Abstract
P-wave morphology reflects atrial remodeling and indicates prognosis after radiofrequency catheter ablation (RFCA) for atrial fibrillation (AF). The impact of p-wave morphology after excluding the effect of pulmonary vein (PV) substrate on outcomes is unknown. We evaluated the p-wave morphology on electrocardiography immediately after PV isolation for clinical outcomes. Eighty-four consecutive patients (47 with paroxysmal AF and 37 with persistent AF) who underwent RFCA were included. P-wave duration (PWD) and amplitude in all leads were examined during sinus rhythm immediately after PV isolation. We evaluated the relationship between electrocardiogram parameters and AF recurrence, according to the type of AF and following ablation, and the correlation with left atrial (LA) volume, low voltage ratio, and fixed conduction time. During 12 months of follow-up, 20 patients experienced recurrence. The cut-off value of PWD > 120 ms in lead I showed a sensitivity of 75% and specificity of 69% for predicting recurrence. PWD was significantly correlated with LA volume, low voltage, and conduction velocity. Significantly higher recurrence rates were observed in patients with PWD > 120 ms than in those with PWD ≤ 120 ms (p < 0.001), and the difference was more pronounced in patients with persistent AF. Multivariate analysis demonstrated that PWD > 120 ms was independently associated with recurrence in the total population (hazard ratio 2.00; 95% confidence interval 1.27–3.22; p = 0.003) and in patients with persistent AF. In conclusion, long PWD after PV isolation predicts AF recurrence, which might be associated with the extent of the LA substrate in persistent AF.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Radiofrequency catheter ablation (RFCA) is an effective method of rhythm control for atrial fibrillation (AF), and its success rate is approximately 70–80% with a single procedure [1]. Pulmonary vein (PV) isolation of AF has a remarkable performance with maintaining sinus rhythm, because ectopic activity in PVs plays the important role of initiating and perpetuating AF [2]. However, there is often a problem of AF recurrence after RFCA, which is mainly because of PV reconnection or substrate remodeling of the left atrium (LA). LA remodeling refers to the spectrum of pathophysiological changes in atrial structure and mechanical and electric functions, which often becomes associated with an increased risk of cardiovascular and embolic events and mortality through an intervention of AF [3,4,5].
P-wave morphology on an electrocardiogram (ECG) can be a simple and non-invasive tool to reflect the process of electrical and structural remodeling, because its duration and amplitude can be associated with atrial conduction and the direction of atrial depolarization and myocardial mass. In particular, prolonged p-wave duration (PWD) has been proven to represent AF-associated atrial structural alterations [6]. There are several studies in which pre-ablation p-wave morphology can be identified as a predictor of AF recurrence after PV isolation [6,7,8,9,10,11,12]. However, standard measurement of PWD on ECG can be calculated at the time of sinus rhythm only. The p-wave morphology during AF rhythm cannot be assessed before the procedure, and therefore, p-wave analysis is not possible prior to ablation in patients with persistent AF. By contrast, there are few prior studies investigating the relationship of post-ablation p-wave morphology with the procedure outcome [13, 14]. The p-wave could also change after catheter ablation because of the modification of atrial myocardial condition. In particular, the p-wave after PV isolation could reflect the remaining atrial substrate in the LA and be a potential marker for estimating LA substrate requiring additional ablation after PV isolation. It is unclear whether p-wave morphology could be a predictor of prognosis and a representative marker for the estimation of additional LA substrate after excluding the effect of arrhythmogenic PV substrate by catheter ablation.
Thus, this study aimed to investigate the relationship between p-wave morphology on ECG immediately after PV isolation and before additional substrate procedure with prognosis after ablation for AF, and to evaluate the correlation between p-wave morphology and LA volume, voltage substrate, and conduction velocity.
Material and methods
Study population
The present study retrospectively analyzed a catheter ablation database at Kasugai Municipal Hospital in Japan. A total of 84 consecutive patients with symptomatic, refractory AF who underwent RFCA for the first time between January 2017 and May 2018 were enrolled. The indication for catheter ablation is in accordance with recent guidelines [15]. All patients underwent preprocedural imaging study using contrast computed tomography (CT), in which replacement fluid was sometimes needed for patients with renal failure. Each three-dimensional (3D) LA anatomical structure was constructed with this imaging to prepare for catheter ablation and to measure prior LA volume. Transthoracic and transesophageal echocardiography were also performed to comprehend information, including the existence or absence of LA thrombus before the procedure. The exclusion criteria for this study were as follows: (i) severe valvular heart disease; (ii) previous RFCA treatment; (iii) follow-up period < 3 months after ablation; (iv) non-radiofrequency, cryoballoon ablation treatment; and (v) failure to restore sinus rhythm by cardioversion after PV isolation. This study was approved by our institutional ethics committee. All patients provided written informed consent prior to the procedure. All examinations and procedures were performed in compliance with the principles of the Declaration of Helsinki.
Catheter ablation and postprocedural follow-up
Antiarrhythmic drugs (AADs) were discontinued for at least five half-lives before catheter ablation in all patients. All ablation procedures, including PV isolation, were performed under the guidance of the 3D-electroanatomical mapping system (CARTO, Biosense Webster) with LA geometry obtained from the CT image. We used a 3.5 mm tip, open-irrigated contact force sensing ablation catheter (25–35 W, 42 ℃, irrigation flow rate of 17–30 mL/min; NaviStar ThermoCool SmartTouch, Biosense Webster, Inc.), a circular mapping catheter (Lasso, Biosense Webster, Inc), and a multi-electrode mapping catheter (PentaRay, Biosense Webster, Inc.). The contact force of the catheter was targeted at 10–25 g, and catheter stability was set with a force–time integral of > 100 g*s. PV isolation was confirmed by the elimination of PV potentials as recorded by a circular mapping catheter and the bi-directional electrical block between the LA and PV. When AF rhythm persisted after PV isolation, external cardioversion was performed to restore sinus rhythm. In addition to circumferential PV isolation, cavotricuspid isthmus (CTI) ablation was applied to prevent a typical common atrial flutter, if necessary. After the procedure, atrial arrhythmia was introduced by rapid atrial stimulus pacing with a high dose of isoproterenol infusion, and then focal ablation for extra PV origin and linear ablation were added for the provoked AF or atrial flutter, if necessary.
All patients were followed-up through the outpatient clinic for 1 year after discharge and were checked for AF recurrence. They were instructed to see their attending physician 1–2 weeks and 1 month after discharge, and thereafter, every 3 months for 1 year. At each follow-up consultation, they underwent 12-lead surface ECG and were asked if they had any symptoms related to the presence of arrhythmia. Twenty-four-hour Holter monitoring was also performed at least 1, 3, and 12 months after discharge. AF recurrence was defined as an episode of AF or atrial tachycardia lasting 30 s or longer after a blanking period of 3 months following the procedure.
P-wave analysis on 12-lead surface ECG
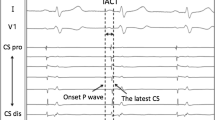
P-wave morphology was examined during sinus rhythm immediately after PV isolation and CTI ablation and before the intravenous infusion of isoproterenol and pacing stimulation. “Post-ablation” p-wave on surface ECG was analyzed with a paper speed of 100 mm/s and more than 32 mm/mV (BARD LabSystem Pro EP; Boston Scientific). The signal from each lead was filtered through a setting between 0.1 and 100 Hz. P-wave morphology in the limbs and precordial leads was analyzed by an experienced investigator who was completely blinded to the outcomes and patient characteristics. The p-wave amplitude (PWA) was measured from the wave’s peak or nadir to the isoelectric line, and the PWD was measured between the p-wave’s beginning and end based on the isoelectric line. The p-wave in lead V1 is pronouncedly biphasic compared with the other leads, and it was divided into positive and negative wave portions based on the isoelectric line, which were evaluated for the outcomes in previous reports [16,17,18]. On the other hand, the parameters in other leads were unified as a simple morphology to be measured using the conventional one.
Measurement of LA volume in CT image
All CT scans were performed on a 64-slice scanner before RFCA. Radiocontrast amount adjusted by the patient’s body weight was administered in 25–30 s. Subsequently, a 3D image, including the LA and PVs, was created, and the final image was constructed to measure LA volume after excluding all PVs.
Measurement of LA local conduction
Post-ablation LA conduction time was measured immediately after PV isolation during sinus rhythm by inserting a fixed electrode catheter (BeeAT, Japan Life Line) into the coronary sinus (CS). This conduction time could represent a local conduction velocity through the fixed distance of the electrode catheter. The proximal side of the electrode catheter was placed at the ostium of the coronary sinus. The time delay between the beginning of the proximal and distal electrode potentials on the four continuous electrodes of the catheter was measured using the LabSystem as described above [19]. In this calculation, the potentials of the four continuous electrodes should have had the same polarity to avoid collision sites of two different conductions in this local area.
Measurement of LA low voltage ratio
An LA voltage map was generated with bipolar electrograms in the multi-electrode mapping catheter (PentaRay, Biosense Webster, Inc.) with 800–1200 points during sinus rhythm after PV isolation. The introduction of atrial arrhythmia by isoproterenol and pacing stimulation was performed after creating the whole voltage map in the LA in all cases. The low-voltage area was defined as an amplitude of < 0.5 mV, and the ratio of the low-voltage area to LA area was calculated using an area measurement software equipped with the CARTO system.
Statistical analyses
Continuous data are expressed as means ± standard deviations, and categorical parameters are presented as numbers (percentages). Comparison of the baseline characteristics and ECG parameters between the recurrence and non-recurrence groups was assessed using Student’s t tests for continuous values and χ2 tests or Fisher’s exact test for categorical values. Intra-class correlation coefficient estimates were used to assess the intra- and inter-observer reproducibility of the ECG parameter measurements. The receiver-operating characteristic (ROC) curves for each parameter were plotted, and the areas under the curves (AUCs) were compared. The cut-off value of the AF recurrence in the parameter was determined based on the ROC curve. Kaplan–Meier survival curve analysis was used to estimate the prognosis, and the difference in the curves was assessed using a log-rank test. The prognostic value of each factor was first evaluated using univariate Cox regression analysis. Significant factors in the univariate analysis were entered into a multivariate Cox regression model to identify independent predictors. Pearson’s correlation coefficient analysis was used to evaluate the relationship between ECG parameters and each LA remodeling parameter. A p value of < 0.05 was considered statistically significant. Statistical analysis was performed with JMP (JMP, SAS Institute Inc., Cary, NC, USA).
Results
Baseline patient characteristics in patients with and without recurrence of AF
In the total population (n = 84), 20 patients had recurrence during the 1 year follow-up period after ablation. Table 1 shows the comparison of clinical characteristics between patients with and without AF recurrence 1 year after ablation. The recurrence group had a significantly larger LA diameter and LA volume than the non-recurrence group. No significant differences in other factors, including the type of AF, comorbidities, and CHADS2 and CHA2DS2-VASc scores, were observed between the two groups.
Among the total population, 47 patients had paroxysmal AF, while 37 patients had persistent AF. Recurrence occurred in 8 patients (17%) with paroxysmal AF and 12 (32%) with persistent AF. Table 2 shows the baseline characteristics of the recurrence and non-recurrence groups for each type of AF. A history of heart failure was more frequent, and the CHADS2 score was significantly higher in the recurrence group than in the non-recurrence group in patients with paroxysmal AF. By contrast, LA volume was larger in the recurrence group than in the non-recurrence group in patients with persistent AF. The recurrence group exhibited a longer local conduction time than the non-recurrence group both in paroxysmal AF and persistent AF. A comparison of the ablation procedure between the recurrence and non-recurrence groups according to the type of AF is shown in Table 3. PV isolation was successfully achieved in all patients. Thirteen patients (28%) with paroxysmal AF and 23 patients (62%) with persistent AF required additional ablation after PV isolation and CTI ablation. The recurrence rate between the additional ablation and non-additional ablation groups was observed in 2/13 (15%) and 6/34 (18%) patients with paroxysmal AF (p > 0.999), and 10/23 (43%) and 2/14 patients (14%) with persistent AF (p = 0.088), respectively. No significant difference in individual procedure outcomes was observed between the recurrence and non-recurrence groups in both the paroxysmal AF and persistent AF groups.
Electrocardiogram characteristics for the prognosis after ablation
Table 4 shows the comparison of the ECG characteristics of the PWD and PWA in all leads between the recurrence and non-recurrence groups. The recurrence group showed a significantly longer PWD in lead I (125.4 ± 12.9 vs. 113.8 ± 11.3 ms, p < 0.001), II (p = 0.003), III (p = 0.006), aVR (p = 0.005), and aVF (p = 0.021) than did the non-recurrence group. There was no significant difference in PWA among all leads between the two groups. Intra- and inter-observer correlation coefficients of the PWD involving leads I, II, III, aVR, and aVF in 30 patients who were randomly selected from the study population were 0.76 [95% confidence interval (CI) 0.56–0.88; p < 0.001] and 0.75 (95% CI 0.55–0.87; p < 0.001), respectively.
Assessment of PWD and its impact on the outcome after ablation according to the cut-off value
We selected five ECG values (PWDs in lead I, II, III, aVR, and aVF) showing a significant association with AF recurrence after ablation, which were presumed reliable parameters for predicting the risk of AF recurrence, and further assessed each property to detect the most useful parameter with a cut-off value on the ROC analysis. Among the five ECG parameters, the ROC curve analysis identified PWD in lead I as the most reliable parameter for predicting AF recurrence after RFCA, with the highest AUC of 0.76 (Fig. 1). The cut-off value of PWD in lead I was determined to be 120 ms, based on the ROC curve with a sensitivity of 75% and specificity of 69% for predicting AF recurrence. Kaplan–Meier survival curve analysis demonstrated a significantly lower rate of AF event-free survival after ablation in patients with PWD > 120 ms than in those with PWD ≤ 120 ms in the entire study population (p < 0.001, Fig. 2).
Predictive value of PWD in lead I
In the entire study population, univariate Cox regression analysis identified LA diameter [hazard ratio (HR) 1.10; 95% CI 1.02–1.18; p = 0.013) and PWD in lead I > 120 ms (HR 2.15; 95% CI 1.38–3.44; p = 0.001) as significant parameters for AF recurrence following ablation. On multivariate analysis, LA diameter (HR 1.08; 95% CI 1.00–1.16; p = 0.049) and the PWD in lead I > 120 ms (HR 2.00; 95% CI 1.27–3.22; p = 0.003) remained independent predictors for AF recurrence (Table 5).
Predictive value of PWD in lead I according to the type of AF and following ablation strategy
The population was divided into patients with paroxysmal AF and persistent AF, and predictors of recurrence were evaluated in each group. Among the patients with persistent AF, the cut-off value of PWD in lead I was determined to be 120 ms for predicting AF recurrence, which was similar to the value analyzed in the total study population. PWD in lead I > 120 ms was the only significant predictor for AF recurrence (HR 3.35; 95% CI 1.70–8.57; p < 0.001) on univariate analysis. By contrast, a history of heart failure (HR 2.65; 95% CI 1.24–6.05; p = 0.013) was the predictor of recurrence in patients with paroxysmal AF (Table 6). PWD was not significantly associated with recurrence of AF in patients with paroxysmal AF.
The population was divided into four groups according to the type of AF and cut-off value of PWD > 120 ms in lead I, and the prognosis of the recurrence was compared among the four groups. Patients with persistent AF and PWD > 120 ms showed the lowest rate of AF event-free survival in comparison with that of the other groups (Supplementary Fig. 1). Furthermore, the total population was alternatively classified into four groups according to the requirement of additional ablation and the cut-off value of PWD in lead I, and the prognosis was similarly compared among the groups. Of note, patients with PWD > 120 ms had a higher rate of AF recurrence regardless of the additional ablation procedure in the Kaplan–Meier survival curve analysis (Supplementary Fig. 2).
When focusing on the group without additional ablation (n = 48) meaning a single PV isolation procedure, the cut-off value of PWD in lead I for recurrence (n = 8) was 122 ms. The PWD in lead I > 122 ms (HR 1.86; 95% CI 1.06–3.28; p = 0.029), CHADS2 score (HR 1.76; 95% CI 1.09–2.78; p = 0.022), and LA diameter (HR 1.10; 95% CI 1.01–1.20; p = 0.036) were significant predictors for AF recurrence in the univariate analysis.
Relation between PWD and other LA remodeling measurements
As described earlier, LA volume, LA local conduction time, and LA low voltage ratio were measured as possible correlative remodeling parameters with PWD. In the total study population, PWD in lead I was significantly correlated with LA volume on 3D-CT (r = 0.394, p < 0.001) and conduction time velocity (r = 0.451; p < 0.001), and was mildly correlated with LA low voltage ratio (r = 0.273, p = 0.017) (Fig. 3). The population was divided into sub-groups of paroxysmal AF and persistent AF, and the correlation was similarly evaluated in each group. Significant correlations were observed between PWD and LA volume (r = 0.591, p = 0.017) and conduction time velocity (r = 0.424; p = 0.011) in patients with persistent AF (Fig. 4). By contrast, the PWD was significantly correlated with conduction time velocity (r = 0.465, p = 0.001) and LA low voltage ratio (r = 0.491, p < 0.001) in patients with paroxysmal AF. We further evaluated the correlation between patients with additional ablation and those without. Both groups exhibited a significant relationship between PWD and LA remodeling parameters (Fig. 5).
The ROC curve analyses to assess the prognostic value of LA volume, LA conduction time, and low voltage ratio for the risk of AF recurrence showed an AUC of 0.74 and a cut-off value of 153.0 mL with a sensitivity of 70% and specificity of 78% in LA volume; an AUC of 0.79, and a cut-off value of 66 ms with a sensitivity of 84% and specificity of 63% in LA conduction time; and an AUC of 0.67 and a cut-off value of 15.7% with a sensitivity of 53% and specificity of 90% in LA low voltage ratio (Fig. 6), respectively.
Discussion
The major findings of the present study are as follows: (i) prolonged PWD on ECG immediately after PV isolation was significantly related to the clinical recurrence of AF after RFCA, (ii) the prognostic value of PWD was stronger in patients with persistent AF than in those with paroxysmal AF, (iii) the longer PWD in lead I was significantly correlated with enlarged LA volume and delayed LA conduction, and (iv) the prognostic value of the longer PWD was definitive for recurrence regardless of the application of ablation in addition to PV isolation.
Many studies have indicated the association of p-wave morphology in ECG with AF recurrence after catheter ablation. A statistically significant difference was reported between the mean PWD of subjects with recurrent AF and those without. In particular, PWD in 12-lead ECG pre-ablation was analyzed in most cases, where the cut-off value was reported to range from 120 to 140 ms [7]. Caldwell et al. studied PWD pre-ablation among paroxysmal AF undergoing PV isolation and reported that PWD > 140 ms predicted AF recurrence with an OR of 2.41 [10]. Mugnai et al. reported a PWD > 120 ms as a predictor of recurrence in patients with drug-resistant symptomatic paroxysmal AF and normal left atrial size [6]. Wu et al. also demonstrated that patients with paroxysmal AF having advanced interatrial block were at an increased risk of AF recurrence after catheter ablation, and defined it as a PWD > 120 ms and biphasic morphology in inferior leads [8]. Among these studies, PWD was identified as the longest PWD in all leads obtained from 12-lead ECG. The cut-off points were different from each other, which was, however, a priori defined. However, our study is unique from the perspective of clinical utility in calculating the cut-off value based on the ROC curves plotted and comparing the AUCs in all leads to scientifically investigate the most reliable value.
As these previous reports indicated, the p-wave morphology of sinus rhythm before ablation was considered to play an important role in predicting the outcome and degree of severity of AF. However, there are few studies that have analyzed the “post-ablation” p-wave morphology. The PWD could change with its morphology after PV isolation. Nakatani et al. determined the coefficient of variation of PWD as an index of atrial conduction heterogeneity with 12-lead ECG and demonstrated that it significantly decreased after catheter ablation in AF-free patients [14]. Other studies reported significantly decreased PWD on the surface ECG as well as signal-averaged ECG and the disappearance of notched p-wave after catheter ablation [11, 20]. Although the pathogenesis of p-wave modification after catheter ablation, such as decreased PWD, is unclear, one possible reason is that it may be caused by the shortening of conduction distance after electrical elimination of the PV. The conductance of the PV area can create atrial late potentials in the p-wave, which indicates prolonged PWD in patients with AF [21]. This hypothesis may also indicate that p-wave morphology analyzed after PV isolation may lead to a more accurate reflection of LA electrical condition except for the component of the PV muscle sleeves. Several studies have verified the relation of p-wave morphologic characteristics with LA remodeling factors, including voltage substrate, conduction, anatomy, stratification of AF, and AF burden [22, 23]. The PWD was also correlated with LA enlargement and volume index, which were measured by M-mode or 3D transthoracic echocardiography [24,25,26]. However, the former studies did not examine the p-wave morphology while focusing on patients with AF scheduled for catheter ablation. In this regard, the PWD immediately after PV isolation examined in the present study may be an accurate marker for representing LA remodeling, which was shown to have a significant correlation with LA low voltage ratio, volume, and conduction time velocity in the total population.
Of interest, the difference in AF free-survival according to the cut-off value of 120 ms for PWD in lead I was more pronounced in persistent AF than in paroxysmal AF. This could be because progressive pathology of persistent AF comes mainly from the LA substrate, and p-wave morphology after PV isolation may strongly reflect uncorrupted LA substrate conditions as mentioned earlier [27]. By contrast, most patients with paroxysmal AF generally have a less damaged, healthy myocardium in the LA after excluding that in PV sleeves. One possible reason why Jadidi et al. could show a strong significance between PWD and several LA remodeling outcomes is because of the inclusion of patients with persistent AF only [28]. It is plausible that PWD immediately after PV isolation had a positive correlation with LA conduction time velocity and especially with LA volume in patients with persistent AF. In addition, PWD in patients with paroxysmal AF had a positive correlation with LA conduction time velocity and LA voltage ratio. It is speculated that anatomic factors might surpass electrical factors in the PWD analysis of persistent AF. There is still no study indicating what the p-wave morphology reflects according to remodeling progression, and this should be examined for future issues.
The present study showed a significantly poor outcome in patients with PWD > 120 ms, regardless of the additional ablation procedure. Moreover, there was a significant relationship between prolonged PWD and AF recurrence in patients undergoing PV isolation without any additional ablation for LA. The significant correlation between the PWD and LA remodeling parameters in the additional and non-additional ablation groups might indicate that the PWD immediately after PV isolation might be a parameter for LA remodeling and an additional ablation strategy. In this context, using the PWD measurement immediately after PV isolation in addition to the conventional strategy of isoproterenol infusion and pacing stimulation might contribute to clinical outcomes. Our results may have a possible clinical implication of PWD assessment for estimating outcomes and deciding an additional ablation after PV isolation. A further prospective investigation with a large sample size will be required to validate PWD following PV isolation and the need for additional ablation in the future.
Study limitations
This was a retrospective study conducted in a single institution. The number of patients enrolled in this study was relatively small. Although p-wave morphology generally reflects both left and right atrial electrical properties, the analysis of atrial volume, low voltage, and conduction time was performed only in the LA in our study. Conduction time was also calculated during sinus rhythm, not under CS proximal pacing, which could not eliminate the influence of activation through the Bachmann bundle. We studied post-ablation p-wave characteristics in patients with paroxysmal and persistent AF undergoing RFCA, and most patients with persistent AF were required to undergo cardioversion after PV isolation. However, cardiac electrophysiological properties after cardioversion are still controversial. The parameters of the p-wave were measured manually, and concern of the measurement variability could remain despite the moderate correlation of inter-and intra-observer variability shown in this study. Because of the limited performance of follow-up examinations (e.g., 12-lead ECG, 24 h Holter monitoring), rhythm assessment in our study could cause underestimation of AF recurrence.
Conclusions
PWD in lead I immediately after PV isolation was the most reliable parameter to predict AF recurrence. In persistent AF, the prognostic value of this parameter was strongly found and might be associated with the extent of the LA substrate. It could also be the prior method for deciding the ablation strategy with the recent developed approaches and management of AF vulnerability [4, 5, 29, 30].
References
Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, Chen PS, Chen SA, Chung MK, Nielsen JC, Curtis AB, Davies DW, Day JD, d’Avila A, de Groot N, Di Biase L, Duytschaever M, Edgerton JR, Ellenbogen KA, Ellinor PT, Ernst S, Fenelon G, Gerstenfeld EP, Haines DE, Haissaguerre M, Helm RH, Hylek E, Jackman WM, Jalife J, Kalman JM, Kautzner J, Kottkamp H, Kuck KH, Kumagai K, Lee R, Lewalter T, Lindsay BD, Macle L, Mansour M, Marchlinski FE, Michaud GF, Nakagawa H, Natale A, Nattel S, Okumura K, Packer D, Pokushalov E, Reynolds MR, Sanders P, Scanavacca M, Schilling R, Tondo C, Tsao HM, Verma A, Wilber DJ, Yamane T (2017) 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 14:e275–e444
Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J (1998) Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 339:659–666
Hoit BD (2014) Left atrial size and function: role in prognosis. J Am Coll Cardiol 63:493–505
Boriani G, Vitolo M, Diemberger I, Proietti M, Valenti AC, Malavasi VL, Lip GYH (2021) Optimizing indices of AF susceptibility and burden to evaluate AF severity, risk and outcomes. Cardiovasc Res. https://doi.org/10.1093/cvr/cvab147
Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL (2021) 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 42:373–498
Mugnai G, Chierchia GB, de Asmundis C, Julia J, Conte G, Sieira-Moret J, Capulzini L, Wauters K, Rodriguez-Manero M, Ciconte G, Baltogiannis G, Di Giovanni G, Saitoh Y, Brugada P (2016) P-wave indices as predictors of atrial fibrillation recurrence after pulmonary vein isolation in normal left atrial size. J Cardiovasc Med (Hagerstown) 17:194–200
Pranata R, Yonas E, Vania R (2019) Prolonged P-wave duration in sinus rhythm pre-ablation is associated with atrial fibrillation recurrence after pulmonary vein isolation-A systematic review and meta-analysis. Ann Noninvasive Electrocardiol 24:e12653
Wu JT, Long DY, Dong JZ, Wang SL, Fan XW, Yang HT, Duan HY, Yan LJ, Qian P, Yang CK (2016) Advanced interatrial block predicts clinical recurrence of atrial fibrillation after catheter ablation. J Cardiol 68:352–356
Park JK, Park J, Uhm JS, Joung B, Lee MH, Pak HN (2016) Low P-wave amplitude (<0.1 mV) in lead I is associated with displaced inter-atrial conduction and clinical recurrence of paroxysmal atrial fibrillation after radiofrequency catheter ablation. Europace 18:384–391
Caldwell J, Koppikar S, Barake W, Redfearn D, Michael K, Simpson C, Hopman W, Baranchuk A (2014) Prolonged P-wave duration is associated with atrial fibrillation recurrence after successful pulmonary vein isolation for paroxysmal atrial fibrillation. J Interv Card Electrophysiol 39:131–138
Okumura Y, Watanabe I, Ohkubo K, Ashino S, Kofune M, Hashimoto K, Shindo A, Sugimura H, Nakai T, Kasamaki Y, Saito S (2007) Prediction of the efficacy of pulmonary vein isolation for the treatment of atrial fibrillation by the signal-averaged P-wave duration. Pacing Clin Electrophysiol 30:304–313
Kaypakli O, Koca H, Şahin DY, Okar S, Karataş F, Koç M (2018) Association of P wave duration index with atrial fibrillation recurrence after cryoballoon catheter ablation. J Electrocardiol 51:182–187
Salah A, Zhou S, Liu Q, Yan H (2013) P wave indices to predict atrial fibrillation recurrences post pulmonary vein isolation. Arq Bras Cardiol 101:519–527
Nakatani Y, Sakamoto T, Mizumaki K, Nishida K, Kataoka N, Tsujino Y, Yamaguchi Y, Inoue H (2016) Coefficient of variation of P-wave duration is a novel atrial heterogeneity index to predict recurrence of atrial fibrillation after catheter ablation. J Cardiovasc Electrophysiol 27:542–548
Nogami A, Kurita T, Abe H, Ando K, Ishikawa T, Imai K, Usui A, Okishige K, Kusano K, Kumagai K, Goya M, Kobayashi Y, Shimizu A, Shimizu W, Shoda M, Sumitomo N, Seo Y, Takahashi A, Tada H, Naito S, Nakazato Y, Nishimura T, Nitta T, Niwano S, Hagiwara N, Murakawa Y, Yamane T, Aiba T, Inoue K, Iwasaki Y, Inden Y, Uno K, Ogano M, Kimura M, Si S, Sasaki S, Satomi K, Shiga T, Suzuki T, Sekiguchi Y, Soejima K, Takagi M, Chinushi M, Nishi N, Noda T, Hachiya H, Mitsuno M, Mitsuhashi T, Miyauchi Y, Miyazaki A, Morimoto T, Yamasaki H, Aizawa Y, Ohe T, Kimura T, Tanemoto K, Tsutsui H, Mitamura H (2021) JCS/JHRS 2019 guideline on non-pharmacotherapy of cardiac arrhythmias. J Arrhythm 37:709–870
Kanzaki Y, Inden Y, Ando M, Kamikubo Y, Ito T, Mizutani Y, Kato H, Fujii A, Yanagisawa S, Hirai M, Murohara T (2016) An ECG index of P-wave force predicts the recurrence of atrial fibrillation after pulmonary vein isolation. Pacing Clin Electrophysiol 39:1191–1197
Doi A, Takagi M, Katayama H, Yoshiyama T, Hayashi Y, Tatsumi H, Yoshiyama M (2018) Diagnostic value of electrocardiographic P-wave characteristics in atrial fibrillation recurrence and tachycardia-induced cardiomyopathy after catheter ablation. Heart Vessels 33:1381–1389
Kubala M, Lucena-Padros I, Xie S, Casado-Arroyo R, Frankel DS, Lin D, Santangeli P, Supple GE, Dixit S, Tschabrunn CM, Liang JJ, Yang J, Hyman MC, Zado ES, Marchlinski FE (2019) P-wave morphology and multipolar intracardiac atrial activation to facilitate nonpulmonary vein trigger localization. J Cardiovasc Electrophysiol 30:865–876
Kanemaru Y, Arima Y, Kaikita K, Kiyama T, Kaneko S, Ito M, Yamabe H, Motozato K, Yamanaga K, Fujisue K, Sueta D, Takashio S, Araki S, Usuku H, Nakamura T, Fukunaga T, Suzuki S, Izumiya Y, Sakamoto K, Soejima H, Yamamoto E, Kawano H, Kanazawa H, Tsujita K (2020) Elongation of the high right atrium to coronary sinus conduction time predicts the recurrence of atrial fibrillation after radiofrequency catheter ablation. Int J Cardiol 300:147–153
Yanagisawa S, Inden Y, Okamoto H, Fujii A, Sakamoto Y, Mamiya K, Tomomatsu T, Shibata R, Murohara T (2020) Electrocardiogram characteristics of P wave associated with successful pulmonary vein isolation in patients with paroxysmal atrial fibrillation: significance of changes in P-wave duration and notched P wave. Ann Noninvasive Electrocardiol 25:e12712
Ogawa M, Kumagai K, Vakulenko M, Yasuda T, Siegerman C, Garfinkel A, Chen PS, Saku K (2007) Reduction of P-wave duration and successful pulmonary vein isolation in patients with atrial fibrillation. J Cardiovasc Electrophysiol 18:931–938
Nakatani Y, Sakamoto T, Yamaguchi Y, Tsujino Y, Kataoka N, Kinugawa K (2020) P-wave vector magnitude predicts the left atrial low-voltage area in patients with paroxysmal atrial fibrillation. J Electrocardiol 59:35–40
Boriani G, Diemberger I, Biffi M, Camanini C, Valzania C, Corazza I, Martignani C, Zannoli R, Branzi A (2005) P wave dispersion and short-term vs. late atrial fibrillation recurrences after cardioversion. Int J Cardiol 101:355–361
Hazen MS, Marwick TH, Underwood DA (1991) Diagnostic accuracy of the resting electrocardiogram in detection and estimation of left atrial enlargement: an echocardiographic correlation in 551 patients. Am Heart J 122:823–828
Andlauer R, Seemann G, Baron L, Dossel O, Kohl P, Platonov P, Loewe A (2018) Influence of left atrial size on P-wave morphology: differential effects of dilation and hypertrophy. Europace 20:iii36–iii44
Kim DH, Kim GC, Kim SH, Yu HK, Choi WG, An IS, Kwan J, Park KS, Lee WH (2007) The relationship between the left atrial volume and the maximum P-wave and P-wave dispersion in patients with congestive heart failure. Yonsei Med J 48:810–817
Nattel S, Burstein B, Dobrev D (2008) Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol 1:62–73
Jadidi A, Muller-Edenborn B, Chen J, Keyl C, Weber R, Allgeier J, Moreno-Weidmann Z, Trenk D, Neumann FJ, Lehrmann H, Arentz T (2018) The duration of the amplified sinus-P-wave identifies presence of left atrial low voltage substrate and predicts outcome after pulmonary vein isolation in patients with persistent atrial fibrillation. JACC Clin Electrophysiol 4:531–543
Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, Fetsch T, van Gelder IC, Haase D, Haegeli LM, Hamann F, Heidbüchel H, Hindricks G, Kautzner J, Kuck KH, Mont L, Ng GA, Rekosz J, Schoen N, Schotten U, Suling A, Taggeselle J, Themistoclakis S, Vettorazzi E, Vardas P, Wegscheider K, Willems S, Crijns H, Breithardt G (2020) Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 383:1305–1316
Potpara TS, Lip GYH, Blomstrom-Lundqvist C, Boriani G, Van Gelder IC, Heidbuchel H, Hindricks G, Camm AJ (2021) The 4S-AF scheme (stroke risk; symptoms; severity of burden; substrate): a novel approach to in-depth characterization (rather than classification) of atrial fibrillation. Thromb Haemost 121:270–278
Acknowledgements
None.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Yanagisawa is affiliated with a department sponsored by Medtronic Japan. All other authors declare that there are no conflicts of interest.
Ethical approval
This study was approved by local institutional ethics committee at Kasugai Municipal Hospital.
Consent to participate
All patients provided written informed consent prior to the procedure.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
380_2021_1932_MOESM1_ESM.tif
Supplementary file1 (TIF 81 KB) Comparison of the Kaplan–Meier survival curves of AF-free survival after the ablation for patients with paroxysmal/persistent AF and with PWD ≤120 ms/ >120 ms in lead I. AF, atrial fibrillation; PWD, p-wave duration
380_2021_1932_MOESM2_ESM.tif
Supplementary file2 (TIF 85 KB) Comparison of the Kaplan–Meier survival curves of AF-free survival after the ablation for patients with additional ablation or non-additional ablation and with PWD ≤120 ms and PWD >120 ms in lead I. AF, atrial fibrillation; PWD, p-wave duration
Rights and permissions
About this article
Cite this article
Ohguchi, S., Inden, Y., Yanagisawa, S. et al. Long P-wave duration immediately after pulmonary vein isolation on radiofrequency catheter ablation for atrial fibrillation predicts clinical recurrence: correlation with atrial remodeling in persistent atrial fibrillation. Heart Vessels 37, 476–488 (2022). https://doi.org/10.1007/s00380-021-01932-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-021-01932-w