Abstract
Purpose
One of the major regulators of gastrointestinal tract development is the hedgehog signaling pathway. The purpose of this study was to evaluate the role of sonic hedgehog (SHh) signaling 24 and 48 h following intestinal ischemia–reperfusion (IR) in a rat.
Materials and methods
Male rats were divided into four experimental groups: (1) Sham-24 h rats underwent laparotomy and were sacrificed after 24 h, (2) Sham-48h rats underwent laparotomy and were sacrificed after 48 h, (3) IR-24h rats underwent occlusion of both superior mesenteric artery and portal vein for 20 min followed by 24 h of reperfusion, and (4) IR-48 h rats underwent ischemia for 20 min followed by 48 h of reperfusion. Intestinal structural changes, enterocyte proliferation and enterocyte apoptosis were determined by immunohistochemistry 24 and 48 h following IR. SHh-related genes and protein expression were determined using real-time PCR, Western blot and immunohistochemistry.
Results
IR-24 rats demonstrated a significant decrease in Shh, Ihh, GIL and Ptch2 mRNA in jejunum and ileum compared to Sham-24 animals that was accompanied by a significant decrease in the number of SHH-positive cells (Immunohistochemistry) in jejunum (2.5-fold decrease) and ileum (37%). After 48 h, IR rats demonstrated a significant increase in Dhh, Ihh, Gil and PTCH2 mRNA in jejunum as well as in Dhh, Ihh, SMO, GIL, PTCH2 mRNA in ileum compared to IR-24 animals that was coincided with increased number of SHH-positive cells in jejunum (2.6-fold increase) and ileum (1.4-fold increase).
Conclusions
24 h following intestinal IR, inhibited cell turnover was associated with inhibited SHh signaling pathway. Signs of intestinal recovery appeared 48 h after IR and were correlated with increase in SHh signaling pathway activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intestinal ischemia–reperfusion (IR) injury is known to give rise to biochemical alterations like formation of radical oxygen species (ROS) [1] and changes in lipid mediator synthesis [2, 3]. The increased production of ROS during ischemia donates to damage of the endothelium [4] and increased epithelial permeability [5] which results in gut barrier dysfunction. Further, there is release of several cytokines and other mediators by polymorphonuclear leucocytes and mast cells infiltrated into intestinal wall causing systemic inflammatory response which could lead to multiple organ failure and death [6, 7]. Recent evidence has shown that IR induces apoptosis which becomes the principal contributor to cell death after IR [8, 9].
The regulation of cell number in the gastrointestinal tract is determined by the balance between enterocytes’ production, differentiation and death via apoptosis. Stem cell proliferation and differentiation which is regulated by multiple signaling pathways is responsible for the regeneration of gastrointestinal epithelium. Several signaling pathways (Wnt/b-catenin, hedgehog, bone morphogenetic protein (BMP), and Notch signaling pathway) [10] are implicated in the control of stem cells activity. Wnt pathway is known to be the main regulator of cell proliferation [11, 12].
Intestinal cell turnover after IR is regulated by shared interactions between the epithelium and the underlying mesenchymal stroma. At the beginning of intestinal villi formation, mesenchymal cells are concentrated close to the epithelium in the estimated sites of new villi to be created. Stem cells situated deeply in the intestinal crypts, start to migrate to the lumen as they differentiate forming the villi epithelium. At the end of the process, these cells are shed either from villi tip or from intestinal epithelium into the lumen. The interaction between the epithelium and extracellular matrix is achieved by adhesions proteins called integrins [13]. Growing evidence suggests that the hedgehog (Hh) signaling pathway is an important mediator of these interactions [14]. Vertebrates express three Hh homologues: desert hedgehog (DHh), Indian hedgehog (IHh), and sonic hedgehog (SHh), of which SHh and IHh are expressed by the intestinal epithelium [15]. The communication between mesenchymal (stromal) cells and epithelial cells in the intestines is carried out by SHh signaling through the expression of bone morphogenetic proteins 2 and 4 (BMP2 and BMP4) which are members of the TGF-β superfamily [16]. The BMP receptors are expressed on the intestinal epithelial cells and mediate signaling through intracellular matrix messenger molecules, SMADs [17].
The purpose of the present study was to evaluate the role of SHh signaling on intestinal stem cells activity and its relation to intestinal cell turnover 24 and 48 h following intestinal IR in a rat.
Materials and methods
Animals
All experiments were done according to the standards for care and use of animal subjects as stated in the Guide for the Care and Use of Laboratory Animals (Rappaport Faculty of Medicine, Technion, Haifa, Israel). Male Sprague–Dawley rats weighing 250–300 g were possessed in a 12-h day and night cycles at 21 °C for 3–5 days before the experiment. The rats were pair fed with standard chow and had free access to water. Animals were fasted for 24 h before the experiment except free access to water.
Experimental design
Animals were divided randomly into four groups: Group A-Sham-24 rats underwent laparotomy with identification and isolation of the superior mesenteric artery (SMA) and portal vein (PV) without vascular occlusion and were sacrificed 24 h after operation; Group B-IR-24 underwent laparotomy and vascular occlusion of both SMA and PV for 20 min followed by 24 h of reperfusion; Group C-Sham-48 rats underwent laparotomy and were sacrificed 48 h after operation, and Group D-IR-48 rats underwent intestinal IR followed by 48 h of reperfusion.
Surgical procedure
Following an overnight fast, the animals were anaesthetized with an intraperitoneal injection of ketamine (90 mg/kg) and xylasine (15 mg/kg). The abdomen was opened through a midline incision. In sham rats, the SMA and PV were isolated without clamping. In IR animals, atraumatic microvascular clamps were used to occlude the SMA and PV for 20 min. During the period of ischemia, the abdominal wall incision was kept approximated to prevent fluid and heat loss. After 20 min of intestinal ischemia the incision was reopened, the clamp was removed, and the ischemic gut was reinserted into the abdomen. Before closure of the abdomen, the rats were resuscitated with a 3 ml intraperitoneal injection of warm 0.9% saline. The abdominal cavity was closed in two layers with a running suture of 3/0Vicryl (Ethicon Corporation, USA) for all operations. The rats were fasted for 6 h but were allowed free access to water following surgery. All animals were sacrificed 24 (Groups A and B) or 48 (Groups C and D) hours after operation. The small bowel was rapidly removed, rinsed with cold isotonic saline and divided into two segments: proximal jejunum and terminal ileum. Each segment was weighed, cut longitudinally and the mucosa was scraped using a glass slide, collected and weighed. Histologic sections were prepared from the proximal jejunum and distal ileum and from comparable sites in control animals.
Intestinal histology
Segments of small bowel were fixed for 24 h in 4% buffered formalin and processed into standard paraffin blocks. Five-micron tissue slices were stained with hematoxylin–eosin. Intestinal mucosal damage was evaluated by two expert investigators blinded to the experimental groups using the criteria of Park [18]. A score scaled at 0–8 represents the severity: 0—normal mucosa, 1—subepithelial space at villus tip, 2—more extended subepithelial space, 3—epithelial lifting along villus sides, 4—denuded villi, 5—loss of villus tissue, 6—crypt layer infarction, 7—transmucosal infarction, 8—transmural infarction.
Immunohistochemistry
Rats were injected with standard 5-bromodeoxyuridine (5-BrdU) labeling reagent (Zymed Lab, Inc, CA) at a dose of 1 ml per 100 g body weight 90 min before sacrifice to detect enterocyte proliferation. Five-micrometer paraffin-embedded slices (5 µm) were deparaffinized with xylene, rehydrated with graded alcohol, and stained with a biotinylated monoclonal anti-BrdU antibody system using BrdU Staining Kit (Zymed Lab, Inc, CA). An index of proliferation was determined as the ratio of crypt cells staining positively for BrdU per 10 crypts.
Immunohistochemistry for Caspase-3 (Caspase-3 cleaved concentrated polyclonal antibody; dilution 1:100; Biocare Medical, Walnut Greek, CA) was performed for identification of apoptotic cells using a combination of streptovidin–biotin–peroxidase method and microwave antigen retrieval on formalin-fixed, paraffin-embedded tissues according to the manufacturers’ protocols. For each group, the number of stained cells was counted in at least ten villi in areas without necrosis. The apoptotic index (AI) was defined as the number of apoptotic cells per ten villi.
To detect SHh signaling pathway activity, immunohistochemistry for SHH (E-1) (SHH monoclonal antibody, dilution 1:50, sc-365112) was performed using a combination of the streptovidin–biotin–peroxidase method and microwave antigen retrieval on formalin-fixed, paraffin-embedded tissues according to the manufacturer’s protocols.
Real-time PCR
RNA was isolated using Trizol (Invitrogen) reagent according to manufacturer’s protocol and quantification of RNA was performed using 260/280 nm spectrophotometry. Thereafter, reverse transcriptase (PrimeScript RT reagent Kit TaKaRa,Japan) was used to convert 500 ng of total RNA into complementary DNA (cDNA) which was then amplified by PCR-Thermal Cycler(2720 Thermal Cycler, ABI, Israel). Gene Expression of Dhh, Shh, Ihh, Smo, Gil and Ptch2 mRNA was determined by quantitative real-time PCR ABI-PRISM 7000 (applied Biosystems, Foster City, CA) on cDNA samples using Cyber Green Master Mix (Takara, Japan) with the exception of template and primers.
Western blotting
Protein extraction from rat ileal tissue samples was performed by homogenizing in RIPA lysis buffer containing 50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 1% NP-40, 2 mM EDTA, supplemented with a cocktail of protease (Roche Diagnostic) and phosphatase cocktail inhibitors (Sigma). The homogenate was centrifuged at 7500 rpm at 4 °C for 15 min and the supernatant was collected. The protein concentrations were determined by Bradford reagent according to manufacturer’s instructions. Samples containing equal amounts of total protein (30 µg) were resolved by SDS–PAGE under reducing conditions. After electrophoresis, proteins were transferred to PVDF membrane and probed with anti-SHH antibody (1:100 dilution, sc-365112), and anti-tubulin antibody (1:1000 dilution, sc-53029). Horseradish peroxidase-conjugated secondary antibody was purchased from Jackson Immuno Research Laboratories Inc. (West Grove, PA) and an enhanced chemiluminescent substrate from Biological Industries (Kibbutz Beth Ha-Emek, Israel).
Statistical analysis
The data are expressed as the mean ± SEM. Statistical analysis of parameters of adaptation, enterocyte proliferation, and apoptosis was performed using Kruskal–Wallis test followed by post hoc test for multiple comparisons with p values of less than 0.05 considered statistically significant.
Results
Microscopic appearance (intestinal damage)
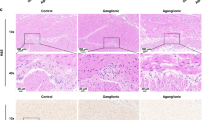
Whereas the jejunum and the ileum of sham animals exhibited normal mucosal architecture with intact villi, IR animals exhibited a significant histologic injury measured by Park’s intestinal injury score [18] in both jejunum and ileum (Fig. 1). IR-24 rats (Group B) demonstrated a significant increase in the mean intestinal injury grade in jejunum (2.5-fold increase, p < 0.001) and ileum (threefold increase, p < 0.001) compared to sham-24 animals (Group A). 48 h following operation, IR rats (Group D) demonstrated a less significant intestinal injury. Although injury score in this group remained higher in ileum (twofold increase, p = 0.002) compared to sham-48 rats, IR-48 rats (Group D) showed a significantly lower histologic score grade in jejunum (twofold decrease, p = 0.01) and ileum (28%, p < 0.05) when compared to IR-24 animals (Group B).
Effect of intestinal IR on intestinal damage score (Park’s injury score). As expected, Sham rats demonstrated a normal histologic architecture. IR rats showed extended subepithelial space and epithelial lifting along the villis sides (Park score 2–3). IR-48 rats exhibited a less marked Park score compared to IR-24 animals. Values are mean ± SEM. IR ischemia–reperfusion. *p < 0.05 IR vs Sham rats, †p < 0.05 IR-48 vs IR-24 rats
Enterocytes’ proliferation and apoptosis
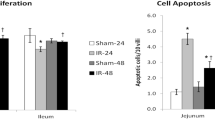
IR-24 (Group B) resulted in a significant decrease in the enterocyte proliferation index in jejunum (125 ± 4 vs 163 ± 16 BrdU positive cells/10 crypts, p < 0.05) and ileum (130 ± 5 vs 160 ± 15 BrdU positive cells/10 crypts, p < 0.05) compared to Sham-24 animals (Group A) (Fig. 2). Although IR-48 rats showed a trend toward decrease in the rates of cell proliferation in jejunum and ileum compared to Sham-48 animals, this trend was not statistically significant. The rates of cell proliferation increased significantly in jejunum (153 ± 7 vs 125 ± 4 BrdU positive cells/10 crypts, p < 0.05) and ileum (146 ± 2 vs 130 ± 5 BrdU positive cells/10 crypts, p < 0.05) of IR-48 rats (Group D) compared to IR-24 animals (Group B).
Effect of intestinal IR on crypt cell proliferation and cell apoptosis. 5-BrdU incorporation into proliferating jejunal and ileal crypt cells was detected with a goat anti-BrdU antibody. Immunochemistry for caspase-3 was used to determine enterocyte apoptosis. Values are mean ± SEM. IR—ischemia–reperfusion. *p < 0.05 IR vs Sham rats, †p < 0.05 IR-48 vs IR-24 rats
The number of apoptotic cells increased significantly 24 h following IR (Group B) in jejunum (fourfold increase, p < 0.05) and ileum (threefold increase, p < 0.05) compared to sham animals (Group A) (Fig. 2). Although IR-48 rats (Group D) demonstrated still greater rates of cell apoptosis in jejunum (twofold increase, p < 0.05) and ileum (50% increase, p < 0.05) compared to Sham-48 animals (Group C), the number of apoptotic cells in this group was lower (twofold decease in jejunum, p < 0.05) and 20% decrease, NS in ileum) compared to IR-24 animals (Group B).
Expression of Hh signaling-related genes (Real-Time PCR)
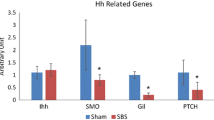
Analysis of Hh-related gene expressions by real-time PCR (Fig. 3) reveals a significant decrease in expression of Shh (fivefold decrease, p < 0.05), Ihh (25% decrease, p < 0.05), Gil (threefold decrease, p < 0.05) and Ptch2 (30% decrease, p < 0.05) mRNA levels in the jejunum, as well as in Dhh (threefold decrease, p < 0.05), Shh (eightfold decrease, p < 0.05), Ihh (30% decrease, p < 0.05), Gil (fourfold decrease, p < 0.05), Smo (2.5-fold decrease, p < 0.05) and Ptch2 (30% decrease, p < 0.05) mRNA levels in the ileum of IR-24 rats compared to sham animals. 48 h following IR, there was a significant increase in Dhh (twofold increase, p < 0.05), Shh (threefold increase, p < 0.05), Ihh (threefold increase, p < 0.05), Gil (threefold increase, p < 0.05), and Ptch2 (2.5-fold increase, p < 0.05) mRNA levels in the jejunum, as well as in Dhh (threefold increase, p < 0.05), Shh (fivefold increase, p < 0.05), Smo (2.5-fold increase, p < 0.05), Gil (tenfold increase, p < 0.05), and Ptch2 (sixfold increase, p < 0.05) mRNA levels in the ileum compared to IR-24 animals.
Expression of SHH protein levels (Western blot)
An increased cell proliferation rates in IR-48 rats (Group D) compared to IR-24 animals was accompanied by a significant increase in SHH protein levels in ileum (threefold increase, p < 0.05) compared to IR-24 animals Group B (Fig. 4). The increased levels of SHH protein in this group was also significantly higher compared to Sham-48 animals (2.2-fold increase, p < 0.05). There was not significant difference in jejunal expression of SHH protein in IR-48 compared to sham 48 h.
Immunohistochemistry
A marked decrease in number of SHH-positive cells was demonstrated in the villi of jejunum (2.5-fold decrease, p < 0.05) and ileum (60% decrease, p < 0.05) of IR-24 rats compared to sham-24 animals (Fig. 5). After 48 h, there was a significant increase in number of SHH-positive cells in villi of both jejunum (threefold increase, p = 0.003) and ileum (40% increase, p < 0.05) of IR-48 rats compared to IR-24 animal.
Discussion
Intestinal IR is a challenging situation with a complex mechanism causing a hazardous damage to the tissue. Initially, the injury to the tissue is caused by decrease in blood supply, which leads to decrease in oxygen delivery, anaerobic metabolism and production of reactive oxygen radicals. Later, there is an accumulation of cytokines, chemokines and oxygen radical species during reperfusion, which is thought to be the reparative phase of the process [7, 8]. These molecules activate inflammatory cells which damage the gut barrier, increase its permeability and translocation of bacteria and may lead to systemic inflammatory response and multiple organ failure [7, 10]. This aggressive inflammatory response, which is known as apoptosis or “programmed cell death”, has been recognized in recent studies to be the key process in intestinal cell death following IR insult [8, 9].
Hedgehog (Hh) signaling pathway is known as an important regulator of tissue growth and cell differentiation in mammal development [15]. According to several studies Hh signaling pathway has a definitive role in several important processes in gastrointestinal tract like regulation of stem cell activity [15, 16], interactions between epithelium and mesenchyme during formation of intestinal villi and proliferation of intestinal epithelium [14, 19].
There are three Hh homologues in mammals, sonic hedgehog (SHh), Indian hedgehog (IHh) and desert hedgehog (DHh). IHh and SHh are known for their important role in intestinal cell proliferation and differentiation. Inhibition of these pathways results in hypoproliferation of intestinal epithelium [15] and consequently inhibition of intestinal epithelial differentiation leading to impaired intestinal absorption [20]. IHh was also discovered previously as an inhibitor of another signaling cascade Wnt that is the main regulator of intestinal cell proliferation [17].
The role of Hh signaling pathway on intestinal stem cells activity following intestinal IR injury has not been previously investigated. We designed the current study to evaluate the role of SHh signaling cascade as a regulator of stem cells activity and its relation to intestinal cell turnover 24 and 48 h following intestinal IR injury in a rat model.
Similar to our previous studies [21, 22], intestinal IR in the current experiment caused a significant mucosal injury after both 24 (Group B) and 48 (Group D) hours of reperfusion. This is evident from the increased Park intestinal injury score, which was observed in both jejunum and ileum; however, the damaging effect was more significant in the ileum. Less significant damage to the proximal jejunum may have been due to collateral blood supply from the celiac axis. In addition, intestinal IR led to intestinal mucosal hypoplasia. IR-24 rats demonstrated a significant decrease in the enterocyte proliferation in jejunum and ileum and concomitant increase in cell apoptosis in jejunum and ileum suggesting inhibited cell turnover. After 48 h, the rates of cell proliferation increased significantly in jejunum and ileum compared to IR-24 animals and was accompanied by a significant decrease in cell apoptosis suggesting increase in cell turnover. Although necrosis is responsible for the intestinal cell death during the ischemic phase, apoptosis or “programmed cell death” has recently been recognized to be a key factor in enterocyte turnover and gut barrier function following an IR insult [23]. ROS and cytokine release during an IR event and intracellular regulatory proteins (e.g., Bcl-2, Bax, Fas, p53) were identified to be involved in apoptosis induction or reduced expression [24]. The reduction of apoptosis and stimulation of cell proliferation and differentiation following IR injury may be an useful therapeutic goal in the future.
Analysis of hedgehog-related gene expressions by real-time PCR has shown a significant decrease in expression of Shh, Ihh, Gil and Ptch2 mRNA levels in the jejunum, as well as in Dhh, Shh, Smo, Gil and PTCH2 mRNA levels in the ileum of IR-24 rats compared to sham animals. IR-48 rats demonstrated a significant increase in Dhh, Shh, Ihh, Gil and PTCH2 mRNA levels in the jejunum, as well as in Dhh, Ihh, Smo, Gil, PTCH2 in the ileum compared to IR-24 animals. In the canonical Shh pathway, the absence of the Shh ligand leads the transmembrane receptor Patched (Ptch) to inhibit the transmembrane receptor Smoothened (Smo). Inhibited Smo causes cleavage of Gli (protein originally isolated in human glioblastoma) to the N-terminal repressor form. Therefore when SHh binds to Ptch, the inhibitory effect on Smo is released and active full-length Gli is transported into the nucleus and activates transcription of Gli-dependent target genes such as Gli1, Ptch1, CyclinD1 and Wnt [25]. Analysis of SHH protein expression was performed by Western blot analysis. 48 h after IR, animals demonstrated a significant up-regulation of SHH protein levels compared to Sham-48 animals that correlated with up-regulated mRNA levels.
A marked decrease in number of SHH-positive cells was demonstrated in the villi of jejunum of IR-24 rats compared to Sham-24 animals. After 48 h, there was a significant increase in number of SHH-positive cells in villi of both jejunum and ileum of IR-48 rats compared to IR-24 animal that was in agreement with increased hedgehog-related gene and protein levels. These changes were correlated with enhanced cell proliferation rates in IR-48 (compared to IR-24) animals. Recent evidence suggests Hh signaling plays a critical role in the regulation of regenerative stem cell proliferation in midguts from fruit fly to human [26]. Depleting Hh from the precursor cells blocks dextran sodium sulfate-stimulated intestinal stem cells (ISCs) proliferation, suggesting that local Hh production is critical for stimulating ISC proliferation.
In conclusion, in a rat model of intestinal IR regulation of stem cell activity is associated with activation of sonic hedgehog pathway. Inhibited cell proliferation 24 h after IR was associated with inhibited SHh signaling cascade, while activated cell turnover 48 h followed IR was correlated with the stimulated SHh pathway. It should be emphasized that hedgehog signaling is essential for intestinal homeostasis and is associated with tissue repair.
References
Carden DL, Granger DN (2000) Pathophysiology of ischemia-reperfusion injury. J Pathol 190:255–266
Kurose I, Argenbright LW, Wolf R et al (1997) Ischemia/reperfusion-induced microvascular dysfunction: role of oxidants and lipid mediators. Am J Physiol 272:H2976–H2982
Mangino MJ, Mangino JE, Murphy MK et al (1996) Arachidonic acid metabolism in intestinal hypothermic preservation injury. Cryobiology 33:404–412
Wolin MS (2000) Interactions of oxidants with vascular signaling systems. Arterioscler Thromb Vasc Biol 20:1430–1442
Mangino JE, Kotadia B, Mangino MJ (1996) Characterization of hypothermic intestinal ischemia-reperfusion injury in dogs. Effects of glycine. Transplantation 62:173–178
Schoenberg MH, Poch B, Younes M et al (1991) Involvement of neutrophils in postischemic damage to the small intestine. Gut 32:905–912
Yamamoto S, Tanabe M, Wakabayashi G et al (2001) The role of tumor necrosis factor-alpha and interleukin-1beta in ischemia-reperfusion injury of the rat small intestine. J Surg Res 99:134–141
Ikeda H, Suzuki Y, Suzuki M et al (1998) Apoptosis is a major mode of cell death caused by ischemia and ischemia/reperfusion injury to the rat intestinal epithelium. Gut 42:530–537
Noda T, Iwakiri R, Fujimoto K et al (1998) Programmed cell death induced by ischemia-reperfusion in rat intestinal mucosa. Am J Physiol Gastrointest Liver Physiol 274:G270–G276
De Santa Barbara P, van den Brink GR, Roberts DJ (2003) Development and differentiation of the intestinal epithelium. Cell Mol Life Sci 60:1322–1323
Haegebarth A, Clevers H (2009) Wnt signaling, Lgr5, and stem cells in the intestine. Am J Pathol 174:15–21
Van de Wetering M, Sancho E, Verweij C (2002) The β-catenin/TCF-4 complex impoves a crypt progenitor phenotype on colorectal cancer cells. Cell 111:241–250
Sukhotnik I, Dorfman T, Halabi S et al (2016) Accelerated intestinal epithelial cell turnover after bowel resection in a rat is correlated with inhibited hedgehog signaling cascade. Pediatr Surg Int 32:1133–1140
Roberts DJ, Smith DM, Goff DJ et al (1998) Epithelial-mesenchymal signaling during the regionalization of the chick gut. Development 125:2791–2801
van den Brink GR (2007) Hedgehog signaling in development and homeostasis of the gastrointestinal tract. Physiol Rev 87:1343–1375
Bitgood MJ, McMahon AP (1995) Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol 172:126–138
Iwamoto M, Hoffenberg EJ, Carethers JM et al (2005) Nuclear accumulation of beta-catenin occurs commonly in the epithelial cells of juvenile polyps. Pediatr Res 57:4–9
Park PO, Haglund U, Bulkley GB et al (1990) The sequence of development of intestinal tissue injury after strangulation ischemia and reperfusion. Surgery 107:574–580
Madison BB, Braunstein K, Kuizon E, Portman K, Qiao XT, Gumucio DL (2005) Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development 132:279–289
Wang LC, Nassir F, Liu ZY, Ling L, Kuo F, Crowell T, Olson D, Davidson NO, Burkly LC (2002) Disruption of hedgehog signaling reveals a novel role in intestinal morphogenesis and intestinal-specific lipid metabolism in mice. Gastroenterology 122:469–482
Haj B, Sukhotnik I, Shaoul R, Pollak Y, Coran AG, Bitterman A, Matter I (2014) Effect of ozone on intestinal recovery following intestinal ischemia-reperfusion injury in a rat. Pediatr Surg Int 30:181–188
Sukhotnik I, Slijper N, Pollak Y, Chemodanov E, Shaoul R, Coran AG, Mogilner JG (2011) Parenteral omega-3 fatty acids (Omegaven) modulate intestinal recovery after intestinal ischemia-reperfusion in a rat model. J Pediatr Surg 46:1353–1360
Noda T, Iwakiri R, Fujimoto K, Matsuo S, Aw TY (1998) Programmed cell death induced by ischemia-reperfusion in rat intestinal mucosa. Am J Physiol 274:G270–G276
Coopersmith CM, O’Donnell D, Gordon JI (1999) Bcl-2 inhibits ischemia-reperfusion-induced apoptosis in the intestinal epithelium of transgenic mice. Am J Physiol 276:G677–G682
Hirata-Tominaga K Nakamura T, Okumura N, Kawasaki S, Kay EP, Barrandon Y, Koizumi N, Kinoshita S (2013) Corneal endothelial cell fate is maintained by LGR5 through the regulation of hedgehog and Wnt pathway. Stem Cells31:1396–1407
Tian A, Jiang J (2015) Hedgehog fuels gut regeneration. J Cell Biol 208:807–819
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not- for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ben-Shahar, Y., Pollak, Y., Bitterman, A. et al. Sonic hedgehog signaling controls gut epithelium homeostasis following intestinal ischemia–reperfusion in a rat. Pediatr Surg Int 35, 255–261 (2019). https://doi.org/10.1007/s00383-018-4406-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-018-4406-2