Abstract
Background and objective
Craniopharyngiomas are locally aggressive neuroepithelial tumors infiltrating nearby critical neurovascular structures. The majority of published surgical series deal with childhood-onset craniopharyngiomas, while the optimal surgical management for adult-onset tumors remains unclear. The aim of this paper is to summarize the main principles defining the surgical strategy for the management of craniopharyngiomas in adult patients through an extensive systematic literature review in order to formulate a series of recommendations.
Material and methods
The MEDLINE database was systematically reviewed (January 1970–February 2019) to identify pertinent articles dealing with the surgical management of adult-onset craniopharyngiomas. A summary of literature evidence was proposed after discussion within the EANS skull base section.
Results
The EANS task force formulated 13 recommendations and 4 suggestions. Treatment of these patients should be performed in tertiary referral centers. The endonasal approach is presently recommended for midline craniopharyngiomas because of the improved GTR and superior endocrinological and visual outcomes. The rate of CSF leak has strongly diminished with the use of the multilayer reconstruction technique. Transcranial approaches are recommended for tumors presenting lateral extensions or purely intraventricular. Independent of the technique, a maximal but hypothalamic-sparing resection should be performed to limit the occurrence of postoperative hypothalamic syndromes and metabolic complications. Similar principles should also be applied for tumor recurrences. Radiotherapy or intracystic agents are alternative treatments when no further surgery is possible. A multidisciplinary long-term follow-up is necessary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Craniopharyngiomas arise from remnants of embryonic epithelial cells of the craniopharyngeal duct or from metaplasia of the pituitary stalk [120, 145]. They are histologically benign but locally aggressive in the sellar region and are associated with a high rate of recurrence and morbidity [157]. Indeed, they have the highest mortality among sellar tumors [14, 172] and the 5-year overall survival depends on the age at diagnosis, tumor size, extension, and recurrence rate [15, 44]. More than a third of craniopharyngiomas occur in the pediatric population where they account for 10% of all intracranial tumors, [15] while they account for about 3% of intracranial tumors for all age groups, [122] with a second peak of incidence at 50–75 years [49].
The majority of studies from literature describe the management of craniopharyngioma in children, while publications related to adult-onset tumors are scarce. There has not been as yet any consensus on the gold standard of treatment with therapeutic options tending to differ between centers, with variability in the choice of the therapeutic approach between surgery (trancranial and/or endoscopic/microscopic endonasal) [2, 17, 25, 57, 60, 62, 87, 116, 131], radiotherapy [5, 117] and intracystic chemotherapy [9, 21, 32, 72, 132]. Also, surgical strategy may vary from an aggressive gross total resection or a maximal safe resection with stalk preservation to biopsy/cyst decompression followed by radiation. The overall survival rate in adults is 89–94% at 5 years and 85–90% at 10 years follow-up [92, 175] but this may vary based on the therapy applied.
The aim of this paper is to summarize the most recent evidence on the surgical treatment of adult patients with craniopharyngiomas, while addressing the more controversial points of the management that exist in current neurosurgical practice.
Methods
The systematic review was conducted according to the PRISMA criteria [112]. The literature search was performed using the MedLine database, including literature from January 1970 to February 2019. The search was conducted using the terms “craniopharyngioma” combined with “epidemiology,” “radiology,” “ophthalmology,” “neuropsychology,” “surgery,” “endoscopy,” “microscopy,” “resection,” “radiation,” “recurrence,” “survival,” and “outcome.” Our search was limited to studies conducted in adults. Additional relevant studies were searched in the reference list of identified studies manually, and through the use of the “related article” tool in PubMed. Duplicate studies were eliminated. Three authors (GC, MM, and RTD) independently reviewed abstracts, full-text articles, and citations to select pertinent studies. A PICO question was formulated to guide the selection process: the population was defined as adult patients with craniopharyngiomas, the intervention was any type of surgery performed and outcomes included endocrinological, visual and clinical outcomes, the extent of resection, recurrence rate and overall survival, early and long-term morbidity, and quality of life. Case reports, preclinical studies, and pediatric trials were excluded.
Only studies in English were included. The methodological quality of selected articles was evaluated using the GRADE system [6] without masking the authorship of the article.
A task force composed of the EANS skull base section along with renowned international experts was constituted to formulate evidence-based recommendations. Consensus was elaborated after a systematic review of literature and direct discussion among the experts. If randomized blinded trials or prospective matched-pair cohort studies were identified, the recommendations were Level A or B. For controlled non-randomized trials or uncontrolled studies the recommendations were Level C or “expert opinion,” respectively (Table 1) [67]. If unanimous responses were recorded, we used the sentence: “we recommend.” Divergent opinions were discussed until a consensus was reached and we used the terms: “we suggest.” After each recommendation or suggestion, the literature supporting the assumption was reported, and in some cases, a remarks section that specified some details or technical/practical issues was included [67].
At the end of this process, we applied the AGREE Reporting Checklist to review and improve the quality and transparency of the manuscript [13].
The authors used the term gross total resection (GTR) to define a macroscopically complete resection, with no residual tumor visible at the postoperative MRI. When residual tumor is present, the term subtotal resection (STR) is used.
Results
We formulated 17 recommendations in total: 13 recommendations and 4 suggestions.
- 1.
Preoperative management: How to evaluate a craniopharyngioma?
- 1.1
We recommend that all patients with a suspected craniopharyngioma undergo cerebral MRI to evaluate the extension and radiological features of the tumor and a cerebral CT scan and/or CT angiogram to determine the presence of calcification, bone erosion, hyperostosis, anterior skull base anatomy and vasculature pertinent to the tumor and thereby to define the appropriate surgical technique and approach (Level C).
- 1.1
- 1.1.1
Evidence
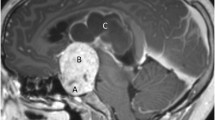
Craniopharyngiomas may present heterogeneous radiological features in the adult population and cerebral MRI with gadolinium enhancement and dedicated pituitary protocols should be performed. They may appear most frequently as sellar and suprasellar lesions with a variable growth pattern within the parasellar and perichiasmatic spaces. They may present as entirely solid or with an associated cystic component and may frequently engulf neurovascular structures in the interpeduncular and suprasellar cisterns. In adults, they arise most commonly posterior to the chiasm and extend posteriorly into the third ventricle [138]. The papillary histopathological type account for at least one-quarter of cases in the adult age group and this finding translates into more solid lesions with fewer calcifications [4, 198], a homogeneous contrast enhancement and a thickened pituitary stalk [193]. Purely intrasellar lesions or isolated intraventricular tumors are rare and generally associated with the papillary histopathological subtype [33, 83]. The differential diagnosis with a pituitary adenoma presenting with a suprasellar extension and a cystic portion remains open till surgery or after the pathological analysis. However, the solid portion of a craniopharyngioma generally enhances on the contrast MRI, more intensely than a classic pituitary adenoma and the normal gland. The MRI provides useful information regarding the tumor characteristics and the relationship with nearby structures, in particular T2-weighted sequences should be performed in the coronal and sagittal planes to study the relationship with the optic chiasm [179] and the floor of the third ventricle and [94] the mammillary body angle, as well as to determine the extension of the tumor and perilesional edema [151,153,154,154]. The localization of the hypothalamo-hypophyseal tract, as well as its displacement and infiltration, might be also be predicted through the use of a diffusion tensor imaging tractography [180].
A complementary cerebral CT scan with or without angiographic sequences with fine cuts through the sella should be performed. This is the gold standard for intralesional calcification and may be helpful in defining cystic portions of the tumor and bony details such as bone erosion, hyperostosis, sphenoid sinus pneumatization, and septations to plan an endoscopic approach. CT angiogram can be added in cases where there is a high suspicion for arterial or venous involvement or in selecting an appropriate surgical approach where there are potential issues with cerebral or cranial base vasculature.
Remarks:
Several surgical classifications have been described based on the relationship of the tumor with the surrounding anatomical structures [74, 94, 142, 143, 155, 156, 168, 191].
Craniopharyngiomas may be classified according to their site of origin and competence of the diaphragm sella. Yasargil et al. classified craniopharyngiomas as purely intrasellar, intra- and suprasellar, supradiaphragmatic parachiasmatic extraventricular, intra- and extraventricular, paraventricular, and purely intraventricular lesions [186]. Wang et al. simplified this classification dividing craniopharyngiomas into subdiaphragmatic with competent diaphragm sellae, subdiaphragmatic with incompetent diaphragm sellae, and supradiaphragmatic [179, 191]. Hoffman et al. also classified craniopharyngiomas on the basis of their relationships with diaphragma sellae, optic chiasm, and third ventricle into subdiaphragmatic, subchiasmatic, prechiasmatic, retrochiasmatic, and intraventricular [75]. These classifications consider the growth pattern of the lesion and the displacement of the optic apparatus, essential factors in the choice of the surgical strategy.
Kassam et al. classified craniopharyngiomas in relation to the infundibulum to plan their surgical access: the preinfundibular, trans-infundibular, and retroinfundibular types can be accessed through the endonasal route while the purely intraventricular subtype requires a transcranial access [94].
The invasiveness and adherence to the hypothalamus should also be carefully analyzed at the preoperative MRI. This was the basis for Puget’s classification, [155] as it represents the most important factor determining the extension of resection and it helps in predicting and limiting the postoperative hypothalamic morbidity [154, 155].
Although these classifications are useful in planning surgical approaches and proposed extent of resection, they are difficult to apply when the tumors are very large. Furthermore, to the best of our knowledge, there is no classification that considers all these factors globally to orient surgeons in choosing between endonasal and trancranial approaches.
- 1.2
We recommend that patients with craniopharyngiomas undergo a complete history and physical examination including the search for hypopituitarism and hypothalamic compromise. Complementary blood tests should be performed to rule out any endocrinological deficit (Level C).
- 1.2.1
Evidence
A complete physical examination should be performed for clinical signs of hypopituitarism or a hypersecretion syndrome that would orient the diagnosis toward a different pituitary pathology. Pituitary function should be evaluated in all sellar/suprasellar lesions even in the absence of clinical manifestations. Anterior panhypopituitarism is recorded in the majority of craniopharyngioma patients [44, 93, 147]. Hypersecretory syndromes should be systematically searched for all sellar lesions through the assessment of serum TSH, free T4, ACTH and morning cortisol, HGH and IGF-1, prolactin, LH, FSH and progesterone, estradiol, and testosterone levels. Diabetes insipidus should also be excluded through a thorough clinical history to search for polydipsia, polyuria, and nocturia, as well as a complete physical evaluation to look for signs of dehydration and biological examination to evaluate if hypernatremia and a low urinary specific gravity and osmolarity are present. The prevalence of diabetes insipidus may be higher than 50% [29].
Furthermore, all patients with craniopharyngiomas should undergo evaluation to determine if hypothalamic functions such as body weight, temperature regulation, and sleep-wake cycles are preserved [55, 199]. The preoperative evaluation of hypothalamic functions deserves increased attention in future studies to evaluate the morbidity and impact of any therapy we apply.
- 1.3
We recommend that all patients with craniopharyngioma undergo an initial ophthalmological evaluation with visual acuity, visual fields and optical coherence tomography (Level C).
- 1.3.1
Evidence
Examination of the visual function is an important element in the diagnosis, monitoring, and prognosis for craniopharyngiomas. Visual decline is one of the most frequent initial manifestations in patients with craniopharyngiomas [150], accounting for two-thirds of clinical presentation in adult-onset tumors [31]. As many as 80% of patients have a visual deficit at the preoperative ophthalmologic evaluation [159, 183]. Visual acuity and visual field defects as well as optic nerve atrophy and papilledema should be carefully evaluated by a neuro-ophthalmologist at diagnosis, because in addition to direct involvement, associated conditions such as hydrocephalus and intracranial hypertension can secondarily impact visual function [31]. Visual loss may be rapidly progressive, and if present, it should motivate an emergent surgery, as it can rapidly lead to permanent blindness [84]. A younger age at diagnosis, edema of the optic nerve, and tumor recurrence are risk factors associated with visual decline in the pediatric population [178]. Data on the adult population are scarce but we can assume that the risk factors are similar.
Optical coherence tomography may represent a valuable tool to evaluate visual damage and predict visual recovery, especially in non-compliant patients [129]. Its utility was widely described in the pediatric population [11, 123] and these findings could also be applied to adults.
We suggest that all patients with craniopharyngiomas undergo a neuropsychological assessment (Expert opinion)
-
1.4.1
Evidence
The impact of craniopharyngiomas on neurocognitive functions in pediatric patients has been studied. Executive functions, memory and learning and fine-motor coordination were impaired in a proportion of pediatric cohorts, where presurgical hypothalamic involvement and impaired visual status were identified as poor prognostic factors [56, 140]. Data in the adult population are limited but it might be of interest to assess neuropsychological functions in the preoperative period and to compare these data with those obtained in the postoperative period. Furthermore, since a significant proportion of patients are subjected to adjuvant radiotherapy a baseline assessment enables tracking neurocognitive changes that may occur after radiation. Some authors reported no changes in neuropsychological performance after surgical procedures [78] while others describe improvement of preoperative cognitive dysfunction and memory [37]. However, reported postoperative morbidity includes cognitive dysfunction with attention deficit, impaired episodic memory, and processing speed [50, 140], which were associated with microstructural alterations of the white matter tracts detected with the use of DTI [51]. A baseline cognitive assessment would thus be helpful in the long-term follow-up of these patients.
- 2
Surgical approach: endoscopic or transcranial?
The choice of the approach will depend to a large degree on the anatomy of the tumor, its extensions, its consistency and last, but not least, the preferences and experience of the surgical team. In general, the best approach should be able to provide the maximal surgical exposure and the most direct trajectory to the tumor limiting brain retraction and minimizing manipulation of neurovascular structures. The best results of surgical treatment depend on the quality of the first surgery and a careful consideration of the ideal approach needs to be taken at initial presentation in order to obtain the best oncological result while minimizing the potential complications associated with craniopharyngioma surgery.
It should be kept in mind that craniopharyngiomas do not always have an identifiable arachnoidal layer separating the tumor from surrounding neurovascular structures unlike meningiomas, schwannomas, and pituitary adenomas that usually do. Moreover, especially at the level of the floor of the 3rd ventricle, craniopharyngiomas may grow in a true subpial fashion making safe GTR of the tumor impossible. These two surgical facts make surgery of craniopharyngiomas extremely challenging if preservation of function is sought and this should be preeminent in the surgeons’ mind while operating on these tumors.
We recommend the use of transcranial skull base approaches for craniopharyngiomas presenting an extension lateral to the internal carotid artery (Level C)
Evidence:
Multiple skull base transcranial approaches have been described to safely resect craniopharyngiomas [116]. These may vary from midline approaches (subfrontal, anterior interhemispheric), anterolateral approaches (pterional, orbitozygomatic, and suprabrow craniotomy) and lateral approaches (subtemporal, transcavernous petrosectomy) [3, 46, 47, 62, 115, 160]. Furthermore, the transcallosal (transcortical/interhemispheric) approaches are used for tumors with a significant intraventricular component [45]. Combined approaches in the same sitting or staged fashion have also been described [191].
The medial subfrontal approach, through the use of a unilateral frontal [160] or a bifrontal transbasal approach [47], offers a direct view of the prechiasmatic space and of the optico-carotid cisterns bilaterally [115] and may be used to perform a trans-lamina terminalis approach to resect craniopharyngiomas with a sellar and prechiasmatic origin and anterior third ventricular extension [115]. In the large series of Du et al., GTR was achieved in 94% of cases treated with this approach with a 12.7% of recurrence and > 90% of surviving patients were living independently at the end of the study [39].
The anterior interhemispheric approach offers not only a direct view of the prechiasmatic space and of the optico-carotid cisterns bilaterally but also to the third ventricular extension via a trans-lamina terminalis approach. The narrow space between the bridging veins (usually < 20 mm in width) is generally sufficient for tumor dissection and this access allows the control and preservation of the anterior communicating complex, mammillary body and fornix, and midline vessels of the interpeduncular cistern [80]. Indeed, small feeding vessels entering the tumor can be identified, coagulated, and cut under direct visual control at the beginning of tumor resection [80].
The lateral subfrontal (pterional) approach allows a large exposure of the suprasellar area through a subfrontal and transsylvian route [62, 191], where the prechiasmatic, optico-carotid, and carotid-oculomotor triangles are used for tumor access [190]. The addition of an orbital or orbitozygomatic osteotomy may increase the access to the suprasellar area and interpeduncular cistern [63].
Complete resection is reported in a variable percentage of cases: Van effenterre et al. described GTR in 59% of cases (operated through a frontolateral approach in 92% of cases) [175], while Gerganov et al. reported GTR in 87.5% of patients with extensive craniopharyngiomas treated through a pterional approach, with no significant postoperative morbidity [62]. Yasargil et al. reported GTR in 90% of cases of their surgical cohort of 144 patients mainly treated through different transcranial approaches (90%) chosen according to tumor’s characteristics [191].
However, traditional transcranial approaches have well-described constraints such as the need for a variable amount of brain retraction and dissection through multiple, long, narrow corridors across major neurovascular structures [165]. The optic apparatus is positioned between the surgeon and the target and the superior pole of the tumor is often situated in the operative blind spot. Also, the vascular supply from the superior hypophyseal arteries to the optic apparatus lies relatively hidden during a lateral approach. Endoscopic assistance can partially solve this problem with angled keyhole endoscopes.
For craniopharyngiomas with a lateral extension beyond the supraclinoid carotid arteries and into the middle cranial fossa, transcranial approaches remain the first choice [165] The technical difficulties in performing very lateral endoscopic approaches associated with the risk of damage to the carotid, posterior communicating artery or thalamoperforators that lie just behind Liliquist’s membrane, make expanded endonasal approaches (EEA) less appealing in these specific cases. Intraoperative vascular injury can be more easily managed through a craniotomy as opposed to an endoscopic approach.
Remarks:
The situation where this limitation of the EEA can be overcome is when the lateral extension of a midline craniopharyngioma is cystic. Decompression of a small cystic component may bring its lateral wall into the view afforded by the endoscope enabling removal and limiting the chances of recurrence.
We recommend performing transcranial approaches for tumors primarily arising from the floor of the 3rd ventricle with an intraventricular location and not extending to the pituitary stalk or the suprasellar space, where the endonasal route can also be performed (Level C)
Evidence
Intrinsic intraventricular craniopharyngiomas account for 3–11% of all craniopharyngiomas [192]. Although the transventricular corridor through either a transcallosal or trancortical-transforaminal approach is generally considered as first surgical options for craniopharyngiomas arising from the floor of the third ventricle and not extending to the pituitary stalk or to the suprasellar space, the interhemispheric transcallosal approach might be associated with less tissue damage and incidence of seizures [105, 192]. From the lateral ventricle, entry into the third ventricle may be performed through the interforniceal, transforaminal, or subchoroidal routes [2]. However, with these approaches, the visualization of the underlying optic apparatus might be limited in some cases and there might be an increased risk of visual deterioration [192]. Alternatively, the subfrontal trans-lamina terminalis approach can be performed to directly access lesions situated in the lower half of the third ventricle, either through a medial subfrontal or a lateral subfrontal approach with a pterional craniotomy [105, 192].
Remarks:
The presence of a tumor projecting through the foramen of Monro leads preference to a transcortical/trans callosal-transforaminal approach [45].
For purely intraventricular craniopharyngiomas the endoscopic endonasal route has been described in some selected cases with encouraging results [24, 52, 135]. The more suitable cases are those with protrusion of the floor of the third ventricle over the suprasellar space and who already have hypopituitarism. The pituitary stalk and gland function will be most likely compromised through the endonasal route. The preoperative presence of empty and deep sella may also favor the endonasal route.
We suggest performing an expanded endonasal transsphenoidal approach as first-line surgical approach for midline and retrochiasmatic craniopharyngiomas without lateral extension (Expert opinion)
Evidence
Expanded endonasal approaches (EEA), through a transtubercular approach (with or without a transplanum route), allow an excellent exposure of the tumor without crossing crucial neurovascular structures [17, 25, 26, 34, 37, 57, 60, 61, 94, 107]. Furthermore, the direct access through the endonasal route has the advantage of early identification of the superior hypophyseal arteries, which are important for the optic apparatus and also of early identification of the chiasm and optic nerves thus avoiding retraction not only of the nerves but also of the basal frontal lobes. The EEA provides an excellent access from the sellar component of the tumor till the floor of the third ventricle allowing resection of the suprasellar, ventricular, and interpeduncular extensions [25, 26, 60, 94, 107, 165].
Since the 1980s, the surgical excision of supradiaphragmatic craniopharyngiomas by using an extended transsphenoidal microsurgical approach has been reported [40, 47, 90, 95, 97, 106], there is no doubt that the use of the endoscope has permitted to overcome most of the limitations of this route in terms of visualization and surgical maneuverability
Several studies have compared the results of transcranial microsurgical versus endoscopic transsphenoidal resections for midline craniopharyngiomas.
Jeswani et al described a similar extent of resection in the 2 subgroups (86% with endoscopic EEA versus 91% with transcranial approaches, p = 0.77) as well as a similar PFS and recurrence rate. Although the rate of CSF leakage was higher with EEA, the rate of cranial nerve injury was higher with transcranial approaches [87]. Moussazadeh et al. reported a higher rate of GTR after endoscopic EEA than transcranial approaches (90 vs 40% respectively, p = 0.009). Endoscopic EEA was also associated with an improved visual outcome (p < 0.05), fewer recurrences and complications (p < 0.001) [131]. In both these studies, patients presented with lesions of similar radiological characteristics [87, 131]. Wannemuehler et al. reported a similar extent of resection between endoscopic EEA and transcranial approaches, with a higher rate of visual improvement in the EEA group (89% vs 25%, p = 0.0075) [182]. Komotar et al. reviewed the surgical series of pediatric and adult craniopharyngiomas and they showed that a greater rate of GTR (67% vs 48%, p < 0.003) and an improved visual outcome (56% vs 33%, p < 0.003) was achieved with endoscopic EEA compared to transcranial approaches [103].
Recent literature is replete with numerous studies that strongly support the use of endoscopic techniques as the approach of choice for suprasellar and retrochiasmatic craniopharyngiomas because of the ability to achieve a high percentage of patients with complete resection (similar to transcranial approaches) while limiting the incidence of neurological and vascular complications [17, 22, 23, 25, 26, 35, 47, 57, 60, 61, 79, 85, 94, 103, 107, 118, 189]. Due to the direct access obtained with ventral approaches, the postoperative morbidity in terms of cranial nerves palsy and postoperative seizures is lower after endoscopic EEA [87, 131]. Through EEA, a careful dissection is possible aided by a direct visualization of the capsule, major vessels, and perforators and also of the walls of the third ventricle (hypothalamus). It is possibly due to this that the preservation of hypothalamic functions is superior with endoscopic EEA when compared to the transcranial approaches [4, 65, 189]. A direct decompression of the optic apparatus may also be performed with a direct visualization and preservation of the superior hypophyseal artery [37, 164]. This may translate into a greater improvement of the postoperative visual outcome [65, 103, 131, 182]. In fact, between 75 and 89% of patients having a preoperative visual impairment showed a recovery in endoscopic series [16, 107, 110, 182]. A prefixed chiasm and the presence of a large tumor extending upward and behind the optic chiasm have been considered for long as relative contraindications to achieve GTR through endoscopic EEA. Nevertheless, recent literature indicates that a narrow corridor between the top of the pituitary gland and the bottom of the chiasm has no relationship with the extent of resection obtained endoscopically [138] and that endoscopic EEA is an effective approach also for retrochiasmatic craniopharyngiomas, even in cases with a low-lying chiasm [99].
Progressive debulking through piecemeal removal before embarking on arachnoid dissection remains an important principle as in other microsurgical procedures. Some skull base surgeons affirm that the visualization of certain portions of retrochiasmatic craniopharyngiomas situated in the interpeduncular cistern and retroinfundibular space may be better visualized and removed through transcranial approaches. However, transcranial approaches may present some disadvantages: through the pterional approach, the contralateral optico-carotid and the hypothalamic surfaces are poorly visualized. The subfrontal approach allows a direct visualization of the third ventricle and hypothalamus but the region beneath the optic chiasm may not be properly visualized [102], and thus, the sellar portion of craniopharyngiomas may not be properly visualized. The petrosal approach was also proposed for large retrochiasmatic craniopharyngiomas [3] but it is technically demanding and time-consuming, not to mention the necessity of retracting the temporal lobe and the difficulty in accessing the most upper portion of the third ventricle.
All these pitfalls of transcranial approaches are avoided with EEA, which provides a ventral approach with direct visualization of the optic chiasm, third ventricle ,and hypothalamus and is thus suitable for the resection of retrochiasmatic craniopharyngiomas [94, 113]. When the craniopharyngioma presents an extension into the interpeduncular fossa, a superior clivectomy with or without posterior clinoidectomy and temporary displacement of the pituitary might be necessary and may help in avoiding petrosectomy.
Remarks:
-
Craniopharyngiomas should be preferentially treated in tertiary referral centers [20]. The choice between transcranial or endoscopic approaches should be based only on the tumor anatomy. Ideally all tertiary referral centers should have equivalent expertise with transcranial and endonasal approaches which will allow the choice of the approach to be independent of the personal preferences of any given surgeon. Size, location, relation to vascular and nervous structures, and tumor consistency should be carefully evaluated preoperatively to choose the best surgical approach.
-
The presence of extensive peripheral calcification may favor the use of transcranial approaches. The performance of an endoscopic dissection of a large calcified craniopharyngioma may be risky, as the basilar artery with the posterior cerebral arteries and its perforators are all situated posterior to the tumor and a blind posterior dissection will need to be performed in this area with endonasal approaches. This is associated with a dramatic increase in the risk of vascular injuries. The transcranial approaches seem to allow superior vascular control with large calcified tumors [41, 197].
-
The accurate knowledge of endonasal anatomy is a fundamental step to safely perform the procedure and avoid serious neurological morbidities.
-
The four-hands technique is used in many centers but the endoscope-holder may represent a valid alternative for bi-manual endoscopic EEA [25, 57, 110, 141, 189].
-
Another key point of EEA is the correct management of perioperative CSF leakage and the prevention of a postoperative one. Multiple skull base reconstruction techniques have been described to address this point [1, 28, 69, 71, 82, 109, 110, 144, 171, 177].
-
The use of multiple working corridors should be considered for the resection of giant craniopharyngiomas extending to multiple anatomical compartments. They should be addressed through the use of combined endonasal and transcranial approaches to obtain a maximal resection while limiting the complication rate. The medial and retrochiasmatic portion of the tumor should be addressed through endonasal approaches, while the portion lateral to ICA bifurcation should be approached through standard transcranial approaches.
We recommend the use of traditional endonasal transsphenoidal approaches for purely intrasellar craniopharyngiomas (Level C)
-
2.4.1
Evidence
The endonasal approach can be tailored according to the surgeon’s needs and tumor characteristics. A standard endonasal endoscopic or microscopic transsphenoidal approach (similar to pituitary adenoma surgery) may be used in selected sellar lesions with a limited and well-defined suprasellar and retrosellar extensions [18, 26, 61]. An enlarged pituitary fossa and a cystic extra-arachnoidal infradiaphragmatic component favor the use of a standard transsphenoidal approach [26].
We recommend performing a careful closure with a nasoseptal flap to limit the risk of postoperative CSF leakage when an extended endonasal approach is performed (Level C)
Evidence
performed for craniopharyngiomas often requires a large bone and dural exposure and the presence of a postoperative CSF leakage is common after a large arachnoid dissection and in cases where a wide opening of cisterns and third ventricle is necessary for tumor resection. CSF leakage has limited the widespread application of endonasal approaches for skull base tumors for a long time [104, 177]. The repair failure rate and postoperative meningitis rates in endoscopic skull base tumor surgery have varied over the last 15 years, but the trend has been encouragingly downward [28].
A multilayer reconstruction with the use of abdominal fat and or a fascia lata patch (if possible around a bone buttress according to the gasket seal closure) [109], combined with a vascularized flap [69] and intercalated by a sealant materials, is the most common technique used to limit CSF leakage and it has been largely described in literature [28, 69, 71, 171]. Surgical series using this technique report a rate of postoperative CSF leakage variable from 23% to less than 4% [25, 28, 42, 107, 110, 121, 141].
Remarks:
The nasoseptal flap is the most used and preferred option because of its large surface and excellent vascular supply with a long pedicle, which allows the correct positioning. Careful placement of the flap is very important, to avoid dead space behind the flap and torsion.
It should be of adequate size and it should be positioned over a bare bone [1] to prevent a postoperative mucocele formation and also to diminish the risk of flap dehiscence [42].
-
The middle or inferior turbinate flap may represent a valid alternative when the nasoseptal flap fails or cannot be performed [28]. Other pediculated flaps, such as the transpterygoid, temporo-parietal fascia, transfrontal pericranial, and Oliver palatal flaps, were described [53, 54, 68, 137, 196]. The Janus flap with a bilateral nasoseptal flap was also described as a safe alternative to cover large bone and dural defects, which are not completely sealed by a unilateral nasoseptal flap [136].
-
Even with revision surgeries for recurrence, the nasoseptal flap should be spared and reused if possible. The use of acoustic Doppler ultrasonography and indocyanine green fluorescence may represent good tools to assess the presence of a viable vascular pedicle [96, 148]
-
The factors correlated with failure of the skull base reconstruction are obesity, lack of buttress and postoperative Valsalva manoeuver [28].
-
The systematic use of a lumbar CSF drain after a multilayer skull base reconstruction is controversial. Some authors affirm that its use may facilitate the healing of the skull base reconstruction [25, 110], while others claim that it carries an infectious risk [28] and may predispose to intracranial hypotension and pneumocephalus, thus limiting its use to persistent CSF leakage after reconstruction [42]. We would suggest their usage based on surgeon experience/preference for primary repair and for all cases following a secondary repair after a persistent postoperative CSF leak.
Extent of resection and hypothalamic involvement: GTR or STR plus radiotherapy?
Craniopharyngiomas have a locally aggressive behavior and the stalk and hypothalamus often have difficult dissection planes. The aim of surgical management is to obtain a safe maximal resection while limiting postoperative morbidity [25, 107, 110].
We recommend performing a GTR when there is no infiltration of the hypothalamus (Level C)
Evidence
A complete resection at first surgical attempt is described as the most effective treatment from an oncological perspective as the treatment of a recurrent lesion may be more complicated [47, 191]. The priority of surgery is to maximize resection while preserving the patient’s long-term functional outcome and quality of life. A balance should be found between tumor removal and damage to nearby critical neurovascular structures [7, 92]. When a dissection plane between the tumor and the hypothalamus is present, GTR should be attempted to limit the long-term risk or recurrence [92, 167, 191] but surgeons should be mindful about tumors with microscopic subpial invasions of the hypothalamus and to perform GTR in such cases may risk injury to the hypothalamus.
We recommend performing STR coupled with adjuvant radiotherapy (STR + XRT) when hypothalamic infiltration is confirmed (hypothalamic-sparing resection) (Level C)
Evidence
The main limitation in performing GTR is the presence of hypothalamic invasion [151], defined as the absence of a surgical plane between the tumor and the hypothalamus. This is the most important predictor of postoperative morbidity and mortality, [151] as the postoperative quality of life should remain a priority.
The handling of the pituitary stalk during surgery is controversial, as its preservation can limit the risk of postoperative endocrine deficits and diabetes insipidus, but this is known to increase the risk of craniopharyngioma recurrence [81, 88, 89, 167, 186, 187, 191]. Patients with hypothalamic disturbances and hypopituitarism have an elevated prevalence of metabolic syndrome and the mortality rate from cardiovascular causes is increased [86, 125]. According to Sughrue et al, patients with GTR experience a 2.5-fold increased risk of developing at least one endocrinopathy compared with patients undergoing STR + XRT and over 10% with GTR had panhypopituitarism [170]. Intentional STR + XRT has thus gained some favor in recent years due to a reduction in morbidity and possibly an equivalent progression-free and overall survival compared to GTR [162, 170, 188] A recent meta-analysis on 759 cases of adult craniopharyngioma showed that despite the recurrence rates favoring GTR, the difference between GTR and STR + XRT did not reach statistical significance (p = 0.18) [31]. The same findings were reported in Zacharia’s meta-analysis on 644 patients, where no survival advantage was associated with GTR [195]. The absence of a clear superiority of GTR in terms of outcome improvement and the higher complication rate of GTR in terms of endocrinological and hypothalamic dysfunctions, associated with greater attention to the quality of life of patients, are therefore changing the paradigm of treatment [181]. The increased use of EEA seems to have improved the rates of achieving hypothalamic preservation regardless of the degree of involvement by the tumor, principally due to the increased visibility of the hypothalamic dissection plane with the tumor [189].
Remarks:
Although the choice to perform intentional STR + XRT is based on the preoperative analysis of radiological features, involvement of neurovascular structures, surgeon’s preference, and experience, the final decision should be made upon surgical exploration and intraoperative findings [181].
Aside from fractionated adjuvant radiotherapy, radiosurgery can also represent an attractive option as an adjuvant therapy after STR [27, 64, 100, 101, 108, 127, 163, 174].
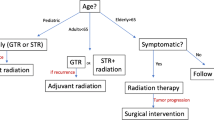
How to treat recurrences and residual tumor progression?
An individualized approach is highly recommended for recurrent tumors and residual tumor after STR + XRT (Level C)
Evidence
Multiple options have been described in the literature to manage recurrent tumors and residual tumors after STR + XRT. A watch and wait strategy, second surgery, radiotherapy, intracystic chemotherapy, and even immunotherapy or target therapies have been described in patients with craniopharyngiomas harboring BRAF V600E mutation [12, 48, 66, 72, 73, 158]. The timing to perform these different options is, however, a matter of debate [12, 48, 72, 73, 158] The tumor progression history is the key factor to consider the best tailored appropriate treatment option for the patient, and the management plan should be based on a multidisciplinary discussion. If a small calcified residual tumor is present, showing no growth at follow-up images, a watchful wait and scan strategy can be adopted, while with rapidly growing residual tumors, an early surgical procedure may be preferred. [25, 61, 110, 173, 181].
Long-term clinico-radiological follow-up is mandatory to evaluate the evolution of residual disease.
We suggest applying the similar surgical principles detailed for primary lesions to the treatment of recurrent craniopharyngiomas that require surgical treatment (Expert opinion)
Evidence
Recurrences are reported in a variable percentage of cases even after GTR (0-62%) [91,93,93] and the surgical approach does not seem to have an impact on the recurrence rate [31]. In addition, the initial tumor size has not been correlated to the recurrence rate [146]. Surgery for recurrent lesions is generally considered to be more difficult than primary surgeries because of the loss of the arachnoid planes and the iatrogenic creation of scars and adhesions [173]. The size and the location of the tumor residue are the main determinants for the choice of the surgical approach [114, 116].
The rates of GTR are significantly inferior to results obtained after primary surgery [23, 92, 185, 191] while the morbidity and mortality rate is considerably higher [23, 92, 126, 130, 185]. GTR for recurrent tumors may vary from 0 to 56% according to the different series considered [47, 91, 92, 169, 173] and only one study reported a GTR rate as high as 78% after EEA for recurrence [37]. The postoperative mortality may vary from 11 to 24% [47, 91, 92, 169]. and is higher in the adult population than in children [92].
The same principles detailed for primary tumors should be applied for recurrent/progressive craniopharyngiomas:
EEA should be strongly considered (with the exceptions previously described) for all midline lesions not crossing the lateral margin of the carotid arteries and optic nerves.
Endonasal approaches should be considered when the first surgery was performed transcranially [23, 36, 98, 173].
Transcranial approaches should be preferentially considered for recurrences in the middle and posterior fossa and lesions limited to the third ventricle [173].
Remarks:
The first surgical attempt remains the best option to obtain a surgical cure.
The use of intraoperative technologies such as image guidance may help in redoing endonasal approaches where the classical landmarks have been removed.
The use of radiotherapy or radiosurgery [117, 134] and of intracystic agents [132] should be discussed in a multidisciplinary meeting and should be considered with cystic lesions, progression of the residual tumor, or, in cases, of tumor recurrence if no further surgery is possible [59, 173].
Postoperative management
The different potential complications of craniopharyngioma treatments necessitate each a specific follow-up.
We recommend a close clinical and endocrinological follow-up in the management of patients with treated craniopharyngiomas (Level C)
Evidence
A clinical follow-up should detect early and late complications such as seizures, hydrocephalus, cerebrovascular injuries, and radiation-induced complications [43].
The development of endocrinological dysfunction is very likely to occur in the early and late postoperative period [16, 25, 57, 107, 110]. Aggressive resections, particularly in the setting of stalk invasion, may increase the rate of postoperative endocrinological deficits [37, 107, 111]. About three-quarters of patients will need a long-term hormonal replacement therapy with growth hormone (GH) and thyroid hormone deficiencies being most frequently observed [92, 158]. GH deficiency is associated with an increased cardiovascular risk [128] and a physiologic substitution seems to have beneficial effects on body fat mass, cholesterol profile and blood pressure [119, 124]. The impact of thyroid substitution is less known but subclinical hypothyroidism may increase the cardiovascular risk [139]. A physiologic substitution of adrenocortical deficit is an important determinant in lowering mortality [10, 166]. Diabetes insipidus is another frequent complication and is permanent in more than half of the cases after surgery [133, 149]. Correct management of dysnatremia represents a priority during the immediate postoperative course as ADH secretion may follow a triphasic course.
However, the complication most difficult to treat remains hypothalamic damage, which may strongly impact the quality of life of these patients. This may present as an obesity-hyperphagia syndrome, sleep cycle disturbances, and temperature dysregulation or behavioral abnormalities [30, 76, 77, 133].
Lifestyle and dietary modifications, antihyperlipemic agents, psychotherapeutic, and bariatric surgery have been used to treat hypothalamic obesity with controversial results. Of late, new agents targeting the hypothalamus (if partially damaged) or other brain or peripheral receptors (if the hypothalamus is completely destroyed) are being tested to improve the management of these patients [176].
Remarks:
We recommend a clinical and endocrinological checkup every 3 months during the first postoperative year to test the different pituitary axes and then a 6-monthly follow-up during the first 5 years postoperatively. After this period, if the patient is stable, an annual follow-up should be performed.
We recommend regular checking of body weight, beginning in the immediate postoperative period, as in some cases, weight gain may be rapid and difficult to reverse.
We recommend a regular check of cardiovascular and cerebrovascular risk factors, as they are strong determinants of the increased mortality in craniopharyngioma patients [14, 147, 172, 184, 194].
We recommend a close ophthalmological follow-up in the management of patients with treated craniopharyngiomas (Level C)
Evidence
Visual complications may strongly impact the quality of life of patients with craniopharyngiomas. Eighty percent of patients may present with a preoperative visual deficit [159, 183] and an improvement is described in 40–60% of patients in the postoperative period, while a visual worsening is described in 5–30% of cases [60, 61, 116, 191]. A regular ophthalmological follow-up should be performed to evaluate the evolution of the visual status as well as for early detection of a tumor recurrence.
Remarks:
We recommend an ophthalmological checkup every 6 months during the first year postoperatively, and annually thereafter.
We suggest assessing neurocognitive functions in the postoperative period (Expert opinion)
Evidence:
Hypothalamic damage is known to be associated with a neurocognitive decline [58, 149] and this impairment, along with memory deficits, increases the postoperative morbidity [19, 38, 147] with consequent reduction in the quality of life [149]. Up to 50% of patients present psychosocial impairment at long-term follow-up due to problems with concentration, memory. and executive function [147]. Recent studies affirm that neurocognitive functions are not impaired when a careful removal of the tumor is performed [78, 93] but these studies had a heterogeneous population in terms of hypothalamic involvement and neuropsychological tests applied and therefore these results are still a matter of debate [50, 51, 140].
Remarks:
A postoperative evaluation and follow-up would help in supporting patients in their daily life activities and in improving their capacity for adaptation.
We recommend an early postoperative MRI to assess the extent of resection and to plan the follow-up. We recommend a close long-term radiological follow-up (Level C)
Evidence
A postoperative MRI may help in evaluating the extent of resection, the decompression of the optic nerves/chiasm and the presence of postoperative complications, such as the presence of intraventricular blood and the ventricular size, to detect the development of early hydrocephalus. This imaging would also detect early ischemic accidents, secondary to vasospasm or intraoperative arterial occlusions.
Remarks:
An early MRI performed during the first 48–72 h after surgery represents a baseline study to compare future exams and to evaluate the radiological evolution of postoperative events [70].
Patients should be further carefully monitored for recurrence or growth of residual disease. A close radiological follow-up with cerebral MRI every 3 months should be performed in the first year after surgery and then every 6 months for the first 5 years. After this period an annual follow-up is recommended for at least 10 years, as craniopharyngiomas are associated with a high risk of local recurrence [8, 23, 157] and less frequently presenting with postoperative intracranial seeding [161]. Most recurrences occur in the first 5 years after treatment [175, 183, 188, 191] but also delayed recurrences were described [31, 92].
The images should be evaluated with an experienced team of neuroradiologists.
Summary
1.1 We recommend that all patients with a suspected craniopharyngioma undergo cerebral MRI to evaluate the extension and radiological features of the tumor and a cerebral CT scan and/or CT angiogram to determine the presence of calcification, bone erosion, hyperostosis, anterior skull base anatomy, and vasculature pertinent to the tumor and thereby to define the appropriate surgical technique and approach (Level C).
1.2 We recommend that patients with craniopharyngiomas undergo a complete history and physical examination including the search for hypopituitarism and hypothalamic compromise. Complementary blood tests should be performed to rule out any endocrinological deficit (Level C).
1.3 We recommend that all patients with craniopharyngioma undergo an initial ophthalmological evaluation with visual acuity, visual fields and optical coherence tomography (Level C).
1.4 We suggest that all patients with craniopharyngiomas undergo a neuropsychological assessment (Expert opinion).
2.1 We recommend the use of transcranial skull base approaches for craniopharyngiomas presenting an extension lateral to the internal carotid artery (Level C).
2.2 We recommend performing transcranial approaches for tumors primarily arising from the floor of the 3rd ventricle with an intraventricular location and not extending to the pituitary stalk or the suprasellar space, where the endonasal route can also be performed (Level C).
2.3 We suggest performing an expanded endonasal transsphenoidal approach as first-line surgical approach for midline and retrochiasmatic craniopharyngiomas without lateral extension (Expert opinion).
2.4 We recommend the use of traditional endonasal transsphenoidal approaches for purely intrasellar craniopharyngiomas (Level C).
2.5 We recommend performing a careful closure with a nasoseptal flap to limit the risk of postoperative CSF leakage when an extended endonasal approach is performed (Level C).
3.1 We recommend performing a GTR when there is no infiltration of the hypothalamus (Level C).
3.2 We recommend performing STR coupled with adjuvant radiotherapy (STR + XRT) when hypothalamic infiltration is confirmed (hypothalamic-sparing resection) (Level C).
4.1 An individualized approach is highly recommended for recurrent tumors and residual tumor after STR + XRT (Level C).
4.2 We suggest applying the similar surgical principles detailed for primary lesions to the treatment of recurrent craniopharyngiomas that require surgical treatment (Expert opinion).
5.1 We recommend a close clinical and endocrinological follow-up in the management of patients with treated craniopharyngiomas (Level C).
5.2 We recommend a close ophthalmological follow-up in the management of patients with treated craniopharyngiomas (Level C).
5.3 We suggest assessing neurocognitive functions in the postoperative period (Expert opinion)
5.4 We recommend an early postoperative MRI to assess the extent of resection and to plan the follow-up. We recommend a close long-term radiological follow-up (Level C).
Conclusion
The initial evaluation of adult patients with craniopharyngiomas should include a clinical, endocrinological, ophthalmological, radiological and neuropsychological assessment. Treatment of these patients should be performed in tertiary referral centers. Based on data from the literature, the endoscopic approach has gained supremacy in the treatment of midline craniopharyngiomas in terms of improved GTR, endocrinological and visual outcomes when compared to standard transcranial approaches. The latter are recommended in cases with lateral extension or with purely intraventricular tumors. Independent of the technique, a safe maximal but hypothalamic-sparing resection should be performed to limit the occurrence of postoperative hypothalamic syndromes and metabolic complications. A close multidisciplinary evaluation is necessary for endocrine, hypothalamic and oncological outcomes to define a long-term treatment plan, tailored to the requirements of each patient. Further clinical studies focused on present-day treatment outcomes of adult-onset craniopharyngiomas would help to better define the optimal management and thereby improve outcomes for these challenging tumors.
References
Abou-Al-Shaar H, Zaidi HA, Cote DJ, Laws ER Jr (2017) Bolstering the Nasoseptal Flap Using Sphenoid Sinus Fat Packing: A Technical Case Report. World neurosurgery 99:813 e811–813 e815
Alli S, Isik S, Rutka JT (2016) Microsurgical removal of craniopharyngioma: endoscopic and transcranial techniques for complication avoidance. J Neuro-Oncol 130:299–307
Al-Mefty O, Ayoubi S, Kadri PA (2008) The petrosal approach for the resection of retrochiasmatic craniopharyngiomas. Neurosurgery 62:ONS331–ONS335 discussion ONS335-336
Apra C, Enachescu C, Lapras V, Raverot G, Jouanneau E (2019) Is gross total resection reasonable in adults with craniopharyngiomas with hypothalamic involvement? World neurosurgery 129:e803–e811
Astradsson A, Munck Af Rosenschold P, Feldt-Rasmussen U, Poulsgaard L, Wiencke AK, Ohlhues L, Engelholm SA, Broholm H, Hansen Moller E, Klose M, Roed H, Juhler M (2017) Visual outcome, endocrine function and tumor control after fractionated stereotactic radiation therapy of craniopharyngiomas in adults: findings in a prospective cohort. Acta Oncol 56:415–421
Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O'Connell D, Oxman AD, Phillips B, Schunemann HJ, Edejer T, Varonen H, Vist GE, Williams JW Jr, Zaza S, Group GW (2004) Grading quality of evidence and strength of recommendations. Bmj 328:1490
Balde NM, Diallo MM, Poirier JY, Sow MS, Brassier G, Lorcy Y (2007) Long-term outcome of the adult onset craniopharyngiomas. Ann Endocrinol 68:186–190
Bao Y, Pan J, Qi ST, Lu YT, Peng JX (2016) Origin of craniopharyngiomas: implications for growth pattern, clinical characteristics, and outcomes of tumor recurrence. J Neurosurg 125:24–32
Bartels U, Laperriere N, Bouffet E, Drake J (2012) Intracystic therapies for cystic craniopharyngioma in childhood. Front Endocrinol 3:39
Bergthorsdottir R, Leonsson-Zachrisson M, Oden A, Johannsson G (2006) Premature mortality in patients with Addison’s disease: a population-based study. J Clin Endocrinol Metab 91:4849–4853
Bialer OY, Goldenberg-Cohen N, Toledano H, Snir M, Michowiz S (2013) Retinal NFL thinning on OCT correlates with visual field loss in pediatric craniopharyngioma. Canadian journal of ophthalmology Journal canadien d’ophtalmologie 48:494–499
Brastianos PK, Shankar GM, Gill CM, Taylor-Weiner A, Nayyar N, Panka DJ, Sullivan RJ, Frederick DT, Abedalthagafi M, Jones PS, Dunn IF, Nahed BV, Romero JM, Louis DN, Getz G, Cahill DP, Santagata S, Curry WT Jr, Barker FG 2nd (2016) Dramatic response of BRAF V600E mutant papillary craniopharyngioma to targeted therapy. J Natl Cancer Inst 108(2). https://doi.org/10.1093/jnci/djv310
Brouwers MC, Kerkvliet K, Spithoff K, ANS C (2016) The AGREE Reporting Checklist: a tool to improve reporting of clinical practice guidelines. Bmj 352:i1152
Bulow B, Attewell R, Hagmar L, Malmstrom P, Nordstrom CH, Erfurth EM (1998) Postoperative prognosis in craniopharyngioma with respect to cardiovascular mortality, survival, and tumor recurrence. J Clin Endocrinol Metab 83:3897–3904
Bunin GR, Surawicz TS, Witman PA, Preston-Martin S, Davis F, Bruner JM (1998) The descriptive epidemiology of craniopharyngioma. J Neurosurg 89:547–551
Campbell PG, McGettigan B, Luginbuhl A, Yadla S, Rosen M, Evans JJ (2010) Endocrinological and ophthalmological consequences of an initial endonasal endoscopic approach for resection of craniopharyngiomas. Neurosurg Focus 28:E8
Cappabianca P, Cavallo LM (2012) The evolving role of the transsphenoidal route in the management of craniopharyngiomas. World neurosurgery 77:273–274
Cappabianca P, Cavallo LM, de Divitiis E (2004) Endoscopic endonasal transsphenoidal surgery. Neurosurgery 55:933–940 discussion 940-931
Carpentieri SC, Waber DP, Scott RM, Goumnerova LC, Kieran MW, Cohen LE, Kim F, Billett AL, Tarbell NJ, Pomeroy SL (2001) Memory deficits among children with craniopharyngiomas. Neurosurgery 49:1053–1057 discussion 1057-1058
Casanueva FF, Barkan AL, Buchfelder M, Klibanski A, Laws ER, Loeffler JS, Melmed S, Mortini P, Wass J, Giustina A, Pituitary Society EGoPT (2017) Criteria for the definition of Pituitary Tumor Centers of Excellence (PTCOE): a Pituitary Society statement. Pituitary 20:489–498
Cavalheiro S, Dastoli PA, Silva NS, Toledo S, Lederman H, da Silva MC (2005) Use of interferon alpha in intratumoral chemotherapy for cystic craniopharyngioma. Childs Nerv Syst 21(8-9):719–724
Cavallo LM, Cappabianca P (2014) Craniopharyngiomas: infradiaphragmatic and supradiaphragmatic type and their management in modern times. World neurosurgery 81:683–684
Cavallo LM, Prevedello DM, Solari D, Gardner PA, Esposito F, Snyderman CH, Carrau RL, Kassam AB, Cappabianca P (2009) Extended endoscopic endonasal transsphenoidal approach for residual or recurrent craniopharyngiomas. J Neurosurg 111:578–589
Cavallo LM, Solari D, Esposito F, Cappabianca P (2013) The endoscopic endonasal approach for the management of craniopharyngiomas involving the third ventricle. Neurosurg Rev 36:27–37 discussion 38
Cavallo LM, Frank G, Cappabianca P, Solari D, Mazzatenta D, Villa A, Zoli M, D'Enza AI, Esposito F, Pasquini E (2014) The endoscopic endonasal approach for the management of craniopharyngiomas: a series of 103 patients. J Neurosurg 121:100–113
Cavallo LM, Solari D, Esposito F, Villa A, Minniti G, Cappabianca P (2014) The role of the endoscopic endonasal route in the management of craniopharyngiomas. World neurosurgery 82:S32–S40
Chung WY, Pan DH, Shiau CY, Guo WY, Wang LW (2000) Gamma knife radiosurgery for craniopharyngiomas. J Neurosurg 93(Suppl 3):47–56
Conger A, Zhao F, Wang X, Eisenberg A, Griffiths C, Esposito F, Carrau RL, Barkhoudarian G, Kelly DF (2018) Evolution of the graded repair of CSF leaks and skull base defects in endonasal endoscopic tumor surgery: trends in repair failure and meningitis rates in 509 patients. J Neurosurg 130(3):861–875. https://doi.org/10.3171/2017.11.JNS172141
Crowley RK, Hamnvik OP, O'Sullivan EP, Behan LA, Smith D, Agha A, Thompson CJ (2010) Morbidity and mortality in patients with craniopharyngioma after surgery. Clin Endocrinol 73:516–521
Crowley RK, Woods C, Fleming M, Rogers B, Behan LA, O'Sullivan EP, Kane T, Agha A, Smith D, Costello RW, Thompson CJ (2011) Somnolence in adult craniopharyngioma patients is a common, heterogeneous condition that is potentially treatable. Clin Endocrinol 74:750–755
Dandurand C, Sepehry AA, Asadi Lari MH, Akagami R, Gooderham P (2017) Adult craniopharyngioma: case series, systematic review, and meta-analysis. Neurosurgery
Dastoli PA, Nicacio JM, Silva NS, Capellano AM, Toledo SR, Ierardi D, Cavalheiro S (2011) Cystic craniopharyngioma: intratumoral chemotherapy with alpha interferon. Arq Neuropsiquiatr 69:50–55
Davies MJ, King TT, Metcalfe KA, Monson JP (1997) Intraventricular craniopharyngioma: a long-term follow-up of six cases. Br J Neurosurg 11:533–541
de Divitiis E, Cappabianca P, Cavallo LM, Esposito F, de Divitiis O, Messina A (2007) Extended endoscopic transsphenoidal approach for extrasellar craniopharyngiomas. Neurosurgery 61:219–227 discussion 228
de Divitiis E, Cavallo LM, Cappabianca P, Esposito F (2007) Extended endoscopic endonasal transsphenoidal approach for the removal of suprasellar tumors: Part 2. Neurosurgery 60:46–58 discussion 58-49
Dhandapani S, Singh H, Negm HM, Cohen S, Souweidane MM, Greenfield JP, Anand VK, Schwartz TH (2017) Endonasal endoscopic reoperation for residual or recurrent craniopharyngiomas. J Neurosurg 126:418–430
Dho YS, Kim YH, Se YB, Han DH, Kim JH, Park CK, Wang KC, Kim DG (2017) Endoscopic endonasal approach for craniopharyngioma: the importance of the relationship between pituitary stalk and tumor. J Neurosurg:1–9
Donnet A, Schmitt A, Dufour H, Grisoli F (1999) Neuropsychological follow-up of twenty two adult patients after surgery for craniopharyngioma. Acta Neurochir 141:1049–1054
Du C, Feng CY, Yuan XR, Liu Q, Peng ZF, Jiang XJ, Li XJ, Xiao GL, Li YF, Xiong T (2016) Microsurgical management of craniopharyngiomas via a unilateral subfrontal approach: a retrospective study of 177 continuous cases. World neurosurgery 90:454–468
Dusick JR, Esposito F, Kelly DF, Cohan P, DeSalles A, Becker DP, Martin NA (2005) The extended direct endonasal transsphenoidal approach for nonadenomatous suprasellar tumors. J Neurosurg 102:832–841
Elliott RE, Jane JA Jr, Wisoff JH (2011) Surgical management of craniopharyngiomas in children: meta-analysis and comparison of transcranial and transsphenoidal approaches. Neurosurgery 69:630–643 discussion 643
Eloy JA, Kuperan AB, Choudhry OJ, Harirchian S, Liu JK (2012) Efficacy of the pedicled nasoseptal flap without cerebrospinal fluid (CSF) diversion for repair of skull base defects: incidence of postoperative CSF leaks. International forum of allergy & rhinology 2:397–401
Erfurth EM, Bulow B, Svahn-Tapper G, Norrving B, Odh K, Mikoczy Z, Bjork J, Hagmar L (2002) Risk factors for cerebrovascular deaths in patients operated and irradiated for pituitary tumors. J Clin Endocrinol Metab 87:4892–4899
Erfurth EM, Holmer H, Fjalldal SB (2013) Mortality and morbidity in adult craniopharyngioma. Pituitary 16:46–55
Evans JJ, Kenning TJ (2014) Craniopharyngiomas; comprehensive diagnosis, treatment and outcome. Elsevier, Oxford
Fahlbusch R, Hofmann BM (2008) Surgical management of giant craniopharyngiomas. Acta Neurochir 150:1213–1226
Fahlbusch R, Honegger J, Paulus W, Huk W, Buchfelder M (1999) Surgical treatment of craniopharyngiomas: experience with 168 patients. J Neurosurg 90:237–250
Fang Y, Cai BW, Zhang H, Liu W, Wu B, Xu JG, You C (2012) Intracystic bleomycin for cystic craniopharyngiomas in children. The Cochrane database of systematic reviews:CD008890
Fernandez-Miranda JC, Gardner PA, Snyderman CH, Devaney KO, Strojan P, Suarez C, Genden EM, Rinaldo A, Ferlito A (2012) Craniopharyngioma: a pathologic, clinical, and surgical review. Head & neck 34:1036–1044
Fjalldal S, Holmer H, Rylander L, Elfving M, Ekman B, Osterberg K, Erfurth EM (2013) Hypothalamic involvement predicts cognitive performance and psychosocial health in long-term survivors of childhood craniopharyngioma. J Clin Endocrinol Metab 98:3253–3262
Fjalldal S, Follin C, Svard D, Rylander L, Gabery S, Petersen A, van Westen D, Sundgren PC, Bjorkman-Burtscher IM, Latt J, Ekman B, Johanson A, Erfurth EM (2018) Microstructural white matter alterations and hippocampal volumes are associated with cognitive deficits in craniopharyngioma. European journal of endocrinology / European Federation of Endocrine Societies 178:577–587
Forbes JA, Ordonez-Rubiano EG, Tomasiewicz HC, Banu MA, Younus I, Dobri GA, Phillips CD, Kacker A, Cisse B, Anand VK, Schwartz TH (2018) Endonasal endoscopic transsphenoidal resection of intrinsic third ventricular craniopharyngioma: surgical results. J Neurosurg:1–11
Fortes FS, Carrau RL, Snyderman CH, Kassam A, Prevedello D, Vescan A, Mintz A, Gardner P (2007) Transpterygoid transposition of a temporoparietal fascia flap: a new method for skull base reconstruction after endoscopic expanded endonasal approaches. Laryngoscope 117:970–976
Fortes FS, Carrau RL, Snyderman CH, Prevedello D, Vescan A, Mintz A, Gardner P, Kassam AB (2007) The posterior pedicle inferior turbinate flap: a new vascularized flap for skull base reconstruction. Laryngoscope 117:1329–1332
Foschi M, Sambati L, Zoli M, Pierangeli G, Cecere A, Mignani F, Barletta G, Sturiale C, Faustini-Fustini M, Milanese L, Cortelli P, Mazzatenta D, Provini F (2017) Site and type of craniopharyngiomas impact differently on 24-hour circadian rhythms and surgical outcome. A neurophysiological evaluation Autonomic neuroscience : basic & clinical 208:126–130
Fournier-Goodnight AS, Ashford JM, Merchant TE, Boop FA, Indelicato DJ, Wang L, Zhang H, Conklin HM (2017) Neurocognitive functioning in pediatric craniopharyngioma: performance before treatment with proton therapy. J Neuro-Oncol 134:97–105
Frank G, Pasquini E, Doglietto F, Mazzatenta D, Sciarretta V, Farneti G, Calbucci F (2006) The endoscopic extended transsphenoidal approach for craniopharyngiomas. Neurosurgery 59:ONS75–ONS83 discussion ONS75-83
Friedman MA, Meyers CA, Sawaya R (2008) Neuropsychological effects of third ventricle tumor surgery. Neurosurgery 62:1093–1100
Frio F, Solari D, Cavallo LM, Cappabianca P, Raverot G, Jouanneau E (2019) Ommaya Reservoir System for the Treatment of Cystic Craniopharyngiomas: Surgical Results in a Series of 11 Adult Patients and Review of the Literature. World neurosurgery
Gardner PA, Kassam AB, Snyderman CH, Carrau RL, Mintz AH, Grahovac S, Stefko S (2008) Outcomes following endoscopic, expanded endonasal resection of suprasellar craniopharyngiomas: a case series. J Neurosurg 109:6–16
Gardner PA, Prevedello DM, Kassam AB, Snyderman CH, Carrau RL, Mintz AH (2008) The evolution of the endonasal approach for craniopharyngiomas. J Neurosurg 108:1043–1047
Gerganov V, Metwali H, Samii A, Fahlbusch R, Samii M (2014) Microsurgical resection of extensive craniopharyngiomas using a frontolateral approach: operative technique and outcome. J Neurosurg 120:559–570
Gonzalez LF, Crawford NR, Horgan MA, Deshmukh P, Zabramski JM, Spetzler RF (2002) Working area and angle of attack in three cranial base approaches: pterional, orbitozygomatic, and maxillary extension of the orbitozygomatic approach. Neurosurgery 50:550–555 discussion 555-557
Gopalan R, Dassoulas K, Rainey J, Sherman JH, Sheehan JP (2008) Evaluation of the role of Gamma Knife surgery in the treatment of craniopharyngiomas. Neurosurg Focus 24:E5
Graffeo CS, Dietrich AR, Grobelny B, Zhang M, Goldberg JD, Golfinos JG, Lebowitz R, Kleinberg D, Placantonakis DG (2014) A panoramic view of the skull base: systematic review of open and endoscopic endonasal approaches to four tumors. Pituitary 17:349–356
Gupta S, Bi WL, Giantini Larsen A, Al-Abdulmohsen S, Abedalthagafi M, Dunn IF (2018) Craniopharyngioma: a roadmap for scientific translation. Neurosurg Focus 44:E12
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ, Group GW (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Bmj 336:924–926
Hackman T, Chicoine MR, Uppaluri R (2009) Novel application of the palatal island flap for endoscopic skull base reconstruction. Laryngoscope 119:1463–1466
Hadad G, Bassagasteguy L, Carrau RL, Mataza JC, Kassam A, Snyderman CH, Mintz A (2006) A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope 116:1882–1886
Hald JK, Eldevik OP, Quint DJ, Chandler WF, Kollevold T (1996) Pre- and postoperative MR imaging of craniopharyngiomas. Acta Radiol 37:806–812
Harvey RJ, Parmar P, Sacks R, Zanation AM (2012) Endoscopic skull base reconstruction of large dural defects: a systematic review of published evidence. Laryngoscope 122:452–459
Hasegawa T, Kondziolka D, Hadjipanayis CG, Lunsford LD (2004) Management of cystic craniopharyngiomas with phosphorus-32 intracavitary irradiation. Neurosurgery 54:813–820 discussion 820-812
Himes BT, Ruff MW, Van Gompel JJ, Park SS, Galanis E, Kaufmann TJ, Uhm JH (2018) Recurrent papillary craniopharyngioma with BRAF V600E mutation treated with dabrafenib: case report. J Neurosurg 1-5. https://doi.org/10.3171/2017.11.JNS172373
Hoffman HJ (1994) Surgical management of craniopharyngioma. Pediatr Neurosurg 21(Suppl 1):44–49
Hoffman HJ, De Silva M, Humphreys RP, Drake JM, Smith ML, Blaser SI (1992) Aggressive surgical management of craniopharyngiomas in children. J Neurosurg 76:47–52
Holmer H, Ekman B, Bjork J, Nordstom CH, Popovic V, Siversson A, Erfurth EM (2009) Hypothalamic involvement predicts cardiovascular risk in adults with childhood onset craniopharyngioma on long-term GH therapy. European journal of endocrinology / European Federation of Endocrine Societies 161:671–679
Holmer H, Pozarek G, Wirfalt E, Popovic V, Ekman B, Bjork J, Erfurth EM (2010) Reduced energy expenditure and impaired feeding-related signals but not high energy intake reinforces hypothalamic obesity in adults with childhood onset craniopharyngioma. J Clin Endocrinol Metab 95:5395–5402
Honegger J, Barocka A, Sadri B, Fahlbusch R (1998) Neuropsychological results of craniopharyngioma surgery in adults: a prospective study. Surg Neurol 50:19–28 discussion 28-19
Honegger J, Buchfelder M, Fahlbusch R (1999) Surgical treatment of craniopharyngiomas: endocrinological results. J Neurosurg 90:251–257
Hori T, Kawamata T, Amano K, Aihara Y, Ono M, Miki N (2010) Anterior interhemispheric approach for 100 tumors in and around the anterior third ventricle. Neurosurgery 66:65–74
Ikeda H, Gotoh H, Watanabe K (2012) Outcome of endoscopy-assisted microscopic extended transsphenoidal surgery for suprasellar adult craniopharyngiomas. Front Endocrinol 3:25
Ishii Y, Tahara S, Teramoto A, Morita A (2014) Endoscopic endonasal skull base surgery: advantages, limitations, and our techniques to overcome cerebrospinal fluid leakage: technical note. Neurol Med Chir 54:983–990
Iwasaki K, Kondo A, Takahashi JB, Yamanobe K (1992) Intraventricular craniopharyngioma: report of two cases and review of the literature. Surg Neurol 38:294–301
Jacobsen MF, Thomsen ASS, Bach-Holm D, Doroudian G, Nissen KR, Fugleholm K, Poulsgaard L, Siersma V, Heegaard S (2018) Predictors of visual outcome in patients operated for craniopharyngioma - a Danish national study. Acta Ophthalmol 96:39–45
Jane JA Jr, Kiehna E, Payne SC, Early SV, Laws ER Jr (2010) Early outcomes of endoscopic transsphenoidal surgery for adult craniopharyngiomas. Neurosurg Focus 28:E9
Jasim S, Alahdab F, Ahmed AT, Tamhane S, Prokop LJ, Nippoldt TB, Murad MH (2017) Mortality in adults with hypopituitarism: a systematic review and meta-analysis. Endocrine 56:33–42
Jeswani S, Nuno M, Wu A, Bonert V, Carmichael JD, Black KL, Chu R, King W, Mamelak AN (2016) Comparative analysis of outcomes following craniotomy and expanded endoscopic endonasal transsphenoidal resection of craniopharyngioma and related tumors: a single-institution study. J Neurosurg 124:627–638
Jung TY, Jung S, Choi JE, Moon KS, Kim IY, Kang SS (2009) Adult craniopharyngiomas: surgical results with a special focus on endocrinological outcomes and recurrence according to pituitary stalk preservation. J Neurosurg 111:572–577
Jung TY, Jung S, Moon KS, Kim IY, Kang SS, Kim JH (2010) Endocrinological outcomes of pediatric craniopharyngiomas with anatomical pituitary stalk preservation: preliminary study. Pediatr Neurosurg 46:205–212
Kaptain GJ, Vincent DA, Sheehan JP, Laws ER Jr (2001) Transsphenoidal approaches for the extracapsular resection of midline suprasellar and anterior cranial base lesions. Neurosurgery 49:94–100 discussion 100-101
Karavitaki N (2014) Management of craniopharyngiomas. J Endocrinol Investig 37:219–228
Karavitaki N, Brufani C, Warner JT, Adams CB, Richards P, Ansorge O, Shine B, Turner HE, Wass JA (2005) Craniopharyngiomas in children and adults: systematic analysis of 121 cases with long-term follow-up. Clin Endocrinol 62:397–409
Karavitaki N, Cudlip S, Adams CB, Wass JA (2006) Craniopharyngiomas. Endocr Rev 27:371–397
Kassam AB, Gardner PA, Snyderman CH, Carrau RL, Mintz AH, Prevedello DM (2008) Expanded endonasal approach, a fully endoscopic transnasal approach for the resection of midline suprasellar craniopharyngiomas: a new classification based on the infundibulum. J Neurosurg 108:715–728
Kato T, Sawamura Y, Abe H, Nagashima M (1998) Transsphenoidal-transtuberculum sellae approach for supradiaphragmatic tumours: technical note. Acta Neurochir 140:715–718 discussion 719
Kerr EE, Jamshidi A, Carrau RL, Campbell RG, Filho LFD, Otto BA, Prevedello DM (2017) Indocyanine green fluorescence to evaluate nasoseptal flap viability in endoscopic endonasal cranial base surgery. Journal of neurological surgery. Part B, Skull base 78:408–412
Kim J, Choe I, Bak K, Kim C, Kim N, Jang Y (2000) Transsphenoidal supradiaphragmatic intradural approach: technical note. Minimally invasive neurosurgery : MIN 43:33–37
Kim SK, Kim YH, Park CK, Kim DG, Jung HW (2014) Extended endoscopic endonasal approach for recurrent or residual adult craniopharyngiomas. Acta Neurochir 156:1917–1922
Kim KH, Kim YH, Dho YS, Kim JH, Hong SD, Choi JW, Seol HJ, Nam DH, Lee JI, Park CK, Kong DS (2018) Is low-lying optic chiasm an obstacle to an endoscopic endonasal approach for retrochiasmatic craniopharyngiomas? (Korean Society of Endoscopic Neurosurgery -003). World neurosurgery 114:e306–e316
Kobayashi T (2009) Long-term results of gamma knife radiosurgery for 100 consecutive cases of craniopharyngioma and a treatment strategy. Progress in neurological surgery 22:63–76
Kobayashi T, Kida Y, Hasegawa T (2003) Long-term results of gamma knife surgery for craniopharyngioma. Neurosurg Focus 14:e13
Komotar RJ, Roguski M, Bruce JN (2009) Surgical management of craniopharyngiomas. J Neuro-Oncol 92:283–296
Komotar RJ, Starke RM, Raper DM, Anand VK, Schwartz TH (2012) Endoscopic endonasal compared with microscopic transsphenoidal and open transcranial resection of craniopharyngiomas. World neurosurgery 77:329–341
Komotar RJ, Starke RM, Raper DM, Anand VK, Schwartz TH (2012) Endoscopic endonasal versus open transcranial resection of anterior midline skull base meningiomas. World neurosurgery 77:713–724
Konovalov AN (2014) Third ventricle craniopharyngiomas. World neurosurgery 82:1023–1025
Kouri JG, Chen MY, Watson JC, Oldfield EH (2000) Resection of suprasellar tumors by using a modified transsphenoidal approach. Report of four cases Journal of neurosurgery 92:1028–1035
Koutourousiou M, Gardner PA, Fernandez-Miranda JC, Tyler-Kabara EC, Wang EW, Snyderman CH (2013) Endoscopic endonasal surgery for craniopharyngiomas: surgical outcome in 64 patients. J Neurosurg 119:1194–1207
Laws ER Jr, Vance ML (1999) Radiosurgery for pituitary tumors and craniopharyngiomas. Neurosurg Clin N Am 10:327–336
Leng LZ, Brown S, Anand VK, Schwartz TH (2008) “Gasket-seal” watertight closure in minimal-access endoscopic cranial base surgery. Neurosurgery 62:ONSE342–ONSE343 discussion ONSE343
Leng LZ, Greenfield JP, Souweidane MM, Anand VK, Schwartz TH (2012) Endoscopic, endonasal resection of craniopharyngiomas: analysis of outcome including extent of resection, cerebrospinal fluid leak, return to preoperative productivity, and body mass index. Neurosurgery 70:110–123 discussion 123-114
Li K, Lu X, Yang N, Zheng J, Huang B, Li L (2015) Association of pituitary stalk management with endocrine outcomes and recurrence in microsurgery of craniopharyngiomas: a meta-analysis. Clin Neurol Neurosurg 136:20–24
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Bmj 339:b2700
Liu JK, Eloy JA (2012) Endoscopic endonasal transplanum transtuberculum approach for resection of retrochiasmatic craniopharyngioma. J Neurosur 32:E2
Liu JK, Cole CD, Kestle JR, Brockmeyer DL, Walker ML (2005) Cranial base strategies for resection of craniopharyngioma in children. Neurosurg Focus 18:E9
Liu JK, Christiano LD, Gupta G, Carmel PW (2010) Surgical nuances for removal of retrochiasmatic craniopharyngiomas via the transbasal subfrontal translamina terminalis approach. Neurosurg Focus 28:E6
Liu JK, Sevak IA, Carmel PW, Eloy JA (2016) Microscopic versus endoscopic approaches for craniopharyngiomas: choosing the optimal surgical corridor for maximizing extent of resection and complication avoidance using a personalized, tailored approach. Neurosurg Focus 41:E5
Losa M, Pieri V, Bailo M, Gagliardi F, Barzaghi LR, Gioia L, Del Vecchio A, Bolognesi A, Mortini P (2018) Single fraction and multisession Gamma Knife radiosurgery for craniopharyngioma. Pituitary 21:499–506
Maira G, Anile C, Rossi GF, Colosimo C (1995) Surgical treatment of craniopharyngiomas: an evaluation of the transsphenoidal and pterional approaches. Neurosurgery 36:715–724
Maison P, Griffin S, Nicoue-Beglah M, Haddad N, Balkau B, Chanson P (2004) Metaanalysis of Blinded RP-CT (2004) Impact of growth hormone (GH) treatment on cardiovascular risk factors in GH-deficient adults: a metaanalysis of blinded, randomized, placebo-controlled trials. J Clin Endocrinol Metab 89:2192–2199
Martinez-Barbera JP (2015) Molecular and cellular pathogenesis of adamantinomatous craniopharyngioma. Neuropathol Appl Neurobiol 41:721–732
Mascarenhas L, Moshel YA, Bayad F, Szentirmai O, Salek AA, Leng LZ, Hofstetter CP, Placantonakis DG, Tsiouris AJ, Anand VK, Schwartz TH (2014) The transplanum transtuberculum approaches for suprasellar and sellar-suprasellar lesions: avoidance of cerebrospinal fluid leak and lessons learned. World neurosurgery 82:186–195
McLendon RE, Rosenblum MK, Bigner DD (2006) Pathology of tumors of the nervous system 7ed. Russell & Rubinstein's, London
Mediero S, Noval S, Bravo-Ljubetic L, Contreras I, Carceller F (2015) Visual outcomes, visual fields, and optical coherence tomography in paediatric craniopharyngioma. Neuro-ophthalmology 39:132–139
Meling TR, Nylen ES (1996) Growth hormone deficiency in adults: a review. Am J Med Sci 311:153–166
Miljic D, Popovic V (2018) Metabolic syndrome in hypopituitarism. Front Horm Res 49:1–19
Minamida Y, Mikami T, Hashi K, Houkin K (2005) Surgical management of the recurrence and regrowth of craniopharyngiomas. J Neurosurg 103:224–232
Minniti G, Saran F, Traish D, Soomal R, Sardell S, Gonsalves A, Ashley S, Warrington J, Burke K, Mosleh-Shirazi A, Brada M (2007) Fractionated stereotactic conformal radiotherapy following conservative surgery in the control of craniopharyngiomas. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 82:90–95
Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Vance ML, Endocrine S (2011) Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96:1587–1609
Moon CH, Hwang SC, Kim BT, Ohn YH, Park TK (2011) Visual prognostic value of optical coherence tomography and photopic negative response in chiasmal compression. Invest Ophthalmol Vis Sci 52:8527–8533
Mortini P, Losa M, Pozzobon G, Barzaghi R, Riva M, Acerno S, Angius D, Weber G, Chiumello G, Giovanelli M (2011) Neurosurgical treatment of craniopharyngioma in adults and children: early and long-term results in a large case series. J Neurosurg 114:1350–1359
Moussazadeh N, Prabhu V, Bander ED, Cusic RC, Tsiouris AJ, Anand VK, Schwartz TH (2016) Endoscopic endonasal versus open transcranial resection of craniopharyngiomas: a case-matched single-institution analysis. Neurosurg Focus 41:E7
Mrowczynski OD, Langan ST, Rizk EB (2018) Craniopharyngiomas: a systematic review and evaluation of the current intratumoral treatment landscape. Clin Neurol Neurosurg 166:124–130
Muller HL, Gebhardt U, Teske C, Faldum A, Zwiener I, Warmuth-Metz M, Pietsch T, Pohl F, Sorensen N, Calaminus G, Study Committee of K (2011) Post-operative hypothalamic lesions and obesity in childhood craniopharyngioma: results of the multinational prospective trial KRANIOPHARYNGEOM 2000 after 3-year follow-up. European journal of endocrinology / European Federation of Endocrine Societies 165:17–24
Niranjan A, Kano H, Mathieu D, Kondziolka D, Flickinger JC, Lunsford LD (2010) Radiosurgery for craniopharyngioma. Int J Radiat Oncol Biol Phys 78:64–71
Nishioka H, Fukuhara N, Yamaguchi-Okada M, Yamada S (2016) Endoscopic endonasal surgery for purely intrathird ventricle craniopharyngioma. World neurosurgery 91:266–271
Nyquist GG, Anand VK, Singh A, Schwartz TH (2010) Janus flap: bilateral nasoseptal flaps for anterior skull base reconstruction. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery 142:327–331
Oliver CL, Hackman TG, Carrau RL, Snyderman CH, Kassam AB, Prevedello DM, Gardner P (2008) Palatal flap modifications allow pedicled reconstruction of the skull base. Laryngoscope 118:2102–2106
Omay SB, Almeida JP, Chen YN, Shetty SR, Liang B, Ni S, Anand VK, Schwartz TH (2017) Is the chiasm-pituitary corridor size important for achieving gross-total resection during endonasal endoscopic resection of craniopharyngiomas? J Neurosurg 129(3):642–647. https://doi.org/10.3171/2017.6.JNS163188
Owen PJ, Lazarus JH (2003) Subclinical hypothyroidism: the case for treatment. Trends Endocrinol Metab 14:257–261
Ozyurt J, Muller HL, Thiel CM (2015) A systematic review of cognitive performance in patients with childhood craniopharyngioma. J Neuro-Oncol 125:9–21
Park HR, Kshettry VR, Farrell CJ, Lee JM, Kim YH, Won TB, Han DH, Do H, Nyguist G, Rosen M, Kim DG, Evans JJ, Paek SH (2017) Clinical outcome after extended endoscopic endonasal resection of craniopharyngiomas: two-institution experience. World neurosurgery 103:465–474
Pascual JM, Carrasco R, Prieto R, Gonzalez-Llanos F, Alvarez F, Roda JM (2008) Craniopharyngioma classification. J Neurosurg 109:1180–1182 author reply 1182-1183
Pascual JM, Prieto R, Carrasco R (2011) Infundibulo-tuberal or not strictly intraventricular craniopharyngioma: evidence for a major topographical category. Acta Neurochir 153:2403–2425 discussion 2426
Patel MR, Taylor RJ, Hackman TG, Germanwala AV, Sasaki-Adams D, Ewend MG, Zanation AM (2014) Beyond the nasoseptal flap: outcomes and pearls with secondary flaps in endoscopic endonasal skull base reconstruction. Laryngoscope 124:846–852
Patel VS, Thamboo A, Quon J, Nayak JV, Hwang PH, Edwards M, Patel ZM (2017) Outcomes after endoscopic endonasal resection of craniopharyngiomas in the pediatric population. World neurosurgery 108:6–14
Pekmezci M, Louie J, Gupta N, Bloomer MM, Tihan T (2010) Clinicopathological characteristics of adamantinomatous and papillary craniopharyngiomas: University of California, San Francisco experience 1985-2005. Neurosurgery 67:1341–1349 discussion 1349
Pereira AM, Schmid EM, Schutte PJ, Voormolen JH, Biermasz NR, van Thiel SW, Corssmit EP, Smit JW, Roelfsema F, Romijn JA (2005) High prevalence of long-term cardiovascular, neurological and psychosocial morbidity after treatment for craniopharyngioma. Clin Endocrinol 62:197–204
Pinheiro-Neto CD, Carrau RL, Prevedello DM, Fernandez-Miranda JC, Snyderman CS, Gardner PA, Kassam AB (2010) Use of acoustic Doppler sonography to ascertain the feasibility of the pedicled nasoseptal flap after prior bilateral sphenoidotomy. Laryngoscope 120:1798–1801
Poretti A, Grotzer MA, Ribi K, Schonle E, Boltshauser E (2004) Outcome of craniopharyngioma in children: long-term complications and quality of life. Dev Med Child Neurol 46:220–229
Prieto R, Pascual JM, Barrios L (2015) Optic chiasm distortions caused by craniopharyngiomas: clinical and magnetic resonance imaging correlation and influence on visual outcome. World neurosurgery 83:500–529
Prieto R, Pascual JM, Rosdolsky M, Castro-Dufourny I, Carrasco R, Strauss S, Barrios L (2016) Craniopharyngioma adherence: a comprehensive topographical categorization and outcome-related risk stratification model based on the methodical examination of 500 tumors. Neurosurg Focus 41:E13
Prieto R, Pascual JM, Barrios L (2017) Optic chiasm distortions in craniopharyngiomas: a sign of hypothalamic involvement. Acta Neurochir 159:1533–1535
Prieto R, Pascual JM, Barrios L (2017) Topographic diagnosis of craniopharyngiomas: the accuracy of MRI findings observed on conventional T1 and T2 images. AJNR Am J Neuroradiol 38:2073–2080
Prieto R, Pascual JM, Rosdolsky M, Barrios L (2017) Preoperative assessment of craniopharyngioma adherence: magnetic resonance imaging findings correlated with the severity of tumor attachment to the hypothalamus. World neurosurgery
Puget S, Garnett M, Wray A, Grill J, Habrand JL, Bodaert N, Zerah M, Bezerra M, Renier D, Pierre-Kahn A, Sainte-Rose C (2007) Pediatric craniopharyngiomas: classification and treatment according to the degree of hypothalamic involvement. J Neurosurg 106:3–12
Qi S, Lu Y, Pan J, Zhang X, Long H, Fan J (2011) Anatomic relations of the arachnoidea around the pituitary stalk: relevance for surgical removal of craniopharyngiomas. Acta Neurochir 153:785–796
Raimondi AJ, Rougerie J (1994) A critical review of personal experiences with craniopharyngioma: clinical history, surgical technique and operative results. 1983. Pediatr Neurosurg 21:134–150 discussion 151-134
Regine WF, Mohiuddin M, Kramer S (1993) Long-term results of pediatric and adult craniopharyngiomas treated with combined surgery and radiation. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 27:13–21
Repka MX, Miller NR, Miller M (1989) Visual outcome after surgical removal of craniopharyngiomas. Ophthalmology 96:195–199
Samii M, Tatagiba M (1997) Surgical management of craniopharyngiomas: a review. Neurol Med Chir 37:141–149
Schmalisch K, Beschorner R, Psaras T, Honegger J (2010) Postoperative intracranial seeding of craniopharyngiomas--report of three cases and review of the literature. Acta Neurochir 152:313–319 discussion 319
Schoenfeld A, Pekmezci M, Barnes MJ, Tihan T, Gupta N, Lamborn KR, Banerjee A, Mueller S, Chang S, Berger MS, Haas-Kogan D (2012) The superiority of conservative resection and adjuvant radiation for craniopharyngiomas. J Neuro-Oncol 108:133–139
Schulz-Ertner D, Frank C, Herfarth KK, Rhein B, Wannenmacher M, Debus J (2002) Fractionated stereotactic radiotherapy for craniopharyngiomas. Int J Radiat Oncol Biol Phys 54:1114–1120
Schwartz TH (2015) Editorial: Does chiasmatic blood supply dictate endonasal corridors? J Neurosurg 122:1163–1164
Schwartz TH, Morgenstern PF, Anand VK (2019) Lessons learned in the evolution of endoscopic skull base surgery. J Neurosurg 130:337–346
Sherlock M, Reulen RC, Alonso AA, Ayuk J, Clayton RN, Sheppard MC, Hawkins MM, Bates AS, Stewart PM (2009) ACTH deficiency, higher doses of hydrocortisone replacement, and radiotherapy are independent predictors of mortality in patients with acromegaly. J Clin Endocrinol Metab 94:4216–4223
Shi XE, Wu B, Fan T, Zhou ZQ, Zhang YL (2008) Craniopharyngioma: surgical experience of 309 cases in China. Clin Neurol Neurosurg 110:151–159
Steno J, Malacek M, Bizik I (2004) Tumor-third ventricular relationships in supradiaphragmatic craniopharyngiomas: correlation of morphological, magnetic resonance imaging, and operative findings. Neurosurgery 54:1051–1058 discussion 1058-1060
Steno J, Bizik I, Steno A, Matejcik V (2014) Recurrent craniopharyngiomas in children and adults: long-term recurrence rate and management. Acta Neurochir 156:113–122 discussion 122
Sughrue ME, Yang I, Kane AJ, Fang S, Clark AJ, Aranda D, Barani IJ, Parsa AT (2011) Endocrinologic, neurologic, and visual morbidity after treatment for craniopharyngioma. J Neuro-Oncol 101:463–476
Thorp BD, Sreenath SB, Ebert CS, Zanation AM (2014) Endoscopic skull base reconstruction: a review and clinical case series of 152 vascularized flaps used for surgical skull base defects in the setting of intraoperative cerebrospinal fluid leak. Neurosurg Focus 37:E4
Tomlinson JW, Holden N, Hills RK, Wheatley K, Clayton RN, Bates AS, Sheppard MC, Stewart PM (2001) Association between premature mortality and hypopituitarism. West Midlands Prospective Hypopituitary Study Group Lancet 357:425–431
Turel MK, Tsermoulas G, Gonen L, Klironomos G, Almeida JP, Zadeh G, Gentili F (2016) Management and outcome of recurrent adult craniopharyngiomas: an analysis of 42 cases with long-term follow-up. Neurosurg Focus 41:E11
Ulfarsson E, Lindquist C, Roberts M, Rahn T, Lindquist M, Thoren M, Lippitz B (2002) Gamma knife radiosurgery for craniopharyngiomas: long-term results in the first Swedish patients. J Neurosurg 97:613–622
Van Effenterre R, Boch AL (2002) Craniopharyngioma in adults and children: a study of 122 surgical cases. J Neurosurg 97:3–11
van Iersel L, Brokke KE, Adan RAH, Bulthuis LCM, van den Akker ELT, van Santen HM (2019) Pathophysiology and individualized treatment of hypothalamic obesity following craniopharyngioma and other suprasellar tumors: a systematic review. Endocr Rev 40:193–235
Van Zele T, Bachert C (2011) Endoscopic skull base reconstruction after endoscopic endonasal approach. B-Ent 7(Suppl 17):41–46
Wan MJ, Zapotocky M, Bouffet E, Bartels U, Kulkarni AV, Drake JM (2018) Long-term visual outcomes of craniopharyngioma in children. J Neuro-Oncol
Wang KC, Hong SH, Kim SK, Cho BK (2005) Origin of craniopharyngiomas: implication on the growth pattern. Child’s nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery 21:628–634
Wang F, Jiang J, Zhang J, Wang Q (2017) Predicting pituitary stalk position by in vivo visualization of the hypothalamo-hypophyseal tract in craniopharyngioma using diffusion tensor imaging tractography. Neurosurg Rev
Wang EW, Zanation AM, Gardner PA, Schwartz TH, Eloy JA, Adappa ND, Bettag M, Bleier BS, Cappabianca P, Carrau RL, Casiano RR, Cavallo LM, Ebert CS Jr, El-Sayed IH, Evans JJ, Fernandez-Miranda JC, Folbe AJ, Froelich S, Gentili F, Harvey RJ, Hwang PH, Jane JA Jr, Kelly DF, Kennedy D, Knosp E, Lal D, Lee JYK, Liu JK, Lund VJ, Palmer JN, Prevedello DM, Schlosser RJ, Sindwani R, Solares CA, Tabaee A, Teo C, Thirumala PD, Thorp BD, de Arnaldo Silva Vellutini E, Witterick I, Woodworth BA, Wormald PJ, Snyderman CH (2019) ICAR: endoscopic skull-base surgery. International forum of allergy & rhinology 9:S145–S365
Wannemuehler TJ, Rubel KE, Hendricks BK, Ting JY, Payner TD, Shah MV, Cohen-Gadol AA (2016) Outcomes in transcranial microsurgery versus extended endoscopic endonasal approach for primary resection of adult craniopharyngiomas. Neurosurg Focus 41:E6
Weiner HL, Wisoff JH, Rosenberg ME, Kupersmith MJ, Cohen H, Zagzag D, Shiminski-Maher T, Flamm ES, Epstein FJ, Miller DC (1994) Craniopharyngiomas: a clinicopathological analysis of factors predictive of recurrence and functional outcome. Neurosurgery 35:1001–1010 discussion 1010-1001
Wijnen M, Olsson DS, van den Heuvel-Eibrink MM, Hammarstrand C, Janssen J, van der Lely AJ, Johannsson G, Neggers S (2018) Excess morbidity and mortality in patients with craniopharyngioma: a hospital-based retrospective cohort study. European journal of endocrinology / European Federation of Endocrine Societies 178:95–104
Wisoff JH (1994) Surgical management of recurrent craniopharyngiomas. Pediatr Neurosurg 21(Suppl 1):108–113
Xiao G, Yuan X, Yuan J, Krumtally NA, Li Y, Feng C, Liu Q, Peng Z, Li X, Ding X (2014) Pituitary stalk management during the microsurgery of craniopharyngiomas. Experimental and therapeutic medicine 7:1055–1064
Yamada S, Fukuhara N, Oyama K, Takeshita A, Takeuchi Y, Ito J, Inoshita N (2010) Surgical outcome in 90 patients with craniopharyngioma: an evaluation of transsphenoidal surgery. World neurosurgery 74:320–330
Yang I, Sughrue ME, Rutkowski MJ, Kaur R, Ivan ME, Aranda D, Barani IJ, Parsa AT (2010) Craniopharyngioma: a comparison of tumor control with various treatment strategies. Neurosurg Focus 28:E5
Yano S, Hide T, Shinojima N (2017) Surgical outcomes of endoscopic endonasal skull base surgery of craniopharyngiomas evaluated according to the degree of hypothalamic extension. World neurosurgery 100:288–296
Yasargil MG (1996) Microneurosurgery. Thieme, New York
Yasargil MG, Curcic M, Kis M, Siegenthaler G, Teddy PJ, Roth P (1990) Total removal of craniopharyngiomas. Approaches and long-term results in 144 patients. J Neurosurg 73:3–11
Yu T, Sun X, Ren X, Cui X, Wang J, Lin S (2014) Intraventricular craniopharyngiomas: surgical management and outcome analyses in 24 cases. World neurosurgery 82:1209–1215
Yue Q, Yu Y, Shi Z, Wang Y, Zhu W, Du Z, Yao Z, Chen L, Mao Y (2017) Prediction of BRAF mutation status of craniopharyngioma using magnetic resonance imaging features. J Neurosurg:1–8
Yuen KCJ, Mattsson AF, Burman P, Erfurth EM, Camacho-Hubner C, Fox JL, Verhelst J, Geffner ME, Abs R (2018) Relative risks of contributing factors to morbidity and mortality in adults with craniopharyngioma on growth hormone replacement. J Clin Endocrinol Metab 103:768–777
Zacharia BE, Bruce SS, Goldstein H, Malone HR, Neugut AI, Bruce JN (2012) Incidence, treatment and survival of patients with craniopharyngioma in the surveillance, epidemiology and end results program. Neuro-oncology 14:1070–1078
Zanation AM, Snyderman CH, Carrau RL, Kassam AB, Gardner PA, Prevedello DM (2009) Minimally invasive endoscopic pericranial flap: a new method for endonasal skull base reconstruction. Laryngoscope 119:13–18
Zimmer LA, Theodosopoulos PV (2009) Anterior skull base surgery: open versus endoscopic. Current opinion in otolaryngology & head and neck surgery 17:75–78
Zoicas F, Schofl C (2012) Craniopharyngioma in adults. Front Endocrinol 3:46
Zoli M, Sambati L, Milanese L, Foschi M, Faustini-Fustini M, Marucci G, de Biase D, Tallini G, Cecere A, Mignani F, Sturiale C, Frank G, Pasquini E, Cortelli P, Mazzatenta D, Provini F (2016) Postoperative outcome of body core temperature rhythm and sleep-wake cycle in third ventricle craniopharyngiomas. Neurosurg Focus 41:E12
Author information
Authors and Affiliations
Contributions
We declare that Mahmoud Messerer had the idea for the article.
Giulia Cossu and Mahmoud Messerer performed the literature search. GC and MM together with Roy Thomas Daniel performed the literature analysis.
GC, MM and Moncef Berhouma drafted the article.
Emmanuel Jouanneau, Luigi M Cavallo, Samer K Elbabaa, Lorenzo Giammattei, Daniele Starnoni, Juan Barges-Coll, Paolo Cappabianca, Vladimir Benes, Mustafa K. Baskaya, Michael Bruneau, Torstein Meling, Karl Schaller, Ari G Chacko, A. Samy Youssef, Diego Mazzatenta, Mario Ammirati, Henry Dufour, Edward Laws, Moncef Berhouma, and Roy Thomas Daniel critically revised the article and gave a substantial contribution in the improvement of the content of the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Comments
The authors are to be congratulated for composing this consensus statement on the present management of craniopharyngiomas in adult patients. The recommendations represent our current understanding of the surgical approaches and alternative treatment strategies for these tumors at first treatment and recurrence.
I personally am in agreement with the consensus recommendations as written. With the identification of targetable mutations in these tumors it will be important to modify recommendations for residual and recurrent tumors as evidence accrues as to the relative advantage of these treatments. As such recommendations may change rapidly.
A minor comment would reflect on the search terms used for the analysis- they have used “microscopic” and “endoscopic” but have not used the terms “transnasal” or “transsphenoidal”. The endoscope and microscope are visualization tools but not approaches in of themselves. With the terms used they have overlooked many papers that have long emphasized the importance of the transnasal approach as a good option for these tumors, before the use of the endoscope was popularized. This advantage of the transnasal approach is that it enables direct visualization of these tumors, along the axis of growth, without traversing the cerebrum in any fashion. Third ventricular extensions of sellar or suprasellar tumors are easily reached under direct visualization. In consideration of the microscopic transnasal approach, this now represents an over 40-year significant experience with the transnasal approach for accessible tumors (without significant lateral extent) that commenced with the microscope but was later facilitated with the endoscope, notably by Laws, Fahlbusch, and Weiss (1,2).
William Couldwell
Utah, USA
1. Couldwell WT, Weiss MH, Rabb C, Liu JK, Apfelbaum RI, Fukushima T. (2004)Variations on the standard transsphenoidal approach to the sellar region, with emphasis on the extended approaches and parasellar approaches: surgical experience in 105 cases. Neurosurgery. 55(3):539–550.
2. Honegger J, Buchfelder M, Fahlbusch R, Däubler B, Dörr HG. (1992)Transsphenoidal microsurgery for craniopharyngioma. Surg Neurol. 37(3):189–196.
3. Laws ER Jr. (1980)Transsphenoidal microsurgery in the management of craniopharyngioma. J Neurosurg. 52(5):661-6
4. Weiss M.H. (1987) The transnasal transsphenoidal approach. In: Apuzzo MLJ (ed) Surgery of the third ventride. Williams & Wilkins, Baltimore, pp 476–494
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pituitaries
Rights and permissions
About this article
Cite this article
Cossu, G., Jouanneau, E., Cavallo, L.M. et al. Surgical management of craniopharyngiomas in adult patients: a systematic review and consensus statement on behalf of the EANS skull base section. Acta Neurochir 162, 1159–1177 (2020). https://doi.org/10.1007/s00701-020-04265-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-020-04265-1