Abstract
Background
Craniopharyngioma has historically been recognized to be a formidable pathology primarily due to its proximity to critical neurovascular structures and the challenging surgical corridors that surgeons have tried to reach this lesion.
Focus of review
In this work, we review the medical and surgical management of these tumors with a focus on clinical presentation, diagnostic identification, surgical approach, and associated adjuvant therapies. We will also discuss our current treatment paradigm using endoscopic, open, and combined approaches to craniopharyngiomas.
Summary
The management of craniopharyngiomas requires a multidisciplinary team of surgeons, endocrinologists, and neuroanesthesiologists as well as neurocritical care specialists to deliver the most comprehensive and safest surgical resection with minimal postoperative morbidity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Craniopharyngiomas (CP) are one of the most challenging childhood brain tumors. Their location in the sellar and suprasellar region frequently causes compression of adjacent critical neurovascular structures and makes them a defiant clinical and surgical entity. Although it is considered histologically benign, it remains a potentially devastating diagnosis—functionally and clinically—for the affected child.
Epidemiology
Craniopharyngiomas (CP) have an overall annual incidence in the USA of 0.13–0.18 per 100,000 persons and constitute about 6–9% of pediatric brain tumors [1, 2]. CP manifests either in children (33–35% of CPs), or older adults [1, 3]. Childhood CPs are more common in children aged 6–10 years followed by 11–15 years [4]. Published series have not demonstrated a clear gender association [2, 5].
Presentation
Clinical manifestations in children with craniopharyngiomas generally correlate with a slow growth pattern, size, and location of the lesion. The primary chief complaint of 60–75% of patients is headache, suggestive of increased intracranial pressure [4, 6]. Complaints of difficulty seeing the school board or sitting closer to the television suggest the presence of visual deficits, which occur in half of patients [6,7,8]. Otherwise, evidence of growth failure, obesity, delayed puberty, and diabetes insipidus related to compression and/or invasion of the hypothalamo-pituitary axis are generally discovered in 20–50% of patients as part of the general endocrinologic evaluation [9]. In cases where obstructive hydrocephalus or compression of the frontal lobes result from a larger lesion, subtle functional decline can be observed with psychomotor slowing or decline in school performance. There can be a significant delay in establishing diagnosis in some due to the slow growth of these tumors [10]. Also, presentation varies in patients based on their ages, with young children manifesting with general symptoms of increased intracranial pressure and hydrocephalus, while adolescents and young adults are diagnosed due to visual deficits and hypopituitarism [10,11,12,13].
Diagnostic evaluation
Imaging
Modern imaging technology such as computed tomography (CT) and magnetic resonance imaging (MRI) offer excellent characterization of CPs. CT scans demonstrate calcifications in up to 90% of pediatric CPs [14, 15]. In general, mixed solid and cystic components of the tumor will usually appear hypodense in comparison to the surrounding cerebral parenchyma. Figure 1 demonstrates a case of CP with mixed solid and cystic components. Fluid within the tumoral cysts will generally be of a slightly greater density than cerebrospinal fluid due to the higher protein content. Also, CT scan can illustrate secondary skull base changes such as enlargement of the sella turcica or erosion of the dorsum sellae or reveal important calcifications that assist in making a diagnosis [15, 16]. These potential alterations in the skull base anatomy are important to note when planning a surgical approach.
Critical information about the tumor and its surrounding neurovascular structures are best seen on MRI. Figure 2 demonstrates a case example a CP with a largely solid component compressing critical neurovascular structures superiorly. Detailed evaluation of the location of the optic nerves and chiasm, pituitary gland and stalk, hypothalamus, circle of Willis vascular anatomy, brainstem, frontal and temporal lobes, as well as ventricles is necessary to determine the safest surgical corridor. Cystic components are generally homogeneously hyperintense on T2-weighted images (WI), and generally hypointense but can vary on T1-WI. Solid portions will have similar appearance as other brain tumors with iso- or hypointense signal on T1-WI, and variable signal on T2-WI [15, 17, 18]. The cyst wall and solid portion typically enhance after intravenous contrast injection [17]. Calcifications are not reliably seen on MRI and can be mistaken for flow voids [19]. Magnetic resonance angiography (MRA) can provide information on the relationship between the tumor and critical vascular structures. Finally, magnetic resonance spectroscopy (MRS) can identify characteristic elevated peaks of lactate or lipids, with only small amounts of other metabolites. This can help differentiate them from gliomas (pattern of choline, n-acetylaspartate, and creatine peaks), and pituitary adenomas (choline peaks, or no elevated metabolites) [20].
Ophthalmologic evaluation
As mentioned, abnormal ophthalmologic findings are present in the majority of patients. Visual acuity and visual field deficits are diagnosed in up to 70–80% of patients during initial assessments [8, 21]. The classically described bitemporal hemianopia from compression of the optic chiasm is present in about half of patients. However, this will vary based on the position of the chiasm in relationship to the tumor (anterior vs. posterior) as well as asymmetric extension of the tumor [22]. Papilledema, reflective of increased intracranial pressure, will manifest in approximately 20% of patients [8].
Endocrinologic evaluation
The hypothalamo-pituitary function is generally compromised in some capacity in the majority of patients. Deficiencies in growth hormone and gonadotropins are reported in 75% and 60% of patients, respectively [10, 23]. A complete and thorough endocrinologic evaluation is extremely important to recognize and correct adrenal dysfunction and/or thyroid dysfunction, which are present in close to a third of patients. Diabetes insipidus, although less frequent, is seen in 10–20% of patients [10, 23]. These deficiencies can result in severe perioperative complications if not addressed properly.
Pathology
According to the World Health Organization classification, CPs are grade I tumors [24]. Two different histopathological subtypes of CPs have been described; adamantinomatous (aCP) and papillary (pCP) [25, 26]. It has long been hypothesized that, although they share common histological features, both entities have different pathogenesis. Recent genetic analyses also support this by demonstrating genetic alterations specific to each subtype [27,28,29,30,31,32,33,34].
The aCP is thought to develop after neoplastic transformation of embryonic epithelial remnants of the Rathke’s pouch, or craniopharyngeal duct [35, 36]. During normal development, the anterior pituitary gland is formed by the ectodermal epithelium that forms the roof of the stomadeum (also known as Rathke’s diverticulum). In parallel, the neuroepithelium from the floor of the third ventricle (infundibular region) forms the posterior pituitary gland and infundibular stalk. Both the ectodermal epithelium and neuroepithelium then come in contact in the sella to form the pituitary gland. Rathke’s pouch stretches and constricts as it forms the craniopharyngeal duct during gestational week 5. Afterwards, it disconnects from the oral epithelium and involutes between the sixth and eighth week. It is thought that remnants of this duct may be the origin of aCPs [30, 37].
Genetic analysis has identified beta-catenin gene mutations in 63–100% of the aCP subtypes [38]. The beta-catenin gene codes for CTNNB1, an adherens junction protein involved in signaling of the Wnt pathway, known to regulate cell proliferation and involved in embryology and tissue development. Dysregulation of this pathway leads to uncontrolled cell proliferation and has been associated with many other types of neoplastic entities [39]. In its normal state, CTNNB1 is phosphorylated by glycogen synthase kinase 3Beta (GSK3B), which leads to the degradation of this protein, stopping it from entering the nucleus to promote cell proliferation. The mutated CTNNB1 protein accumulates within the nucleus and upregulates the signaling pathway, leading to uncontrolled cell proliferation [28, 40]. The identification of nuclear beta-catenin protein in the pathological analysis of a sellar region tumor allows to differentiate aCPs from other tumors [41].
The adamantinomatous subtype is the most common subtype overall and can occur at any age. Childhood craniopharyngiomas are almost exclusively the adamantinomatous subtype [4, 7, 11]. On histopathological evaluation, it generally has three layers: (1) inferior or basal layer composed of small cells, (2) a middle layer formed of loose stellate cells, and (3) top layer of larger, keratinized, and flat cells along the cyst lumen. This last layer can lead to the formation of keratin nodules (“wet” keratin) and occasionally accumulate calcium salts [26]. The cyst fluid also contains desquamated epithelial cells and cholesterol, giving the fluid its characteristic “motor oil” appearance. The solid component of the tumor can also have microscopic “finger-like” invasion of the surrounding neural tissue. The latter can demonstrate a gliotic reaction leading to reactive astrocytes and Rosenthal fibers [26]. It has been suggested that this gliotic tissue allows a surgical plane between brain and tumor leading to increased extent of resection [11]. However, invasion of critical neural structures such as the hypothalamus has also led to devastating morbidity in aggressive surgical resections [42].
In contrast to aCP, the squamous papillary CP subtype is almost exclusively seen in adults and frequently presents as a solid tumor with little mineralization. On histopathological analysis, epithelial cells in pseudopapillary architecture are seen with a similar appearance to metaplastic respiratory epithelium. “Wet” keratin nodules are typically absent [43]. In contrast to the embryonic origin of the aCP, the pCP is thought to form from metaplasia or somatic mutations of differentiated cells [44]. In 57% to 100% of pCPs, a BRAF V600E (Val 600 Glu) mutation has been identified and appears to be exclusive to this subtype. This BRAF mutation leads to uncontrolled activation of the mitogen-activated protein kinase (MAPK) pathway and cell proliferation. This mutation has been linked to the development of many other tumors including malignant melanoma and papillary thyroid carcinoma and has also led to the development of interesting new targeted therapies that could potentially apply to pCPs [45, 46].
Management
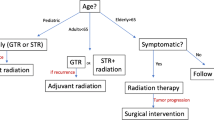
CPs are benign and rarely undergo malignant transformation. However, their proximity to vital neurovascular structures make complete extirpation of the mass challenging and recurrence of craniopharyngiomas remains a significant problem. Complete surgical resection of the tumor can be curative; however, resection with irradiation of residual tumor is a common treatment algorithm when complete resection cannot be attained with acceptably low risk to these critical structures. Adjuvant therapies such as radiosurgery or intracystic/local therapy may also have a role in select tumors and patients [47].
Surgical management
Prior authors have demonstrated that the most important factor in long-term disease control is extent of resection [47]. However, there is no consensus on the ideal approach to CP surgery. A number of prospective and retrospective studies have demonstrated that the 10-year recurrence rate following gross total resection (GTR) is 0–50% and 25–100% with subtotal resection (STR) [42, 48]. Some advocate STR with postoperative radiation, which will be discussed in detail. Regardless of the goals of surgical resection (GTR versus STR with radiation), balance must be obtained between resecting as much tumor as possible while preserving nearby neurovascular structures [49]. The approach to CP surgery in which the surgical target is at the skull base should be determined on a case by case basis according to patient factors (development of sinuses, ability to tolerate general anesthesia, size, and anatomic location of the tumor), surgeon experience, and goals of the procedure [47].
Open approaches
A number of open transcranial approaches exist to access the skull base and multiple modifications for every technique have been described. For the purpose of this review, we will categorize open techniques into anterior, lateral, and intraventricular techniques [47, 50]. These techniques can be combined or utilized with endoscopic techniques to address specific anatomic subsites if necessary.
Anterior
Anterior approaches can be further subcategorized into anteromedial and anterolateral approaches. Anteromedial visualization has been advocated for large craniopharyngiomas residing posterior to the optic chiasm and superior in the hypophyseal axis with extension into the third ventricle [50, 51]. An anteromedial view can be obtained using a bifrontal transbasal or frontobasal interhemispheric approach. These exposures generally require bicoronal incision, removal of bifrontal bone flap, and potentially obliteration of the frontal sinus with pericranial flap depending on surgeon preference. Authors have noted that the anterolateral view is limited in access to the lamina terminalis due to the oblique view into the third ventricle, while the anteromedial approach accesses the lamina terminalis in a more direct fashion [51, 52].
Anterolateral approaches access the sellar region at an oblique angle using a unilateral subfrontal or transsylvian view. The ability to address lateral encroachment of the tumor into the Sylvian space, short working distance between surgeon and tumor, and familiarity with the surgical steps for many neurosurgeons are advantages of this approach [50, 53, 54]. Pterional, frontolateral, and orbitozygomatic approaches are included in this category [53, 55]. Smaller tumors may also be approached using a modified eyebrow or lateral supraorbital incision if less superoinferior angulation is required.
Lateral
The lateral approach has a more limited role in addressing sellar and parasellar lesions due to oblique angle of approach and relative ease of midline access via endoscopic endonasal or open craniotomy procedures noted above. However, some authors have advocated for a posterior petrosal/presigmoid transtentorial open resection for tumors with significant retrochiasmatic involvement. Advantages include improved access to tumors posterior to the optic apparatus with a caudal to cranial angle of visualization allowing the surgeon to objectively inspect the relationship between tumor and neurovascular structures and less pituitary gland and stalk retraction during craniopharyngioma resection [56, 57]. The lateral approach does access the skull base via mastoidectomy, but the smaller pediatric mastoid cavity may add complexity to this approach [58, 59]. Other disadvantages include possible venous infarct due to vein of Labbe injury or ligation of the superior petrosal sinus and prolonged temporal lobe retraction [50]. Several reports describe using a subtemporal approach to address craniopharyngiomas with benefits including smaller incision, smaller craniotomy, and potentially lower incidence of CSF leak due to avoidance of the mastoid system. However, GTR is much more difficult due to the significantly smaller operating corridor [60]. As a result, the subtemporal approach alone has very limited utility in most CP surgery.
Intraventricular
Transcortical and transcallosal approaches facilitate access to craniopharyngiomas involving the ventricular system. The transcortical approach is more useful in cases where the lateral ventricle has become dilated or the tumor involves only one ventricle; however, visualization of the ipsilateral hypothalamic wall is limited [50]. The transcallosal approach allows midline access to the ventricles and does not require passing through the cortex and bilateral hypothalamic wall visualization [50, 61]. Neither approach adequately visualizes suprasellar masses and there are significant risks including venous and arterial infarct, memory loss from injury to fornix, and seizures (in the transcortical approach), leading to the relegation of these approaches to purely intraventricular craniopharyngiomas [50, 55].
Endoscopic approaches
There are multiple open approaches to the skull base, all of which require some degree of brain or critical neurovascular structure retraction for adequate exposure which contributes to morbidity [49]. Subsequently, transnasal microsurgical techniques were used to access the midline skull base, but these approaches have gradually been supplanted by the endoscopic endonasal approach (EEA) as familiarity with endoscopy, the team approach to surgery involving both neurosurgical and otolaryngologic personnel, and neuronavigation software have progressed [62, 63]. Advantages of EEA include improved visualization with panoramic endoscopic view, midline exposure, and the ability to dissect the undersurface of the optic chiasm and hypothalamic walls under direct visualization. Lack of external excision and no need for bone transposition as in open craniofacial techniques are ancillary benefits of EEA. Collaboration between otolaryngologists and neurosurgeons has been integral to the widespread adoption of this approach, combining expertise in endoscopic sinonasal dissection and intimate familiarity with intracranial anatomy and pathology as well as the microdissection techniques necessary for skull base surgery [64, 65].
Adoption of endoscopic techniques in the pediatric population has lagged behind the adult population due to concern that endonasal surgery may interfere with craniofacial growth centers and that the smaller nasal passages of children and underdeveloped paranasal sinuses may prevent safe visualization of the skull base and associated lesions. There have been several studies demonstrating that pediatric EEA is safe and efficacious [62, 66, 67]. There have been no reported aberrations in skull development following endoscopic skull base surgery; however, it should be noted that given the relatively recent adoption of EEA in pediatric patients, long-term outcomes will need to be analyzed before conclusions can be made. Potential drawbacks to EEA include relative learning curve for endoscopic visualization, and potentially less ability to control significant bleeding compared to open approaches [68, 69]. Figures 3 and 4 demonstrate a case of a 17-year-old male who presented with a craniopharyngioma managed surgically via an EEA; depicted are preoperative and postoperative scans of the case and a surgical montage of the case as seen through the endoscope.
A case of a 17-year-old male who presented with a craniopharyngioma that was managed surgically via an expanded endoscopic endonasal approach. Images demonstrated here are preoperative and postoperative CT and MRI scans. a Sagittal preoperative CT scan demonstrating a sellar craniopharyngioma with suprasellar extension and characteristic calcifications that are commonly seen in this pathology. b Sagittal and c coronal T1-weighted MRI images with contrast of the same patient preoperatively. d Postoperative sagittal CT and e postoperative sagittal and f postoperative coronal T1-weighted MRI scans with contrast demonstrating gross total resection, preservation of the pituitary stalk and good approximation of the nasoseptal flap to the skull base opening
Surgical still photographs taken with an intraoperative endoscope of the craniopharyngioma case presented in Fig. 3 via a transsphenoidal approach. a Endoscopic view of the sellar region and its septations from a transsphenoidal approach. b Drilling of the sella and tuberculum sellae exposing the necessary dura for access to the tumor. c Incision of the dura to the pituitary compartment. d Expanded dural opening to expose the suprasellar compartment with the presentation of the tumor behind a thin veil of arachnoid. e Resection of the tumor using microinstruments and suction. f Mobilization of the tumor under the superior hypophyseal artery. g Identification of the tumor and its exophytic relationship to the stalk, white asterisk denotes the pituitary stalk and the black asterisk represents the tumor. h Resection of the attachment of the tumor to the stalk, again noted are the pituitary stalk denoted by the white asterisk and the tumor denoted by the black asterisk. i Resection of the last remnants of tumor from the suprasellar space. j View of the suprasellar space demonstrating gross total resection and preservation of the stalk. k Reconstruction of the dural opening and skull base using a DuraGen inlay placed in a gasket seal type arrangement. l Elevation of the nasoseptal flap with good approximation to the skull base
Endoscopic versus open approaches
As mentioned previously, surgical approach should be determined on an individual basis according to surgeon preference, experience, tumor extent, and involvement of adjacent structures [50]. Relationship of the tumor to the optic apparatus, pituitary stalk, and third ventricle are of particular importance and several authors have designed classification systems to represent the position and extent of the craniopharyngioma [50]. The role of EEA has increased as instrumentation and surgeon familiarity increase over time. The endoscopic approach can be used for sellar, subdiaphragmatic, supradiaphragmatic, and retrochiasmatic lesions including those that involve the third ventricle [64, 70, 71]. With careful patient selection, EEA has been shown to be feasible even in revision and recurrent cases of craniopharyngioma regardless of the initial procedure’s approach [72, 73]. Lesions that deviate significantly from the midline are less amenable to endoscopic approach from the transsphenoidal corridor. Extension laterally into the Sylvian fissure, superiorly into the interhemispheric fissure, or with encasement of neurovascular structures (due to limited ability to perform microvascular repair in the event of an injury) are contraindications to the endoscopic technique [50, 74]. However, some authors argue that EEA is the approach of choice for all craniopharyngiomas, excluding purely intraventricular tumors, providing comparable outcomes to traditional open approaches [73]. Indications and contraindications to EEA are presented in Table 1. The role of EEA continues to increase and limits of this technique have yet to be fully defined. Figure 5 demonstrates the utility of EEA in the management of a CP where residual tumor remained primarily in the sella following an open approach. The patient underwent reoperation via EEA to achieve a gross total resection. This case underscores the versatility of EEA in treating sellar and suprasellar disease.
Sagittal T1-weighted MRI images after initial open resection (a) and subsequent expanded endonasal resection (b) of the craniopharyngioma presented in Fig. 2. In the post-transcranial scan (a), note residual tumor in the sella (asterisk). The post-endonasal contrasted MRI (b) demonstrates gross total resection of disease and the nasoseptal flap is well visualized (arrow)
Postoperative management
All pediatric patients are admitted following skull base surgery and reconstruction for monitoring. Given the high likelihood for postoperative diabetes insipidus and frequent monitoring and medication adjustments this entails, patients are admitted to the intensive care unit following open or endonasal approaches to address craniopharyngioma. Prophylactic antibiotics are administered for a minimum of 7 days if absorbable packing is placed. Antibiotics are continued until the packing is removed in clinic or the operating room to reduce the risk of toxic shock syndrome. As the primary concerns driving the use of antibiotics in the postoperative period is the risk of meningitis and toxic shock syndrome (TSS), a fourth-generation cephalosporin is typically selected as this provides antimicrobial penetration to the cerebrospinal fluid and covers the staphylococcal and streptococcal species that would precipitate TSS. In the presence of true anaphylactic penicillin allergy, alternative regimens include vancomycin and sulfamethoxazole with trimethoprim. It should be noted that there is limited prospective data to support antibiotic use postoperatively following skull base surgery and future studies are needed to determine appropriate antibiotic choice and duration. One study has reported no intracranial infections following 24–48 h of antibiotic use following EEA for skull base surgery, suggesting that this may be sufficient [75].
The use of lumbar drains is variable following craniopharyngioma surgery in pediatric patients. This is typically not employed following open approaches, but cerebrospinal fluid diversion is heterogeneously utilized following endonasal approaches in an effort to decrease the incidence of postoperative CSF leak. While the adult literature supports avoidance of lumbar drains in standard endonasal approaches, there is no pediatric-specific data to guide the use of CSF diversion. As the success of endonasal reconstruction relies to a certain extent on compliance of the patient with nasal precautions and avoidance of actions that increase intracranial pressure, the younger pediatric population may represent a special population at higher risk for reconstruction failure given their limited ability to comply with these restrictions [76]. As such, the decision to place a lumbar drain is personalized to each patient; however, effort is made to avoid CSF drainage in the majority of patients [76]. In general, we consider placement of a lumbar drain in cases of failure. Nasal precautions are instituted with the patient being instructed to sneeze with mouth open and avoid nose blowing until the reconstruction is determined to be well healed, typically 4–6 weeks following surgery. Nasal tubes (nasogastric tubes, deep nasal suctioning, nasopharyngeal airways, impedance monitors) are avoided to prevent inadvertent disruption of the repair and potential catastrophy. Nasal cannula is avoided to prevent postoperative nasal drying with humidified face mask utilized if supplemental oxygen is required. The head of bed is elevated 15–30° immediately postoperatively [77].
Nasal saline spray is started on the day of surgery to facilitate clearance of mucous and crusting. After splints and non-absorbable packing are removed, nasal saline irrigations are then utilized when tolerated, most frequently in patients > 6 years of age and with appropriate education and positive reinforcement. If irrigations are not tolerated, nasal saline spray is continued. Debridement of excess mucous and visualization of the repair is attempted approximately 5–7 days after surgery. This may be accomplished at the bedside in older children and teens, but patient maturity and tolerance of instrumentation may require sedation for debridement and splint removal.
Close collaboration with pediatric endocrinology to aggressively manage pre- and postoperative hypopituitarism is essential. Patients are monitored closely for diabetes insipidus in the immediate postoperative setting and started on replacement hormone therapy accordingly.
Adjuvant therapy
Radiation therapy
The earliest studies on pediatric craniopharyngioma advocated for aggressive radical resection for disease control, and there are a number of studies that demonstrate that suggest that long-term disease control is improved in GTR compared with subtotal resection [42, 78, 79]. In more recent years, focus has shifted to the corresponding morbidity of aggressive GTR and associated endocrine and quality of life problems that result from this approach. One study by Ali et al. demonstrated that quality of life was inversely related to the extent of resection [80]. There has accordingly been a shift in surgical planning with some surgeons preferring to perform STR with planned radiation therapy in an attempt to preserve as much pituitary and cranial nerve function as possible. It should be noted that long-term quality of life data for endoscopic endonasal surgery is somewhat lacking given the relatively recent adoption of this technique. Overall, the decision to pursue GTR versus STR with radiation is made on an individual basis with maximal resection without compromise of nearby structures (hypothalamus, optic nerves, etc.) being the preferred approach to optimize both quality of life and disease control [47].
Clinical trials comparing STR to STR in addition to radiation have shown a decrease in tumor recurrence from 55–85 to 0–20% after radiation therapy [47, 81,82,83]. Interestingly, several studies have not shown a survival benefit to starting radiation therapy in the immediate postoperative period versus at the time of disease progression [84, 85]. There has been a trend due to this data to delay radiation therapy until progression of disease is proven in order to delay and minimize cognitive side effects in the pediatric patient. Radiation side effects can include memory loss, optic nerve damage, brain necrosis, hypothalamic injury, and endocrine dysfunction, making judicious use important in minimizing morbidity [47]. Proton-based radiation, stereotactic radiosurgery, and intensity-modulated radiation therapy have shown promise in localizing delivered radiation and minimizing damage to surrounding structures. Data from proton-based radiation treatment algorithms has been encouraging due to lower whole brain and overall body radiation than traditional radiation protocols, but the impact on functional outcomes has yet to be fully assessed [86, 87].
Outcomes
Disease control
Disease control is variable, with reports of disease control without recurrence ranging from 44 to 93% [49, 73, 88, 89]. As mentioned above, gross total resection (GTR) of disease at the first surgical effort has been demonstrated to be associated with decreased likelihood for recurrence, as between 70 and 94% of patients with incomplete resection were noted to recur [89,90,91]. Not surprisingly, increased experience in the management of craniopharyngioma increases the likelihood of achieving gross total resection (when desired) [68].
The use of radiotherapy in the management of craniopharyngioma both at primary surgery and with recurrent disease also impacts disease control. As mentioned above, in the setting of subtotal resection, the addition of radiotherapy with either proton therapy or more conventional delivery methods has been employed with the evidence of improved disease control similar to that of gross total resection with rates from 77 to 100% at 10-year follow up [92, 93]. In a recent meta-analysis designed to evaluate the efficacy of the combination of subtotal resection and radiotherapy vs. gross total resection with respect to disease control, a pooled analysis of nearly 750 patients revealed no significant difference in disease control between groups, further supporting the potential for subtotal resection and postoperative radiation [94]. Of note, in this analysis, there was insufficient data to assess the impact of radiation on complications, an important factor considering the impact of complications on quality of life [95, 96]. In the KRANIOPHARYNGEOM 2007 prospective analysis of patients with craniopharyngioma, the authors advocate for a strategy focusing more on quality of life than disease eradication given the variable rates of long-term disease control and significant consequences of morbidity related to optic and hypothalamic dysfunction that may result from attempts at resection of tumor invading or compromising these areas [93]. However, the impact of radiotherapy on these structures and attendant impact on quality of life is not included in the discussion. Both neurosurgeons and neuroradiologists demonstrate a high degree of accuracy assessing preoperative hypothalamic tumor involvement, suggesting the ability to preoperatively identify patients at greatest risk for hypothalamic injury from surgery or radiotherapy and tailor treatment plans accordingly [97].
The impact of surgical approach on disease control also bears mentioning, with more recent reports in adults utilizing endonasal approaches reporting favorable resection rates as compared to case-matched transcranial approaches [98, 99]. In pediatric series, historic rates of craniopharyngioma resection range from 20 to 75%, while in more recent endonasal series, gross total resection rates range from 56 to 94% supporting improved outcomes with the judicious use of EEA [10, 12, 49, 64, 66, 68]. Another approach-specific concern in pediatric craniopharyngioma is whether sphenoid pneumatization impacts resection. A recent retrospective analysis of 27 patients with varying degrees of pneumatization demonstrated no differences in resection rates by pneumatization pattern further supporting the notion that degree of sphenoid pneumatization does not impact resectability via EEA [100].
Vision
Vision preservation in the management of pediatric craniopharyngioma is generally good and a transition toward endonasal approaches allowing for improved visualization of the optic chiasm during tumor microdissection has facilitated safe dissection of disease from around the optic nerves and chiasm without additional visual disturbance [68, 99]. The rate of improvement in vision is also very high, with 75–100% demonstrating at least some degree of visual improvement postoperatively [68, 73, 95, 101]. Multiple authors report improved visual outcomes utilizing endonasal approaches rather than transcranial approaches, with both greater likelihood of visual improvement and less tendency toward detrimental effects to the visual system following surgery [49, 73, 101]. These recent reports are in keeping with prior studies with similarly improved visual outcomes with endonasal approaches [102,103,104].
While rates of improvement in vision following surgery are encouraging, the visual system is often compromised preoperatively and to a greater degree in the pediatric patient presenting with craniopharyngioma than their adult counterparts [105]. Attaining complete normalization of vision is more challenging, with 25–67% experiencing normalization of vision postoperatively [68, 73, 105]. The incidence of persistent visual disturbances following surgical management of pediatric craniopharyngioma is high, with 48–75% of patients followed long-term with some degree of reported visual disturbance [10, 95, 105]. As deficits in visual function have been associated with decreased quality of life in long-term follow up [95], early diagnosis and definitive management to preserve residual vision and avoid surgical morbidity to the visual system are paramount.
Endocrine function
Endocrine function is notoriously poor following craniopharyngioma treatment with both radiation and surgical management resulting in high rates of both posterior and anterior pituitary dysfunction. Hypopituitarism occurs frequently following surgical management of craniopharyngioma with incidences ranging from 57 to 98%, with both incidence and impact more significant in the pediatric population [49, 66, 68, 73, 105]. Permanent diabetes insipidus also is seen in 64–80% of pediatric patients post-intervention with the presence of transient diabetes insipidus being the rule rather than the exception in the immediate postoperative period [66, 73, 105, 106].
The impact of radiotherapy on long-term endocrine function is less well established in the craniopharyngioma literature. However, recent publications focusing on radiotherapy involving the hypothalamic-pituitary axis (HPA) for other indications cite long-term endocrine dysfunction in nearly half of patients, with growth hormone secretion most likely affected while thyroxine is most radioresistant [107]. These authors identified that a radiation dose of 27 Gray (Gy) to any volume of the hypothalamus increased the likelihood of endocrinopathy fourfold, supporting the notion that the hypothalamus is more radiosensitive to the effects of radiation than adjacent pituitary tissue [107, 108].
In addition to primary pituitary dysfunction, the incidence of increased weight is elevated after craniopharyngioma management due to a host of factors from pituitary dysfunction to steroid replacement to hypothalamic injury. Rates of obesity range from 40 to 80% following craniopharyngioma surgery [10, 42, 49, 88, 95, 105]. Obesity is also known to have a significant impact on long-term quality of life, emphasizing the importance of preoperative discussion regarding these risks and plans for continued collaboration with ancillary services such as bariatric surgery and psychiatry that may help mitigate the impact on quality of life [96].
Complications
Other complications that bear mentioning include postoperative cerebrospinal fluid (CSF) leak, meningitis, hydrocephalus, and cranial neuropathies. In recent pediatric-specific series addressing endonasal craniopharyngioma management, the rate of CSF leak ranges from 10 to 23%, with transition to utilization of the nasoseptal flap reported to decrease CSF leak rates to ~ 11% [49, 66, 73]. In open transcranial approaches, the rate of CSF leak is considerably lower. The incidence of meningitis is between 6 and 12% and hydrocephalus occurs postoperatively in approximately 15% [49, 66, 73]. Cranial neuropathy following surgery can be seen in up to 20%, but this is typically transient [49, 66, 73]. Extensive tumor involvement, prior radiation, and revision surgery have been associated with increased perioperative complications [49, 73].
Quality of life
Optimal care of a patient with craniopharyngioma requires an individualized approach identifying disease- and patient-specific factors impacting resectability, long-term quality of life, patient age, and surgical and radiation risks with sometimes competing goals of complete disease eradication and avoidance of morbidity. In a recent pediatric sample with nearly two decades of follow up, quality of life was decreased as compared to national averages but differences were not significant [95]. Within this cohort, visual deficits were associated with lower quality of life while there was no clear association between rate of endocrine dysfunction, extent of surgery, and use of radiotherapy and quality of life.
Results related to weight and impact on quality of life are mixed, with mean BMI demonstrating no impact on quality of life while the incidence of obesity was significantly higher in patients with lower quality of life [95]. However, long-term quality of life in the KRANIOPHARYNGEOM 2007 prospective multinational randomized study was noted to be significantly decreased in the presence of obesity [96]. Recurrence of disease and presence and extent of complications also negatively impact quality of life [95]. Appropriate endocrine follow-up and replacement can also positively impact quality of life, especially in the case of growth hormone insufficiency, one of the most frequently deficient hormones following craniopharyngioma management [109]. The consensus regarding optimization of quality of life revolves around preserving optic function, preserving hypothalamic function, optimizing endocrine function, and avoiding complications [95, 96, 110].
Mortality
Perioperative mortality is very low in the management of craniopharyngioma—less than 1% in Bakhsheshian et al.’s 2016 national database review, with recent publications with similarly low figures while prior reports of mortality up to 9% prior to the trend toward subtotal resection in the presence of extensive involvement of critical structures [49, 54, 73, 102, 106]. Five to 10-year survival is also quite high with reports ranging from 85 to 92% [93, 105]. After 20 years, overall survival falls to approximately 80% and stabilizes at that level over the following decade [105]. Recurrence of disease has been associated with decreased 10-year survival, with a nearly 30% decrement in survival in those with recurrent craniopharyngioma as compared to patients without recurrence of disease [73].
Conclusion
Pediatric patients presenting with craniopharyngioma represent a distinct cohort from their adult counterparts from both a genetic and clinicopathologic standpoint. Surgical management remains the mainstay of primary therapy for pediatric craniopharyngioma with transition to endonasal approaches in favorably located lesions providing improved visual outcomes and comparable resection rates. The role of gross total resection vs. near total resection with the potential for postoperative radiotherapy remains a point of contention as the quality of life detriment related to surgical injury of the hypothalamic and optic structures is well established while the impact of radiotherapy on these structures is less well defined, especially in the pediatric patient in whom the cumulative effects of radiotherapy have a greater time horizon in which to present. Nevertheless, approaching the pediatric craniopharyngioma patient with intent to optimize long-term quality of life and survival by means of resection that spares the hypothalamic and optic structure has become the current standard of care. Further research regarding the long-term impact of near total resection with radiation and gross total resection on hypothalamic and visual outcomes as well as recurrence and survival will be required to confirm this approach.
References
Yang I, Sughrue ME, Rutkowski MJ, Kaur R, Ivan ME, Aranda D, Barani IJ, Parsa AT (2010) Craniopharyngioma: a comparison of tumor control with various treatment strategies. Neurosurg Focus 28(4):E5
Bunin GR, Surawicz TS, Witman PA, Preston-Martin S, Davis F, Bruner JM (1998) The descriptive epidemiology of craniopharyngioma. J Neurosurg 89(4):547–551
Hoffman HJ, Hendrick EB, Humphreys RP, Buncic JR, Armstrong DL, Jenkin RD (1977) Management of craniopharyngioma in children. J Neurosurg 47(2):218–227
Muller HL (2010) Childhood craniopharyngioma: current controversies on management in diagnostics, treatment and follow-up. Expert Rev Neurother 10(4):515–524
Sorva R, Heiskanen O (1986) Craniopharyngioma in Finland. A study of 123 cases. Acta Neurochir 81(3–4):85–89
Ohmori K, Collins J, Fukushima T (2007) Craniopharyngiomas in children. Pediatr Neurosurg 43(4):265–278
Muller HL (2010) Childhood craniopharyngioma—current concepts in diagnosis, therapy and follow-up. Nat Rev Endocrinol 6(11):609–618
Abrams LS, Repka MX (1997) Visual outcome of craniopharyngioma in children. J Pediatr Ophthalmol Strabismus 34(4):223–228
Muller HL (2008) Childhood craniopharyngioma. Recent advances in diagnosis, treatment and follow-up. Horm Res 69(4):193–202
Karavitaki N, Brufani C, Warner JT, Adams CBT, Richards P, Ansorge O, Shine B, Turner HE, Wass JAH (2005) Craniopharyngiomas in children and adults: systematic analysis of 121 cases with long-term follow-up. Clin Endocrinol 62(4):397–409
Weiner HL, Wisoff JH, Rosenberg ME, Kupersmith MJ, Cohen H, Zagzag D, Shiminski-Maher T, Flamm ES, Epstein FJ, Miller DC (1994) Craniopharyngiomas: a clinicopathological analysis of factors predictive of recurrence and functional outcome. Neurosurgery 35(6):1001–1010 discussion 1010-1001
Van Effenterre R, Boch AL (2002) Craniopharyngioma in adults and children: a study of 122 surgical cases. J Neurosurg 97(1):3–11
Hoff JT, Patterson RH Jr (1972) Craniopharyngiomas in children and adults. J Neurosurg 36(3):299–302
Schroeder JW, Vezina LG (2011) Pediatric sellar and suprasellar lesions. Pediatr Radiol 41(3):287–298 quiz 404-285
Harwood-Nash DC (1994) Neuroimaging of childhood craniopharyngioma. Pediatr Neurosurg 21(Suppl 1):2–10
Tsuda M, Takahashi S, Higano S, Kurihara N, Ikeda H, Sakamoto K (1997) CT and MR imaging of craniopharyngioma. Eur Radiol 7(4):464–469
Sartoretti-Schefer S, Wichmann W, Aguzzi A, Valavanis A (1997) MR differentiation of adamantinous and squamous-papillary craniopharyngiomas. AJNR Am J Neuroradiol 18(1):77–87
Freeman MP, Kessler RM, Allen JH, Price AC (1987) Craniopharyngioma: CT and MR imaging in nine cases. J Comput Assist Tomogr 11(5):810–814
Caruso RD, Rosenbaum AE, Sherry RG, Wasenko JJ, Joy SE, Hochhauser L, Chang JK (1998) Pituitary gland. Variable signal intensities on MRI. A pictorial essay. Clin Imaging 22(5):327–332
Sutton LN, Wang ZJ, Wehrli SL, Marwaha S, Molloy P, Phillips PC, Zimmerman RA (1997) Proton spectroscopy of suprasellar tumors in pediatric patients. Neurosurgery 41(2):388–394 discussion 394-385
Laws ER Jr (1994) Transsphenoidal removal of craniopharyngioma. Pediatr Neurosurg 21(Suppl 1):57–63
Duff J, Meyer FB, Ilstrup DM, Laws ER Jr, Schleck CD, Scheithauer BW (2000) Long-term outcomes for surgically resected craniopharyngiomas. Neurosurgery 46(2):291–302 discussion 302-295
Honegger J, Buchfelder M, Fahlbusch R (1999) Surgical treatment of craniopharyngiomas: endocrinological results. J Neurosurg 90(2):251–257
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131(6):803–820
Adamson TE, Wiestler OD, Kleihues P, Yasargil MG (1990) Correlation of clinical and pathological features in surgically treated craniopharyngiomas. J Neurosurg 73(1):12–17
Miller DC (1994) Pathology of craniopharyngiomas: clinical import of pathological findings. Pediatr Neurosurg 21(Suppl 1):11–17
Apps JR, Martinez-Barbera JP (2016) Molecular pathology of adamantinomatous craniopharyngioma: review and opportunities for practice. Neurosurg Focus 41(6):E4
Hussain I, Eloy JA, Carmel PW, Liu JK (2013) Molecular oncogenesis of craniopharyngioma: current and future strategies for the development of targeted therapies. J Neurosurg 119(1):106–112
Robinson LC, Santagata S, Hankinson TC (2016) Potential evolution of neurosurgical treatment paradigms for craniopharyngioma based on genomic and transcriptomic characteristics. Neurosurg Focus 41(6):E3
Larkin SJ, Ansorge O (2013) Pathology and pathogenesis of craniopharyngiomas. Pituitary. 16(1):9–17
Holsken A, Sill M, Merkle J et al (2016) Adamantinomatous and papillary craniopharyngiomas are characterized by distinct epigenomic as well as mutational and transcriptomic profiles. Acta Neuropathol Commun 4:20
Goschzik T, Gessi M, Dreschmann V, Gebhardt U, Wang L, Yamaguchi S, Wheeler DA, Lauriola L, Lau CC, Müller HL, Pietsch T (2017) Genomic alterations of adamantinomatous and papillary craniopharyngioma. J Neuropathol Exp Neurol 76(2):126–134
Malgulwar PB, Nambirajan A, Pathak P, Faruq M, Suri V, Sarkar C, Jagdevan A, Sharma BS, Sharma MC (2017) Study of beta-catenin and BRAF alterations in adamantinomatous and papillary craniopharyngiomas: mutation analysis with immunohistochemical correlation in 54 cases. J Neuro-Oncol 133(3):487–495
Martinez-Barbera JP (2015) Molecular and cellular pathogenesis of adamantinomatous craniopharyngioma. Neuropathol Appl Neurobiol 41(6):721–732
Goldberg GM, Eshbaugh DE (1960) Squamous cell nests of the pituitary gland as related to the origin of craniopharyngiomas. A study of their presence in the newborn and infants up to age four. Arch Pathol 70:293–299
Karavitaki N, Cudlip S, Adams CB, Wass JA (2006) Craniopharyngiomas. Endocr Rev 27(4):371–397
Prabhu VC, Brown HG (2005) The pathogenesis of craniopharyngiomas. Childs Nerv Syst 21(8–9):622–627
Yoshimoto K, Hatae R, Suzuki SO, Hata N, Kuga D, Akagi Y, Amemiya T, Sangatsuda Y, Mukae N, Mizoguchi M, Iwaki T, Iihara K (2018) High-resolution melting and immunohistochemical analysis efficiently detects mutually exclusive genetic alterations of adamantinomatous and papillary craniopharyngiomas. Neuropathology. 38(1):3–10
Anastas JN, Moon RT (2013) WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer 13(1):11–26
Youngblood JL, Coleman TF, Davis SW (2018) Regulation of pituitary progenitor differentiation by beta-catenin. Endocrinology 159(9):3287–3305
Buslei R, Nolde M, Hofmann B, Meissner S, Eyupoglu IY, Siebzehnrübl F, Hahnen E, Kreutzer J, Fahlbusch R (2005) Common mutations of beta-catenin in adamantinomatous craniopharyngiomas but not in other tumours originating from the sellar region. Acta Neuropathol 109(6):589–597
Elliott RE, Hsieh K, Hochm T, Belitskaya-Levy I, Wisoff J, Wisoff JH (2010) Efficacy and safety of radical resection of primary and recurrent craniopharyngiomas in 86 children. J Neurosurg Pediatr 5(1):30–48
Crotty TB, Scheithauer BW, Young WF Jr et al (1995) Papillary craniopharyngioma: a clinicopathological study of 48 cases. J Neurosurg 83(2):206–214
Hunter IJ (1955) Squamous metaplasia of cells of the anterior pituitary gland. J Pathol Bacteriol 69(1–2):141–145
Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, Hamid O, Schuchter L, Cebon J, Ibrahim N, Kudchadkar R, Burris HA III, Falchook G, Algazi A, Lewis K, Long GV, Puzanov I, Lebowitz P, Singh A, Little S, Sun P, Allred A, Ouellet D, Kim KB, Patel K, Weber J (2012) Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 367(18):1694–1703
Martinez-Gutierrez JC, D'Andrea MR, Cahill DP, Santagata S, Barker FG 2nd, Brastianos PK (2016) Diagnosis and management of craniopharyngiomas in the era of genomics and targeted therapy. Neurosurg Focus 41(6):E2
Graffeo CS, Perry A, Link MJ, Daniels DJ (2018) Pediatric Craniopharyngiomas: a primer for the skull base surgeon. J Neurol Surg B Skull Base 79(1):65–80
Mortini P, Gagliardi F, Boari N, Losa M (2013) Surgical strategies and modern therapeutic options in the treatment of craniopharyngiomas. Crit Rev Oncol Hematol 88(3):514–529
Patel VS, Thamboo A, Quon J, Nayak JV, Hwang PH, Edwards M, Patel ZM (2017) Outcomes after endoscopic endonasal resection of craniopharyngiomas in the pediatric population. World Neurosurg 108:6–14
Liu JK, Sevak IA, Carmel PW, Eloy JA (2016) Microscopic versus endoscopic approaches for craniopharyngiomas: choosing the optimal surgical corridor for maximizing extent of resection and complication avoidance using a personalized, tailored approach. Neurosurg Focus 41(6):E5
Liu JK, Christiano LD, Gupta G, Carmel PW (2010) Surgical nuances for removal of retrochiasmatic craniopharyngiomas via the transbasal subfrontal translamina terminalis approach. Neurosurg Focus 28(4):E6
Liu JK (2013) Modified one-piece extended transbasal approach for translamina terminalis resection of retrochiasmatic third ventricular craniopharyngioma. Neurosurg Focus 34(1 Suppl):Video 1
Gerganov V, Metwali H, Samii A, Fahlbusch R, Samii M (2014) Microsurgical resection of extensive craniopharyngiomas using a frontolateral approach: operative technique and outcome. J Neurosurg 120(2):559–570
Yasargil MG, Curcic M, Kis M, Siegenthaler G, Teddy PJ, Roth P (1990) Total removal of craniopharyngiomas. Approaches and long-term results in 144 patients. J Neurosurg 73(1):3–11
Liu JK, Cole CD, Kestle JR, Brockmeyer DL, Walker ML (2005) Cranial base strategies for resection of craniopharyngioma in children. Neurosurg Focus 18(6a):E9
Hakuba A, Nishimura S, Inoue Y (1985) Transpetrosal-transtentorial approach and its application in the therapy of retrochiasmatic craniopharyngiomas. Surg Neurol 24(4):405–415
Kunihiro N, Goto T, Ishibashi K, Ohata K (2014) Surgical outcomes of the minimum anterior and posterior combined transpetrosal approach for resection of retrochiasmatic craniopharyngiomas with complicated conditions. J Neurosurg 120(1):1–11
Lee DH, Jun BC, Kim DG, Jung MK, Yeo SW (2005) Volume variation of mastoid pneumatization in different age groups: a study by three-dimensional reconstruction based on computed tomography images. Surg Radiol Anat 27(1):37–42
Al-Mefty O, Ayoubi S, Kadri PA (2007) The petrosal approach for the total removal of giant retrochiasmatic craniopharyngiomas in children. J Neurosurg 106(2 Suppl):87–92
Wong RH, De Los Reyes K, Alikhani P et al (2015) The subtemporal approach to retroinfundibular craniopharyngiomas: a new look at an old approach. Oper Neurosurg (Hagerstown, Md) 11(4):495–503
Chamoun R, Couldwell WT (2013) Transcortical-transforaminal microscopic approach for purely intraventricular craniopharyngioma. Neurosurg Focus 34(1 Suppl):Video 4
Rastatter JC, Snyderman CH, Gardner PA, Alden TD, Tyler-Kabara E (2015) Endoscopic endonasal surgery for sinonasal and skull base lesions in the pediatric population. Otolaryngol Clin N Am 48(1):79–99
Jankowski R, Auque J, Simon C, Marchal JC, Hepner H, Wayoff M (1992) Endoscopic pituitary tumor surgery. Laryngoscope 102(2):198–202
Gardner PA, Prevedello DM, Kassam AB, Snyderman CH, Carrau RL, Mintz AH (2008) The evolution of the endonasal approach for craniopharyngiomas. J Neurosurg 108(5):1043–1047
Kassam A, Carrau RL, Snyderman CH, Gardner P, Mintz A (2005) Evolution of reconstructive techniques following endoscopic expanded endonasal approaches. Neurosurg Focus 19(1):E8
Chivukula S, Koutourousiou M, Snyderman CH, Fernandez-Miranda JC, Gardner PA, Tyler-Kabara EC (2013) Endoscopic endonasal skull base surgery in the pediatric population. J Neurosurg Pediatr 11(3):227–241
Gump WC (2015) Endoscopic endonasal repair of congenital defects of the anterior skull base: developmental considerations and surgical outcomes. J Neurol Surg B Skull Base 76(4):291–295
Kshettry VR, Do H, Elshazly K, Farrell CJ, Nyquist G, Rosen M, Evans JJ (2016) The learning curve in endoscopic endonasal resection of craniopharyngiomas. Neurosurg Focus 41(6):E9
Sylvester PT, Moran CJ, Derdeyn CP, Cross DWT, Dacey RG, Zipfel GJ, Kim AH, Uppaluri R, Haughey BH, Tempelhoff R, Rich KM, Schneider J, Chole RA, Chicoine MR (2016) Endovascular management of internal carotid artery injuries secondary to endonasal surgery: case series and review of the literature. J Neurosurg 125(5):1256–1276
Conger AR, Lucas J, Zada G, Schwartz TH, Cohen-Gadol AA (2014) Endoscopic extended transsphenoidal resection of craniopharyngiomas: nuances of neurosurgical technique. Neurosurg Focus 37(4):E10
Gardner PA, Kassam AB, Snyderman CH, Carrau RL, Mintz AH, Grahovac S, Stefko S (2008) Outcomes following endoscopic, expanded endonasal resection of suprasellar craniopharyngiomas: a case series. J Neurosurg 109(1):6–16
Cavallo LM, Prevedello DM, Solari D, Gardner PA, Esposito F, Snyderman CH, Carrau RL, Kassam AB, Cappabianca P (2009) Extended endoscopic endonasal transsphenoidal approach for residual or recurrent craniopharyngiomas. J Neurosurg 111(3):578–589
Koutourousiou M, Gardner PA, Fernandez-Miranda JC, Tyler-Kabara EC, Wang EW, Snyderman CH (2013) Endoscopic endonasal surgery for craniopharyngiomas: surgical outcome in 64 patients. J Neurosurg 119(5):1194–1207
Cappabianca P, Cavallo LM (2012) The evolving role of the transsphenoidal route in the management of craniopharyngiomas. World Neurosurg 77(2):273–274
Brown SM, Anand VK, Tabaee A, Schwartz TH (2007) Role of perioperative antibiotics in endoscopic skull base surgery. Laryngoscope 117(9):1528–1532
Tien DA, Stokken JK, Recinos PF, Woodard TD, Sindwani R (2016) Cerebrospinal fluid diversion in endoscopic skull base reconstruction: an evidence-based approach to the use of lumbar drains. Otolaryngol Clin N Am 49(1):119–129
Tien DA, Stokken JK, Recinos PF, Woodard TD, Sindwani R (2016) Comprehensive postoperative management after endoscopic skull base surgery. Otolaryngol Clin N Am 49(1):253–263
Hoffman HJ, De Silva M, Humphreys RP, Drake JM, Smith ML, Blaser SI (1992) Aggressive surgical management of craniopharyngiomas in children. J Neurosurg 76(1):47–52
Mortini P, Losa M, Pozzobon G, Barzaghi R, Riva M, Acerno S, Angius D, Weber G, Chiumello G, Giovanelli M (2011) Neurosurgical treatment of craniopharyngioma in adults and children: early and long-term results in a large case series. J Neurosurg 114(5):1350–1359
Ali ZS, Bailey RL, Daniels LB, Vakhshori V, Lewis DJ, Hossain AT, Sitterley KY, Lee JYK, Storm PB, Heuer GG, Stein SC (2014) Comparative effectiveness of treatment options for pediatric craniopharyngiomas. J Neurosurg Pediatr 13(2):178–188
Lichter AS, Wara WM, Sheline GE, Townsend JJ, Wilson CB (1977) The treatment of craniopharyngiomas. Int J Radiat Oncol Biol Phys 2(7–8):675–683
Merchant TE, Kiehna EN, Sanford RA, Mulhern RK, Thompson SJ, Wilson MW, Lustig RH, Kun LE (2002) Craniopharyngioma: the St. Jude Children's Research Hospital experience 1984-2001. Int J Radiat Oncol Biol Phys 53(3):533–542
Stripp DC, Maity A, Janss AJ et al (2004) Surgery with or without radiation therapy in the management of craniopharyngiomas in children and young adults. Int J Radiat Oncol Biol Phys 58(3):714–720
Hetelekidis S, Barnes PD, Tao ML, Fischer EG, Schneider L, Scott RM, Tarbell NJ (1993) 20-year experience in childhood craniopharyngioma. Int J Radiat Oncol Biol Phys 27(2):189–195
Moon SH, Kim IH, Park SW, Kim I, Hong S, Park CI, Wang KC, Cho BK (2005) Early adjuvant radiotherapy toward long-term survival and better quality of life for craniopharyngiomas—a study in single institute. Child's Nerv Syst 21(8–9):799–807
Beltran C, Roca M, Merchant TE (2012) On the benefits and risks of proton therapy in pediatric craniopharyngioma. Int J Radiat Oncol Biol Phys 82(2):e281–e287
Fitzek MM, Linggood RM, Adams J, Munzenrider JE (2006) Combined proton and photon irradiation for craniopharyngioma: long-term results of the early cohort of patients treated at Harvard cyclotron laboratory and Massachusetts General Hospital. Int J Radiat Oncol Biol Phys 64(5):1348–1354
Harrington MH, Casella SJ (2012) Pituitary tumors in childhood. Curr Opin Endocrinol Diabetes Obes 19(1):63–67
Unsinn C, Neidert MC, Burkhardt JK, Holzmann D, Grotzer M, Bozinov O (2014) Sellar and parasellar lesions - clinical outcome in 61 children. Clin Neurol Neurosurg 123:102–108
Shi X, Zhou Z, Wu B et al (2017) Outcome of radical surgical resection for craniopharyngioma with hypothalamic preservation: a single-center retrospective study of 1054 patients. World Neurosurg 102:167–180
Tomita T, Bowman RM (2005) Craniopharyngiomas in children: surgical experience at Children's memorial hospital. Child's Nerv Syst 21(8–9):729–746
Iannalfi A, Fragkandrea I, Brock J, Saran F (2013) Radiotherapy in craniopharyngiomas. Clin Oncol (R Coll Radiol (Great Britain)) 25(11):654–667
Muller HL (2014) Craniopharyngioma. Endocr Rev 35(3):513–543
Wang G, Zhang X, Feng M, Guo F (2018) Comparing survival outcomes of gross total resection and subtotal resection with radiotherapy for craniopharyngioma: a meta-analysis. J Surg Res 226:131–139
Yano S, Kudo M, Hide T, Shinojima N, Makino K, Nakamura H, Kuratsu JI (2016) Quality of life and clinical features of long-term survivors surgically treated for pediatric craniopharyngioma. World Neurosurg 85:153–162
Muller HL (2013) Childhood craniopharyngioma. Pituitary. 16(1):56–67
Muller HL, Reichel J, Boekhoff S et al (2018) Low concordance between surgical and radiological assessment of degree of resection and treatment-related hypothalamic damage: results of KRANIOPHARYNGEOM 2007. Pituitary 21(4):371–378
Moussazadeh N, Prabhu V, Bander ED, Cusic RC, Tsiouris AJ, Anand VK, Schwartz TH (2016) Endoscopic endonasal versus open transcranial resection of craniopharyngiomas: a case-matched single-institution analysis. Neurosurg Focus 41(6):E7
Zacharia BE, Amine M, Anand V, Schwartz TH (2016) Endoscopic endonasal management of craniopharyngioma. Otolaryngol Clin N Am 49(1):201–212
Kuan EC, Kaufman AC, Lerner D, Kohanski MA, Tong CCL, Tajudeen BA, Parasher AK, Lee JYK, Storm PB, Palmer JN, Adappa ND (2019) Lack of sphenoid pneumatization does not affect endoscopic endonasal pediatric skull base surgery outcomes. Laryngoscope 129(4):832–836
Yano S, Hide T, Shinojima N (2017) Surgical outcomes of endoscopic endonasal skull base surgery of craniopharyngiomas evaluated according to the degree of hypothalamic extension. World Neurosurg 100:288–296
Fahlbusch R, Honegger J, Paulus W, Huk W, Buchfelder M (1999) Surgical treatment of craniopharyngiomas: experience with 168 patients. J Neurosurg 90(2):237–250
Chakrabarti I, Amar AP, Couldwell W, Weiss MH (2005) Long-term neurological, visual, and endocrine outcomes following transnasal resection of craniopharyngioma. J Neurosurg 102(4):650–657
Elliott RE, Jane JA Jr, Wisoff JH (2011) Surgical management of craniopharyngiomas in children: meta-analysis and comparison of transcranial and transsphenoidal approaches. Neurosurgery 69(3):630–643 discussion 643
Wijnen M, van den Heuvel-Eibrink MM, Janssen J et al (2017) Very long-term sequelae of craniopharyngioma. Eur J Endocrinol 176(6):755–767
Bakhsheshian J, Jin DL, Chang KE, Strickland BA, Donoho DA, Cen S, Mack WJ, Attenello F, Christian EA, Zada G (2016) Risk factors associated with the surgical management of craniopharyngiomas in pediatric patients: analysis of 1961 patients from a national registry database. Neurosurg Focus 41(6):E8
Jalali R, Maitre M, Gupta T, Goda JS, Shah N, Krishna U, Swamidas J, Kannan S, Dutta D, Sarin R (2019) Dose constraint model to predict neuroendocrine dysfunction in Young patients with brain tumours: data from a prospective study. Pract Radiat Oncol 9:e362–e371
Follin C, Erfurth EM (2016) Long-term effect of cranial radiotherapy on pituitary-hypothalamus area in childhood acute lymphoblastic leukemia survivors. Curr Treat Options in Oncol 17(9):50
Heinks K, Boekhoff S, Hoffmann A, Warmuth-Metz M, Eveslage M, Peng J, Calaminus G, Müller HL (2018) Quality of life and growth after childhood craniopharyngioma: results of the multinational trial KRANIOPHARYNGEOM 2007. Endocrine. 59(2):364–372
Muller HL (2017) Risk-adapted, long-term management in childhood-onset craniopharyngioma. Pituitary. 20(2):267–281
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Drapeau, A., Walz, P.C., Eide, J.G. et al. Pediatric craniopharyngioma. Childs Nerv Syst 35, 2133–2145 (2019). https://doi.org/10.1007/s00381-019-04300-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-019-04300-2