped Sur 17(10):790–794
Ge L, Gou Q, Zhao J et al (2021) The study on the mechanism of BMP-9 in liver fibrosis of biliary atresia. Tianjin Med J 49(10):1020–1025. https://doi.org/10.11958/20210765
Zhao J, Xu X, Gou Q et al (2022) TGF-β1-mediated leukocyte cell-derived chemotaxin 2 Is associated with liver fibrosis in biliary atresia. Front Pediatr 10:901888. https://doi.org/10.3389/fped.2022.901888
Zhang S, Chen Y, Gao T, Zhan J (2017) Effects of RhoA, Rac1 and Cdc42 on liver fibrosis in patients with biliary atresia. Chin J Pediatr Surg 38(11):816–821. https://doi.org/10.3760/cma.j.issn.0253-3006.2017.11.003
Gou Q, Zheng Q, Zhao J et al (2021) Expression and mechanism of Hedgehog signaling pathway in liver fibrosis of biliary atresia. Chin J Pediatr Surg 42(9):774–780. https://doi.org/10.3760/cma.j.cn421158-20210106-00008
Zheng Q, Gou Q, Zhao J et al (2022) Mechanism of epidermal growth factor in hepatic fibrosis of biliary atresia. Chin J Pediatr Surg 43(4):310–315. https://doi.org/10.3760/cma.j.cn421158-20210105-00014
Zheng Q, Li M, Chen L et al (2023) Potential therapeutic target of EGF on bile duct ligation model and biliary atresia children. Pediatr Res 94(4):1297–1307. https://doi.org/10.1038/s41390-023-02592-4
Wei Y, Ding M, Gao T, Hu X, Zhan J (2016) The expression of HIF-1αand VEGF in the patients with biliary atresia. J Clin Ped Sur 1:34–37. https://doi.org/10.3969/j.issn.1671-6353.2016.01.010
Zhao J, Dou R, Zheng Q et al (2020) Expression and clinical significance of LECT2 in biliary atresia hepatic fibrosis. Chin J Pediatr Surg 41(7):633–639. https://doi.org/10.3760/cma.j.cn421158-20200222-00110
Jia J, Zhan J, Yu C, Xiong X, Hu X (2019) Expression and significance of Notch-1, Jagged-1 and Hes-1 in liver fibrosis of children with biliary atresia. Chin J Pediatr Surg 40(5):399–403. https://doi.org/10.3760/cma.j.issn.0253-3006.2019.05.004
Abudureyimu A, Lin F, Wang H et al (2021) Activation of Notch signaling pathway collaborated with macrophages for promoting liver fibrosis in biliary atresia. J Clin Ped Sur 20(4):376–381. https://doi.org/10.12260/lcxewkzz.2021.04.014
Guan Z, Zhan J, Hu X, Luo X, Bao G, Liu Y (2012) The assessment and significance of liver fibrosis in children with biliary atresia. Chin J Pediatr Surg 33(11):815–819. https://doi.org/10.3760/cma.j.issn.0253-3006.2012.11.004
Ding M, Zhan J, Liu D, Zhang H, Wei Y, Gao T (2015) Grading of hepatic fibrosis in biliary atresia. Chin J Pediatr Surg 36(11):866–872. https://doi.org/10.3760/cma.j.issn.0253-3006.2015.11.016
Yu C, Zhan J, Gao W, Wang Z (2017) Clinicopathological analysis with different native liver survivals for biliary atresia after Kasai. J Clin Ped Sur 16(6):552–558. https://doi.org/10.3969/j.issn.1671-6353.2017.06.007
Xiong X, Zhan J, Yu C, Hu X, Zhao L (2018) Relationship between native liver survival and ductular reaction in biliary atresia. J Clin Ped Sur 17(11):814–820. https://doi.org/10.3969/j.issn.1671-6353.2018.11.004
Iordanskaia T, Hubal MJ, Koeck E, Rossi C, Schwarz K, Nadler EP (2013) Dysregulation of upstream and downstream transforming growth factor-β transcripts in livers of children with biliary atresia and fibrogenic gene signatures. J Pediatr Surg 48(10):2047–2053. https://doi.org/10.1016/j.jpedsurg.2013.03.047
Xu F, Liu C, Zhou D, Zhang L (2016) TGF-β/SMAD pathway and its regulation in hepatic fibrosis. J Histochem Cytochem 64(3):157–167. https://doi.org/10.1369/0022155415627681
Jiang Y, Wu C, Boye A et al (2015) MAPK inhibitors modulate Smad2/3/4 complex cyto-nuclear translocation in myofibroblasts via Imp7/8 mediation. Mol Cell Biochem 406(1–2):255–262. https://doi.org/10.1007/s11010-015-2443-x
Mu D, Cambier S, Fjellbirkeland L et al (2002) The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol 157(3):493–507. https://doi.org/10.1083/jcb.200109100
Breitkopf-Heinlein K, Meyer C, König C et al (2017) BMP-9 interferes with liver regeneration and promotes liver fibrosis. Gut 66(5):939–954. https://doi.org/10.1136/gutjnl-2016-313314
Addante A, Roncero C, Almalé L et al (2018) Bone morphogenetic protein 9 as a key regulator of liver progenitor cells in DDC-induced cholestatic liver injury. Liver Int 38(9):1664–1675. https://doi.org/10.1111/liv.13879
Chen Y, Fan Y, Guo DY et al (2020) Study on the relationship between hepatic fibrosis and epithelial-mesenchymal transition in intrahepatic cells. Biomed Pharmacother 129:110413. https://doi.org/10.1016/j.biopha.2020.110413
Wang JY, Cheng H, Zhang HY et al (2019) Suppressing microRNA-29c promotes biliary atresia-related fibrosis by targeting DNMT3A and DNMT3B. Cell Mol Biol Lett 24:10. https://doi.org/10.1186/s11658-018-0134-9
Cui S, Leyva-Vega M, Tsai EA et al (2013) Evidence from human and zebrafish that GPC1 is a biliary atresia susceptibility gene. Gastroenterology 144(5):1107-1115.e3. https://doi.org/10.1053/j.gastro.2013.01.022
Tang V, Cofer ZC, Cui S, Sapp V, Loomes KM, Matthews RP (2016) Loss of a candidate biliary atresia susceptibility gene, add3a, causes biliary developmental defects in zebrafish. J Pediatr Gastroenterol Nutr 63(5):524–530. https://doi.org/10.1097/MPG.0000000000001375
Sheng W, Tang J, Cao R, Shi X, Ma Y, Dong M (2022) Numb-PRRL promotes TGF-β1- and EGF-induced epithelial-to-mesenchymal transition in pancreatic cancer. Cell Death Dis 13(2):173. https://doi.org/10.1038/s41419-022-04609-y
Sakata K, Eda S, Lee ES, Hara M, Imoto M, Kojima S (2014) Neovessel formation promotes liver fibrosis via providing latent transforming growth factor-β. Biochem Biophys Res Commun 443(3):950–956. https://doi.org/10.1016/j.bbrc.2013.12.074
Zhou Y, Jiang M, Tang ST et al (2017) Laparoscopic finding of a hepatic subcapsular spider-like telangiectasis sign in biliary atresia. World J Gastroenterol 23(39):7119–7128. https://doi.org/10.3748/wjg.v23.i39.7119
Xu M, Xu HH, Lin Y et al (2019) LECT2, a ligand for Tie1, plays a crucial role in liver fibrogenesis. Cell 178(6):1478-1492.e20. https://doi.org/10.1016/j.cell.2019.07.021
Qiu JL, Zhang GF, Chai YN et al (2022) Ligustrazine attenuates liver fibrosis by targeting miR-145 mediated transforming growth factor-β/Smad signaling in an animal model of biliary atresia. J Pharmacol Exp Ther 381(3):257–265. https://doi.org/10.1124/jpet.121.001020
Meng L, Liu J, Wang J et al (2021) Characteristics of the gut microbiome and IL-13/TGF-β1 mediated fibrosis in post-Kasai cholangitis of biliary atresia. Front Pediatr 9:751204. https://doi.org/10.3389/fped.2021.751204
Yang Y, Liu YJ, Tang ST et al (2013) Elevated Th17 cells accompanied by decreased regulatory T cells and cytokine environment in infants with biliary atresia. Pediatr Surg Int 29(12):1249–1260. https://doi.org/10.1007/s00383-013-3421-6
Vejchapipat P, Theamboonlers A, Poomsawat S, Chittmittrapap S, Poovorawan Y (2008) Serum transforming growth factor-beta1 and epidermal growth factor in biliary atresia. Eur J Pediatr Surg 18(6):415–418. https://doi.org/10.1055/s-2008-1038950
Ramm GA, Nair VG, Bridle KR, Shepherd RW, Crawford DH (1998) Contribution of hepatic parenchymal and nonparenchymal cells to hepatic fibrogenesis in biliary atresia. Am J Pathol 153(2):527–535. https://doi.org/10.1016/S0002-9440(10)65595-2
Lee SY, Chuang JH, Huang CC et al (2004) Identification of transforming growth factors actively transcribed during the progress of liver fibrosis in biliary atresia. J Pediatr Surg 39(5):702–708. https://doi.org/10.1016/j.jpedsurg.2004.01.030
Siyu P, Junxiang W, Qi W, Yimao Z, Shuguang J (2022) The role of GLI in the regulation of hepatic epithelial-mesenchymal transition in biliary atresia. Front Pediatr 10:861826. https://doi.org/10.3389/fped.2022.861826
Harada K, Sato Y, Ikeda H et al (2009) Epithelial-mesenchymal transition induced by biliary innate immunity contributes to the sclerosing cholangiopathy of biliary atresia. J Pathol 217(5):654–664. https://doi.org/10.1002/path.2488
Deng YH, Pu CL, Li YC et al (2011) Analysis of biliary epithelial-mesenchymal transition in portal tract fibrogenesis in biliary atresia. Dig Dis Sci 56(3):731–740. https://doi.org/10.1007/s10620-010-1347-6
Xiao Y, Zhou Y, Chen Y et al (2015) The expression of epithelial-mesenchymal transition-related proteins in biliary epithelial cells is associated with liver fibrosis in biliary atresia. Pediatr Res 77(2):310–315. https://doi.org/10.1038/pr.2014.181
Chu AS, Diaz R, Hui JJ et al (2011) Lineage tracing demonstrates no evidence of cholangiocyte epithelial-to-mesenchymal transition in murine models of hepatic fibrosis. Hepatology 53(5):1685–1695. https://doi.org/10.1002/hep.24206
Scholten D, Osterreicher CH, Scholten A et al (2010) Genetic labeling does not detect epithelial-to-mesenchymal transition of cholangiocytes in liver fibrosis in mice. Gastroenterology 139(3):987–998. https://doi.org/10.1053/j.gastro.2010.05.005
Taura K, Iwaisako K, Hatano E, Uemoto S (2016) Controversies over the epithelial-to-mesenchymal transition in liver fibrosis. J Clin Med 5(1):9. https://doi.org/10.3390/jcm5010009
Robertson H, Kirby JA, Yip WW, Jones DEJ, Burt AD (2007) Biliary epithelial-mesenchymal transition in posttransplantation recurrence of primary biliary cirrhosis. Hepatology 45(4):977–981. https://doi.org/10.1002/hep.21624
Acknowledgements
Funding by Tianjin Key Medical Discipline (Specialty) Construction Project, the Tianjin Science and Technology Program (No.21ZXGWSY00070) and the Tianjin Applied Basic Research Project (No.22JCZDJC00290).
Author information
Authors and Affiliations
Contributions
Conception and design: Jianghua Zhan, Shaowen Liu; administrative support: Jianghua Zhan; drawing of figures: Qianhui Yang; data extraction: Shaowen Liu, Yu Meng, Qianhui Yang; manuscript writing: all authors. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
This is a systematic review. No ethical approval is required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhan, J., Liu, S., Meng, Y. et al. Systematic review of the mechanism and assessment of liver fibrosis in biliary atresia. Pediatr Surg Int 40, 205 (2024). https://doi.org/10.1007/s00383-024-05778-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s00383-024-05778-x