Abstract
Purpose
Notch and Wnt/β-catenin signaling are responsible for regulation of intestinal stem cells (ISCs) proliferation and differentiation. The purpose of the study was to evaluate Wnt/β-catenin and Notch signaling roles in regulation of ISC differentiation following ischemia–reperfusion (IR) injury in a rat.
Methods
Rats were assigned into two groups: Sham rats underwent laparotomy without vascular intervention and IR rats underwent occlusion of SMA and portal vein for 20 min followed by 48 h of reperfusion. Wnt/β-catenin and Notch-related gene expression were determined using Real-Time PCR. Enterocyte proliferation, differentiation and Wnt-related proteins were determined by immunohistochemistry.
Results
IR rats demonstrated a significant decrease in β-catenin gene expression, a decrease in cyclin D1 and β-catenin positive cells in jejunum and ileum compared to Sham rats. IR rats demonstrated a significant increase in Notch-related gene expression in jejunum and ileum compared to Sham rats. The number of secretory cells was higher mainly in the jejunum and number of absorptive cells was significantly lower in jejunum and lower in ileum in IR rats compared to Sham rats.
Conclusions
Intestinal stem-cell differentiation is toward secretory cells 48 h after IR injury; however, Wnt/β-catenin pathway inhibition and Notch-related gene expression stimulation suggest crosstalk between pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemia–reperfusion intestinal (IR) injury is a complex process which leads to intestinal mucosal cell death during ischemia by a decrease in oxygen delivery, dropping of cellular energy stores and accumulation of toxic metabolites. Furthermore, the reintroduction of oxygen during reperfusion may initiate a cascade of events that exacerbates intestinal tissue injury via formation of reactive oxygen species (ROS) and nitrogen species [1]. The increase in ROS and nitrogen species production strengthens damage to the endothelium and increase permeability of the epithelium [2], which leads to translocation of bacteria and their products into the systemic circulation, infiltration of polymorphonuclear leukocytes and mast cells that secrete several cytokines (TNF-α, IL-1 and IL-8), platelet activating factor, eicosanoids, leukotrienes and other mediators, which can promote a systemic inflammatory response, multiple organ failure and even death [3, 4]. Additionally, nowadays, it is known that apoptosis triggered by IR is a significant, and perhaps the principal contributor to cell death after IR [5, 6].
The process of intestinal cells regeneration, which is also known as intestinal cell turnover is possible due to intestinal stem cells (ISCs) proliferation and differentiation to absorptive and secretory types of cells, which migrate and replace the preceding intestinal epithelial layer. The regeneration rate of the intestinal cells is balanced by stem-cell proliferation and differentiation on the one hand and on apoptosis of the cells on the other hand, and is regulated by interaction of the epithelium and the stromal layer of intestinal tissue [7].
Four main signaling pathways known as Hedgehog, Bone Morphogenetic Protein (BMP), Wnt/b-catenin and Notch regulate proliferation and differentiation of ISCs [8]. ISCs are capable to divide either to intestinal absorptive cells called enterocytes or to secretory cells which are goblet cells, endocrine cells and Paneth cells [9, 10]. Wnt/b-catenin signaling contains two pathways, β-catenin-dependent (canonical) and β-catenin-independent (non-canonical) [11, 12]. It is known that the canonical Wnt signaling pathway is involved in cell proliferation and differentiation through transcription of Wnt responsive genes in the cell nucleus like c-Myc [13]. Notch signaling is also believed to drive intestinal stem cells differentiation through activating target genes in the nucleus like Hes-1 which is responsible for differentiation of precursor cells into enterocytes [14, 15]. It is known that when Notch signaling is inhibited the ISCs differentiate to secretory cells and when Notch signaling is activated the differentiation of ISCs to secretory cells is ceased [10, 14, 16].
The purpose of this study was to evaluate the roles of Notch and Wnt/b-catenin signaling in ISC differentiation process after ischemia–reperfusion injury in a rat.
Materials and methods
Animals
Experimental animals were handled according to the standards of care and use of animals as mentioned in the Guide for the Care and Use of Laboratory Animals (Sourasky Faculty of Medicine, Tel Aviv, Israel). Sprague–Dawley male rats (weighing 250–270 g) were stored in pairs at 21 °C regime and on 12-h day and night cycles for 7 days prior to the experiment. The animals were fed with standard chow and had free access to water and were only allowed access to water 24 h before the operation.
Experimental design
The rats were randomly divided into two experimental groups. Group A (Sham) rats underwent laparotomy, identification of the superior mesenteric artery (SMA) and portal vein (PV) without their occlusion and were sacrificed 48 h after operation. Group B (IR) underwent laparotomy in which both SMA and PV were occluded for 20 min followed by 48 h of reperfusion. All animals were sacrificed by carbon dioxide (CO2) inhalation according to AVMA Guidelines for the Euthanasia of Animals.
Surgical procedure
The experimental animals were anaesthetized by injection of ketamine (90 mg/kg) and xylazine (15 mg/kg) subcutaneously (SC) after an overnight fast. Opening the abdomen was done using a midline laparotomy. In the sham groups, the SMA and PV were isolated but not occluded. In the intervention group, the SMA and PV were occluded using atraumatic microvascular clamps, causing intestinal ischemia. During ischemic period, the abdominal wound was covered by wet gauze to prevent heat and fluid loss. After 20 min, the abdomen was re-opened and the clamp was removed and the ischemic bowel was returned into the abdomen. The abdominal cavity was washed with a 3-ml IP injection of 0.9% saline before closure. The abdomen was closed using 3/0 Vicryl (Ethicon Corporation, USA) in two layers of running suture. The rats were allowed free access to water 6 h after operation. The rats were sacrificed 48 h after operation.
Intestinal microscopic appearance
During sacrificing the animals after operation, the small intestine was extracted, excised and rinsed with cold 0.9% saline and divided into two segments: proximal jejunum and terminal ileum. Both segments were cut longitudinally and in each the mucosa was scraped using a glass slide and collected. Histologic sections were performed from the bowel segments by fixation in 4% buffered formalin for 24 h and processed into paraffin blocks. Five-micrometer paraffin-embedded tissue slices (5 µm) were stained with hematoxylin and eosin. The villus height and crypt depth for each specimen were measured using an objective mounted micrometer (X100 magnification) and an optical microscope (10X100 magnification). Villus height and crypt depth measurement consists of the mean of five villi and crypts.
Real-time PCR
Trizol (Invitrogen) reagent was used to isolate ribonucleic acid (RNA) according to the manufacturer’s protocol. Spectrophotometry of 260/280 nm was used to quantify the extracted RNA. Thereafter, 500 ng of total RNA was converted into complementary DNA (cDNA) by reverse transcriptase (qScriptcDNA Synthesis Kit Quantabio, USA). Next, cDNA was amplified by PCR-Thermal Cycler (2720 Thermal Cycler, ABI, Israel). Gene expression of β-catenin, cyclin D1, c-Myc, Jag2, DLL was determined by quantitative real-time polymerase chain reaction (PCR) ABI-PRISM 7000 (applied Biosystems, Foster City, California, USA) on cDNA samples using PerfeCTa SYBR Green FastMix, Low ROX (Quantabio, USA) except for template and primers.
Immunohistochemistry
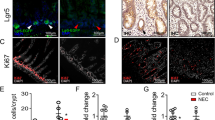
The proliferation of crypt cells was evaluated using immunohistochemistry for nuclear protein Ki-67. Tissue slices were stained with Ki-67 monoclonal antibody (Ki-67 a recombinant monoclonal antibody, dilution 1:200). The index of proliferation was set as the ratio of positive stained crypt cells for Ki-67 per ten crypts. The detection of Wnt/β-catenin signaling pathway was done using immunohistochemistry for β-catenin (β-catenin cleaved polyclonal antibody, dilution 1:100) and Cyclin D1 (Cyclin D1 cleaved polyclonal antibody, dilution 1:50). Positive cells were identified using a combination of streptavidin–biotin–peroxidase method and microwave antigen retrieval on formalin-fixed, paraffin-embedded tissues in accordance with manufacturer's protocols. β-Catenin staining distribution was scored as M (membranous) when the intercellular borders of enterocytes were stained positively; H (heterogeneous) when the intercellular borders of some enterocytes were stained positively; C (cytoplasmic) when the enterocytes cytoplasm were stained. The immunostaining strength was graded as strong ( +), weak ( ±), and absent (-).
Intestinal epithelial cell proliferation
To estimate the differentiation of intestinal cells to specialized secretory cells, additional sections of jejunum and ileum were stained with hematoxylin and eosin to detect enterocytes and Paneth cells, Alcian blue for detection of goblet cells according to Myer's protocol [17]. For determination of enteroendocrine cells, immunohistochemistry with anti-chromogranin (Abcam 151601; 1:1250; Abcam, Cambridge, UK), diluted in 1%BSA/PBS, following antigen retrieval in citrate buffer for 15 min. Secondary antibody was biotinylated goat anti-rabbit, detected with the ABC system (Vector Laboratories, PK-6100) and DAB chemistry to give a brown stain.
Statistical analysis
All data expressed as the Mean ± SEM. Kruskal–Wallis test was used for statistical analysis of intestinal cell proliferation and differentiation, followed by post hoc test for multiple comparisons. Statistical significance was considered if p value was < 0.05.
Results
Intestinal epithelial cell proliferation and microscopic bowel appearance
We have demonstrated a decrease in intestinal cell proliferation index 48 h after IR injury in the jejunum (112.6 ± 23.2 vs. 168.3 ± 21 Ki-67 positive cells/10 crypts) and in the ileum (98.4 ± 25 vs. 199 ± 38 Ki-67 positive cells/10 crypts; p < 0.05) compared to Sham rats (Fig. 1). We have also demonstrated a significant decrease in villus height in the jejunum (2027.2 ± 268.2 vs. 2695.3 ± 213.4 µm; p < 0.05) and a trend toward decrease in villus height in the ileum (2120.5 ± 179.8 vs. 2209.3 ± 128.7 µm) compared to Sham animals (Fig. 2). There was no a significant difference in crypt depth in both jejunum and ileum between IR and Sham groups.
Wnt/β-catenin and Notch signaling-related genes (real-time PCR)
We evaluated Wnt/β-catenin and Notch-related gene expressions using real-time PCR and we have demonstrated a significant decrease in the expression of β-catenin mRNA level in the jejunum (20-fold decrease; p < 0.05) and in the ileum (13-fold decrease; p < 0.05) of IR rats compared to Sham rats (Fig. 3). There were no differences in the expression of C-Myc and Cyclin D1 mRNA levels 48 h after IR compared to Sham group. Regarding Notch-related gene expression, we have shown an increase of JAG2 mRNA level in the jejunum (1.5-fold increase, NS) and in the ileum (5.3-fold increase; p < 0.05) as well as a significant increase of DLL mRNA level in the jejunum (8.2-fold increase; p < 0.05) and in the ileum (1.5-fold increase; p < 0.05) of IR rats compared to Sham rats.
Effect of intestinal ischemia–reperfusion on Wnt/β-catenin and Notch signaling-related genes. A: Gene expression of β-catenin, C-Myc and Cyclin D1 mRNA was determined by quantitative real-time PCR. B: Gene expression of Jag 2 and DLL mRNA was determined by quantitative real-time PCR. Values are mean ± SEM. IR: ischemia–reperfusion. *P < 0.05 IR rats vs sham rats
Immunohistochemistry and cell differentiation
Immunohistochemistry for β-catenin in IR rats revealed a weak immunolabeling distributed throughout the enterocytes cytoplasm, suggesting lower activity of Wnt/β-catenin signalling, while in Sham rats, the immunolabeling was strongly distributed throughout the enterocytes cytoplasm (Fig. 4). IR rats also demonstrated a slight decrease in the number of Cyclin D1 positive cells in both jejunum (152 ± 8 vs. 161 ± 22) and ileum (178 ± 9 vs. 183 ± 10) compared to Sham animals (Fig. 4).
Immunohistochemistry for Cyclin D1 and β-catenin was performed for identification of positive cells using a combination of the streptavidin–biotin–peroxidase method and microwave antigen retrieval on formalin-fixed, paraffin-embedded tissues according to the manufacturer’s protocols. Values are mean ± SEM. IR: ischemia–reperfusion. *P < 0.05 IR rats vs sham rats
Intestinal stem cell differentiation
IR rats demonstrated an increase in the number of goblet cells in the jejunum (15% increase) as well as an increase in the number of enteroendocrine cells in the jejunum (25% increase) and a decrease in the ileum (30% decrease) compared to sham animals (Fig. 5). IR rats demonstrated also an increase in the number of Paneth cells in jejunal crypt (31% increase) and a significant decrease in those cells in the ileum (34% decrease, p < 0.05) compared to control animals. With regard to intestinal absorptive cells, IR rats demonstrated a significant decrease in the number of enterocytes in the jejunum (317 ± 49 vs. 506 ± 49, p < 0.05) and in the ileum (410 ± 39 vs. 572 ± 71, p < 0.05) compared to sham rats. When calculated per 100 cells per villus/crypt unit, IR rats have shown a significant increase in the number of goblet cells in the jejunum (7.1 ± 1 vs. 3.8 ± 0.6, p < 0.05) and in the ileum (6.2 ± 0.8 vs. 4.6 ± 0.5) as well as an increase in the number of enteroendocrine cells in the jejunum (0.2 ± 0.1 vs. 0.1 ± 0.02) and a significant increase in the number of Paneth cells/100 cells in the jejunum (0.8 ± 0.2 vs. 0.3 ± 0.04, p < 0.05) and a slight decrease in the number of those cells in the ileum (0.6 ± 0.1 vs. 0.7 ± 0.1) compared to Sham rats. Regarding intestinal absorptive cells, the number of enterocytes/100 cells decreased significantly in the jejunum (92 ± 1.3 vs. 96 ± 0.6, p < 0.05) and in the ileum (93 ± 0.9 vs. 95 ± 0.6) in the IR rats compared to Sham animals.
Effect of intestinal ischemia–reperfusion on the cell differentiation in the remaining small intestine. A: staining with hematoxylin and eosin, Alcian blue, and anti-chromogranin was used in enterocytes, goblet cells, enteroendocrine cells, and Paneth cells. B: the number of enterocytes decreased, while the number of goblet cells, enteroendocrine cells, and Paneth cells increased in IR (vs. sham) rats when calculated per 100 cells. Values are mean ± SEM. IR: ischemia–reperfusion. *P < 0.05 IR rats vs sham rats
Discussion
Intestinal ischemia–reperfusion injury is a complex process causing damage to the intestinal wall, especially to the vulnerable layer, the epithelium. The regeneration process of the intestinal epithelial cells is known as intestinal cell turnover is based on the proliferation, differentiation and apoptosis of intestinal epithelial layer. There are four main signaling pathways which regulate intestinal stem cells (ISCs) activity and are known as Wnt/β-catenin, hedgehog, bone morphogenetic protein (BMP) and Notch [7, 8]. ISCs can be divided into two progenitor cells which are known as absorptive and secretory cells. The absorptive cells are known as enterocytes, whereas the secretory cells are known as goblet cells, enteroendocrine cells and Paneth cells [8, 13, 18].
Both Wnt/β-catenin and Notch signaling pathways are known to play a vital role as regulators of stem cells activity in the gastrointestinal tract [13, 19, 20]. Wnt/β-catenin has two separate pathways; canonical or Wnt/β-catenin-dependent pathway and a non-canonical or Wnt/β-catenin-independent pathway [21]. The Wnt/β-catenin-dependent pathway is activated when Wnt ligand binds to a receptor complex. Wnt ligand binding causes a phosphorylation of low-density lipoprotein receptor-related protein 5/6 (LRP5/6). The phosphorylated LRP5/6 binds to Axin and this complex proteins which is known as β-catenin destruction complex dissociate. Then, the stable β-catenin penetrates the cell’s nucleus and binds to transcription factors (TCF) which leads to transcription of Wnt-related genes like c-Myc which in turn leads to cell proliferation [13]. When Wnt ligand is absent, β-catenin is part of a multiprotein β-catenin destruction complex, while it is phosphorylated and degraded by ubiquitin–proteosome complex. Notch signaling pathway is composed of four single trans-membrane Notch receptors (Notch 1–4) and 5 single Delta-like ligands (DLL) 1, 3, 4 and Jagged (Jag) 1, 2 [22]. Notch pathway is activated when a ligand binds to a neighboring receptor. The binding of ligand to its receptor leads to series cleavage of Notch receptor and release of NICD (Notch intracellular domain). NICD penetrates the cell’s nucleus and binds to a complex of transcription factors to promote transcription of Notch potential target genes like HES 1, 5, 7 and HERP 1, 2, 3 [14].
Intestinal epithelia cells have multiple functions such as digestion and absorption of nutrients and protection against luminal pathogens and are localized along a vertical axis in crypts and villi. The epithelium includes four differentiated cell lineages, the absorptive enterocytes and the secretory goblet, enteroendocrine and Paneth cells. All these mature cell lineages are proliferated and differentiated from stem cells residing along the crypts bases and maintained the homeostasis of this organized multicellular tissue [9, 23,24,25]. The self-renew of the epithelial cells throughout life or after injury like IR is made by proliferation of several types of precursor cells in the lower part of the crypts. The proliferation continues for several rounds till these proliferating precursor cells differentiate into one of the mature cell lineages and move upward to the top of the villi except Paneth cells which move down to the base of the crypts [26, 27].
According to previous studies, Wnt/β-catenin and Notch signaling pathway has a vital role in the regulation of intestinal stem cell proliferation and differentiation toward intestinal absorptive or secretory cells [13, 18, 28, 29]. Based on number of studies, canonical Wnt/β-catenin signaling has a pivotal role as a regulator of stem cell proliferation and also of the transit-amplifying cells in intestinal crypts [30]. It has been shown that when there are mutations in APC or β-catenin components of Wnt signaling and is overactivated, there is an overproliferation of epithelial cells and failure of them to differentiate [31,32,33]. However, when Wnt signaling is inhibited by overexpression of Dkk1 inhibitor or alternatively in β-catenin or TCF4 knockout mice, epithelial cell proliferation ceased [34,35,36,37]. Additionally other studies stated about the differentiation of progenitor cells to absorptive cells rather than secretory cells due to stimulation by Notch signaling pathway [38, 39]. Another substantial knowledge is the fundamental roles of bHLH (basic helix–loop–helix) transcription factors as part of Notch signaling pathway in the regulating of intestinal epithelial cells differentiation [40]. A primary evidence about the role of Notch signaling in intestinal cell differentiation was in Hes1 knockout mice [41]. Hes1 is a known bHLH-type transcription repressor which Notch signaling activates its expression [42, 43]. Deletion of a Hes1 gene resulted in amplified generation of secretory cells such as goblet, enteroendocrine and Paneth cells [40]. On the other hand, inhibition of Notch signaling pathway by blocking the release of NICD resulted in suppression of progenitor cells to proliferate and converted them into secretory cells [44]. However, the regulation of ISCs and their differentiation process following IR injury in a rat has not been investigated thoroughly.
The purpose of the study was to evaluate the roles of Wnt/β-catenin and Notch signaling pathways in the regulation of ISCs’ differentiation process following IR injury in a rat model. In our previous study, we had demonstrated that 48 h following intestinal IR Notch signaling pathway was inhibited. This inhibition was based on a decrease in Notch-related proteins expression, Notch-1 and Hes-1 and a decrease in the number of Notch-related positive cells. However, signs of Notch signaling pathway activation emerged and characterized by increase in Notch-related gene expression particularly in the jejunum [45].
To evaluate the roles of Wnt/β-catenin and Notch signaling and explore the crosstalk between pathways in the regulation of ISCs proliferation and differentiation following IR injury, we examined Wnt/β-catenin and Notch signaling-related gene expression using real-time PCR. We have demonstrated a significant decrease in the expression of β-catenin mRNA level in the jejunum and in the ileum of IR rats compared to Sham rats. The expression of C-Myc and Cyclin D1 mRNA levels was a slight higher in the IR rats particularly in the jejunum compared to Sham rats but without statistical significance. Regarding Notch-related gene expression, we have demonstrated an increase of JAG2 mRNA level in the jejunum and a significant increase in the ileum and also a significant increase of DLL mRNA level in the jejunum and in the ileum of IR rats compared to Sham rats.
The significance decrease in the expression of β-catenin mRNA level was coincided with a trend of a slight decrease in the number of Cyclin D1 positive cells in both jejunum and ileum compared to Sham rats and a weak immunolabeling of β-catenin distributed throughout the enterocytes’ cytoplasm compared to Sham rats where the immunolabeling distributed strongly throughout the enterocytes’ cytoplasm in both jejunum and ileum. The decrease in the number of positive cells to Cyclin D1 along with immunolabeling reduction of β-catenin throughout enterocytes’ cytoplasm suggests lower activity of Wnt/β-catenin signaling in IR rats compared to Sham animals.
Next, we have determined the differentiation of intestinal stem cells in relation to Wnt/β-catenin and Notch signaling activity. Analysis of secretory cells differentiation 48 h following IR injury revealed an increase in the number of goblet cells in the jejunum with no difference in the number of goblet cells in the ileum. An increase in the number of enteroendocrine cells in the jejunum and a decrease in the ileum compared to Sham rats when calculated by 10 villi. There was also an increase in the number of Paneth cells in jejunum and a significant decrease in those cells in the ileum compared to Sham rats when calculated by 10 crypts. However, while secretory cells were calculated per 100 cells per villus/crypt unit, IR rats demonstrated a significant increase in the number of goblet cells in the jejunum and also an increase in the number of those cells in the ileum compared to Sham rats. An increase in the number of enteroendocrine cells in the jejunum and no difference in the number of those cells in the ileum compared to Sham rats. In addition, IR rats demonstrated a significant increase in the number of Paneth cells/100 cells in the jejunal crypt and only a slight decrease in the number of those cells in the ileal crypt compared to Sham rats. While analysis of absorptive cells revealed a significant decrease in the number of enterocytes in jejunum and ileum of IR rats compared to Sham rats. Additionally, while absorptive cells were calculated per 100 cells per villus/crypt unit, the number of enterocytes decreased significantly in the jejunum and decreased less in the ileum of IR rats compared to Sham animals.
According to the depicted roles of Wnt/β-catenin and Notch signaling in regulating the proliferation and differentiation of intestinal crypt cells progenitors, a crosstalk model was suggested. Both Wnt/β-catenin and Notch signaling are required to activate multipotent progenitor cells to proliferate. As soon as Notch signaling stops activating some progenitor cells, their proliferation ceases and they are directed toward secretory fate. However, if Wnt/β-catenin signaling stops activation of some of these progenitor cells, they differentiate into absorptive cells [40]. There are a few hypotheses about the mechanisms involved in this complicated integration between Wnt/β-catenin and Notch signaling for stem cells fate decision. One of the assumptions is that Notch signaling is the principal regulator of cell fate decision and Wnt/β-catenin signaling is a modulator of Notch signaling activity. This assumption is based on the activity of Notch and Wnt/β-catenin signaling as regulators of colon cancer cells proliferation in vitro. According to Leow et al., Hath1 mRNA (the human homolog of Math1—a repressed gene by Hes1) is inhibited by an overactivated Wnt/β-catenin signaling in colon cancer cells [46]. Another possible assumption about the mechanism of crosstalk between Wnt/β-catenin and Notch signaling might be an interaction between both signaling pathways in a certain cell cycle control step. This possible hypothesis is based on the modulation of p21CIPI/WAF1, a cyclin-dependent kinase inhibitor (CKI) by Wnt/β-catenin and Notch signaling that might regulate the G1/S cell cycle transition in progenitor cells [40].
Based on the studies depicted, complex interactions and intracellular communications between Wnt/β-catenin and Notch signaling pathways dominate intestinal stem cell fate determination. However, more studies are needed to clarify this integration.
In conclusion, intestinal stem-cell differentiation is toward secretory cells 48 h after IR injury. Enterocytes inhibition is dominate; however, Wnt β/catenin pathway inhibition and Notch-related gene expression stimulation start to emerge suggest crosstalk between the two pathways.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Carden DL, Granger DN (2000) Pathophysiology of ischemia–reperfusion injury. J Pathol 190:255–266. https://doi.org/10.1002/(SICI)1096-9896(200002)190:3%3c255::AID-PATH526%3e3.0.CO;2-6
Mangino JE, Kotadia B, Mangino MJ (1996) Characterization of hypothermic intestinal ischemia–reperfusion injury in dogs. Effects Glycine Transpl 62:173–178. https://doi.org/10.1097/00007890-199607270-00005
Schoenberg MH, Poch B, Younes M et al (1991) Involvement of neutrophils in postischemic damage to the small intestine. Gut 32:905–912. https://doi.org/10.1136/gut.32.8.905
Yamamoto S, Tanabe M, Wakabayashi G et al (2001) The role of tumor necrosis factor-alpha and interleukin-1beta in ischemia–reperfusion injury of the rat small intestine. J Surg Res 99:134–141. https://doi.org/10.1006/jsre.2001.6106
Ikeda H, Suzuki Y, Suzuki M et al (1998) Apoptosis is a major mode of cell death caused by ischemia and ischemia/reperfusion injury to the rat intestinal epithelium. Gut 42:530–537. https://doi.org/10.1136/gut.42.4.530
Noda T, Iwakiri R, Fujimoto K et al (1998) Programmed cell death induced by ischemia–reperfusion in rat intestinal mucosa. Am J PhysiolGastrointest Liver Physiol 274:G270–G276. https://doi.org/10.1152/ajpgi.1998.274.2.G270
Ben-Shahar Y, Pollak Y, Bitterman A et al (2019) Sonic hedgehog signaling controls gut epithelium homeostasis following intestinal ischemia–reperfusion in a rat. Pediatr Surg Int 35:255–261. https://doi.org/10.1007/s00383-018-4406-2. (Epub 2018 Nov 1)
De Santa BP, van den Brink GR, Roberts DJ (2003) Development and differentiation of the intestinal epithelium. Cell Mol Life Sci 60:1322–1323. https://doi.org/10.1007/s00018-003-2289-3
Cheng H, Leblond CP (1974) Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. Unitarian theory of the origin of the four epithelial cell types. Am J Anat 141:537–561. https://doi.org/10.1002/aja.1001410407
Vander Flier LG, Clevers H (2009) Stem cells, self-renewal, and differentiation in the intestinal epithelium. Ann Rev Physiol 71:241–260. https://doi.org/10.1146/annurev.physiol.010908.163145
Katoh M (2007) WNT signaling pathway and stem cell signaling network. Clin Cancer Res 13:4042–4045. https://doi.org/10.1158/1078-0432.CCR-06-2316
Turashvili G, Bouchal J, Burkadze G, Kolar Z (2006) Wnt signaling pathway in mammary gland development and carcinogenesis. Pathobiology 73:213–223. https://doi.org/10.1159/000098207
van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, Tjon-Pon-Fong M, Moerer P, van den Born M, Soete G, Pals S, Eilers M, Medema R, Clevers H (2002) The beta-catenin/TCF4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 18(111):241–250. https://doi.org/10.1016/s0092-8674(02)01014-0
Riccio O, van Gijn ME, Bezdek AC, Pellegrinet L, van Es JH, Zimber-Strobl U, Strobl LJ, Honjo T, Clevers H, Radtke F (2008) Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27 Kip1and p57 Kip2. EMBO Rep 9:377–383. https://doi.org/10.1038/embor.2008.7. (Epub 2008 Feb 15)
Schroder N, Gossler A (2002) Expression of Notch pathway components in fetal and adult mouse small intestine. Gene Expr Patterns 2:247–250. https://doi.org/10.1016/s1567-133x(02)00060-1
Fre S, Huyghe M, Mourikis P, Robine S, Louvard D (2005) Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature 435:964–968. https://doi.org/10.1038/nature03589
Myers RB, Fredenburgh JL, Grizzle WE (2008) Carbohydrates. Theory and Practice of Histological Techniques(Bancroft JD, Gamble M, edi-tors). Philadelphia, PA, Churchill Livingstone Elsevier, pp 161–186
Haegebarth A, Clevers H (2009) Wntsignaling, Lgr5, and stem cells in the intestine. Am J Pathol 174:15–21. https://doi.org/10.2353/ajpath.2009.080758
Clevers H (2006) Wnt/beta-catenin signaling in development and disease. Cell 127:469–480. https://doi.org/10.1016/j.cell.2006.10.018
MacDonald BT, Tamai K, He X (2009) Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 17:9–26. https://doi.org/10.1016/j.devcel.2009.06.016
Eisenmann DM (2005) Wnt signaling. WormBook. https://doi.org/10.1895/wormbook.1.7.1
Kopan R, Ilagan MXG (2009) The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137:216–233. https://doi.org/10.1016/j.cell.2009.03.045
Bjerknes M, Cheng H (1999) Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology 116(7–14):9869596. https://doi.org/10.1016/S0016-5085(99)70222-21:STN:280:DyaK1M%2Fosl2mtA%3D%3D
Wong MH, Saam JR, Stappenbeck TS, Rexer CH, Gordon JI (2000) Genetic mosaic analysis based on Cre recombinase and navigated laser capture microdissection. Proc Natl Acad Sci USA 97(12601–12606):11050178. https://doi.org/10.1073/pnas.2302379971:CAS:528:DC%2BD3cXotFygtLo%3D
Marshman E, Booth C, Potten CS (2002) The intestinal epithelial stem cell. BioEssays 24(91–98):11782954. https://doi.org/10.1002/bies.10028
Booth D, Potten CS (2001) Protection against mucosal injury by growth factors and cytokines. J Natl Cancer Inst Monogr 29:16–20. https://doi.org/10.1093/oxfordjournals.jncimonographs.a003433
Podolsky DK (1999) Mucosal immunity and inflammation. V. Innate mechanisms of mucosal defense and repair: the best offense is a good defense. Am J Physiol. https://doi.org/10.1152/ajpgi.1999.277.3.G495
Pellegrinet L, Rodilla V, Liu Z, Chen S, Koch U, Espinosa L, Kaestner KH, Kopan R, Lewis J, Radtke F (2011) Dll1- and Dll4-mediated Notch signaling are required for homeostasis of intestinal stem cells. Gastroenterology 140:1230–1240. https://doi.org/10.1053/j.gastro.2011.01.005
Van Es JH, van Gijn ME, Riccio O, vanden Born M, Vooijs M, (2005) Notch/gamma-secretase inhibition turns proliferativecells in intestinal crypts and adenomas into goblet cells. Nature 435:959–963. https://doi.org/10.1038/nature03659
Kinzler KW, Vogelstein B (1996) Lessons from hereditary colorectal cancer. Cell 87(159–170):8861899. https://doi.org/10.1016/S0092-8674(00)81333-11:CAS:528:DyaK28XmsVGhs7g%3D
Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C et al (1992) Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science 256:668–670. https://doi.org/10.1126/science.1350108
Sansom OJ, Reed KR, Hayes AJ, Ireland H, Brinkmann H, Newton IP et al (2004) Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev 18:1385–1390. https://doi.org/10.1101/gad.287404
Andreu P, Colnot S, Godard C, Gad S, Chafey P, Niwa-Kawakita M et al (2005) Crypt-restricted proliferation and commitment to the Paneth cell lineage following Apc loss in the mouse intestine. Development 132:1443–1451. https://doi.org/10.1242/dev.01700. (Epub 2005 Feb 16)
Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ et al (1998) Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet 19:379–383. https://doi.org/10.1038/1270
Pinto D, Gregorieff A, Begthel H, Clevers H (2003) Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev 17:1709–1713. https://doi.org/10.1101/gad.267103
Ireland H, Kemp R, Houghton C, Howard L, Clarke AR, Sansom OJ et al (2004) Inducible Cre-mediated control of gene expression in the murine gastrointestinal tract: effect of loss of beta-catenin. Gastroenterology 126:1236–1246. https://doi.org/10.1053/j.gastro.2004.03.020
Kuhnert F, Davis CR, Wang HT, Chu P, Lee M, Yuan J et al (2004) Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci USA 101:266–271. https://doi.org/10.1073/pnas.2536800100
Artavanis-Tsakonas S, Rand MD, Lake RJ (1999) Notch signaling: cell fate control and signal integration in development. Science 284:770–776. https://doi.org/10.1126/science.284.5415.770
Gridley T (1997) Notch signaling in vertebrate development and disease. Mol Cell Neurosci 9:103–108. https://doi.org/10.1006/mcne.1997.0610
Nakamura T, Tsuchiya K, Watanabe MJ (2007) Crosstalk between Wnt and Notch signaling in intestinal epithelial cell fate decision. Gastroenterol 42:705–710. https://doi.org/10.1007/s00535-007-2087-z. (Epub 2007 Sep 25)
Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M et al (2000) Control of endodermal endocrine development by Hes-1. Nat Genet 24:36–44. https://doi.org/10.1038/71657
Sasai Y, Kageyama R, Tagawa Y, Shigemoto R, Nakanishi S (1992) Two mammalian helix-loop-helix factors structurally related to Drosophila hairy and Enhancer of split. Genes Dev 6:2620–2634. https://doi.org/10.1101/gad.6.12b.2620
Jarriault S, Brou C, Logeat F, Schroeter EH, Kopan R, Israel A (1995) Signalling downstream of activated mammalian Notch. Nature 377:355–358. https://doi.org/10.1038/377355a0
Gerbe F, van Es JH, Makrini L, Brulin B, Mellitzer G, Robine S, Romagnolo B, Shroyer NF, Bourgaux JF, Pignodel C, Clevers H, Jay P (2011) Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J Cell Biol 192:767–780. https://doi.org/10.1083/jcb.201010127
Ben-Shahar Y, Abassi Z, Pollak Y, Bitterman A, Kreizman-Shefer H, Koppelman T, Fuhrer AE, Hayari L, Sukhotnik I (2019) Accelerated cell turnover 48 h after intestinal ischemia is NOTCH independent. Pediatr Surg Int 35:1413–1420. https://doi.org/10.1007/s00383-019-04569-z. (Epub 2019 Oct 1PMID: 31576469)
Leow CC, Romero MS, Ross S, Polakis P, Gao WQ (2004) Hath1, down-regulated in colon adenocarcinomas, inhibits proliferation and tumorigenesis of colon cancer cells. Cancer Res 64:6050–6057. https://doi.org/10.1158/0008-5472.CAN-04-0290
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Y. B. participated in design, operated the animals, analysed and interpreted the results and wrote the main manuscript text, V. V. and K. K. participated in design and operated the animals, Y. P. analysed and interpreted the results, I. S. participated in design, operated the animals, analysed and interpreted the results and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ben-Shahar, Y., Vasserman, V., Pollak, Y. et al. The mechanism of intestinal stem cells differentiation after ischemia–reperfusion injury in a rat model. Pediatr Surg Int 40, 23 (2024). https://doi.org/10.1007/s00383-023-05610-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s00383-023-05610-y