Abstract
Background
Complex ablation for persistent atrial fibrillation (AF) aims to modify the arrhythmogenic substrates to become incapable to perpetuate the arrhythmia. Ablation-associated determinants of atrial tachycardia (AT) rather than AF recurrences are unknown. The aim of the study was to evaluate the association between the type of arrhythmia recurrence and electrophysiological findings during redo procedures.
Methods
A total number of 384 consecutive patients with persistent AF underwent complex ablation consisting of PV isolation (PVI), biatrial electrogram-guided ablation, and linear ablation with the desired procedural endpoint of AF termination. Electrophysiological findings during redo procedures and its relation to AR type are the subject of this study.
Results
Overall, 177 (46%) patients underwent a second procedure. Patients with AT recurrences had significantly more often persistent PVI (47 vs. 25%; P = 0.002). Moreover, a higher number of recovered PVs were associated with AF recurrence (3 PVs recovered, AF = 16.1% vs. AT = 5.2%; P = 0.02; 4 PVs recovered, AF = 18.5% vs. AT = 6.3%; P = 0.01), regardless of the extent of substrate ablation during the first procedure.
Conclusions
Durable PV isolation but not the extent of atrial substrate ablation determines the type of arrhythmia recurrence. Thus, the PVs may represent dominant perpetuators (and not only triggers) of persistent AF even in the presence of a significantly modified atrial substrate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Complex catheter ablation for persistent atrial fibrillation (AF) has the aim to eliminate AF triggers and to modify the atrial substrate to become incapable to perpetuate this complex arrhythmia [1, 2]. Pulmonary vein (PV) isolation is commonly the first step in complex catheter ablation procedures for persistent AF [1, 3]. The pathophysiological basis is the elimination of focal AF triggers [4]. Data from previous studies on persistent AF ablation suggests that additional substrate modification is mandatory to achieve atrial tachycardia (AT) rather than AF recurrences [5,6,7]. However, the role of electrically recovered PVs in the presence of a complex modified atrial substrate for the type of arrhythmia recurrence after catheter ablation for persistent AF is still unknown.
The objective of the presented study was to evaluate the association between the type of arrhythmia recurrence and electrophysiological findings during redo procedures.
2 Methods
2.1 Study population
The patient cohort consists of 384 patients with persistent AF undergoing complex catheter ablation, performed between August 2011 and April 2016 at the University Hospital Mainz. Patients with long-standing persistent AF (> 1 year) or AF episodes < 7 days were excluded.
All study patients had to be older than 18 years and a BMI < 35 kg/m2. A second procedure due to arrhythmia recurrence (AR) was done in 177 patients (46%).
The study was approved by the local ethics committee and complied with the provisions of the Declaration of Helsinki. Written informed consent was obtained from all patients.
2.2 Electrophysiologic study and ablation
The procedure was performed in deep sedation with the use of propofol. Cardiac chambers and function were assessed, and intracardiac thrombi were excluded by echocardiography. Bipolar endocardial electrograms were continuously monitored and stored in the LabSystem® Pro (Bard EP Inc., Lowell, MA, USA). Electroanatomic mappings were created with the NavX™ Ensite™ System (St. Jude Inc.) or CARTO System (Biosense-Webster).
The same catheter material and access to the left atrium was used, and the follow-up was organized in the same manner, as described in a recent work of our group [8].
2.3 Catheter ablation protocol in the first procedure
A complex ablation approach for persistent AF was performed as described previously: [1, 8,9,10]. In brief, first a circumferential PV Isolation (PVI) was performed. Electrogram-guided ablation followed if AF did not terminate during PVI and AFCL was higher than 150ms. If AF cycle length was very short after PV isolation (< 150ms), electrical cardioversion was performed after PVI and linear lesion ablation at the LA roof and mitral isthmus was performed.
Target sites of electrogram-guided ablation were areas harboring complex fractionated potentials (CFAEs), continuous electric activity, activation gradients between proximal and distal ablation catheter bipoles, or atrial regions with short cycle lengths. The desired endpoint of electrogram-guided ablation was termination into AT or SR. In case of termination to AT, all subsequent ATs were mapped and ablated in order to achieve SR. Furthermore, if extensive CFAE ablation was required at the LA roof or mitral isthmus region, roof line or mitral isthmus ablation was performed with the endpoint of bidirectional block. The sequence of linear ablation was based on the discretion of the operator.
After termination to SR, no further attempts were made to re-induce AT or AF. The endpoint of bidirectional block of linear ablation was evaluated by differential pacing maneuvers [9].
2.4 Second procedure ablation protocol
The first step of the second procedure was evaluation of PV recovery and re-isolation of reconnected PVs. The following procedural strategy was performed according to the spontaneous baseline rhythm at the beginning of the procedure.
If the patient presented in SR, the clinical arrhythmia was induced by atrial burst stimulation. If the patient presented in AT, the clinical AT and all subsequent and inducible arrhythmias were mapped and ablated. In case of spontaneous AF at the beginning of the procedure, PV isolation was followed by electrogram-guided ablation as described above. Procedural endpoint was termination of all subsequent arrhythmias to achieve SR.
2.5 Follow-up
Follow-up included 48 h of continuous in-hospital heart rhythm monitoring. During follow-up, the patients received 48-h Holter ECG at 3, 6, and 12 months after ablation and further follow-up visits with Holter ECGs every 6 months afterwards. If patients suffer from symptoms suggestive of arrhythmia recurrence, additional follow-up visits with Holter monitoring or event recorder documentation were performed. A blanking period of 90 days post-ablation was included.
2.6 Mapping of atrial tachycardias
ATs were diagnosed conventionally with entrainment and activation mapping. ATs were categorized as macro-reentry, if perfect entrainment (PPI less than 30 ms) could be observed in > 3 different atrial segments. Focal AT was defined as a local early activity with CL variance of 15%, centrifugal activation, and ablation termination at a specific location. Localized reentry was defined as a region which covered > 75% of the AT CL, showed centrifugal atrial activation, and revealed an entrainment post-pacing interval of less than 30 ms [11].
2.7 Statistical analysis
This was a prospective observational non-randomized study. All continuous variables are specified as mean with standard deviation. Statistical significance was estimated with Students t test or Mann–Whitney U test. Categorical variables were analyzed with the Fisher exact test. Comparison of categorical variables was performed using odds ratios. All univariate factors with a P value < 0.10 were included in a logistic regression model. The adjusted receiver operating characteristic (ROC) was investigated, with calculating the area under the curve (AUC) for PV recovery and type of arrhythmia. Time-dependent AR was investigated with cumulative hazard analysis and Wald test. All significance tests were two-tailed with rejecting the null hypothesis at P < 0.05. Statistical analysis was performed with the R programming language (R Foundation for Statistical Computing, R Development Core Team, Vienna, Austria).
3 Results
3.1 Catheter ablation of the index procedure
After PV isolation was performed in all 384 study patients, 314 (81.8%) patients underwent electrogram-guided ablation, resulting in AF termination in 221 (57.6%). Linear ablation was performed in 287 patients (74.7%). Sixty patients (15.6%) were treated by PV isolation and additional linear ablation. Of them, 35 patients (9%) had a very short AF cycle length (less than 150 ms) after pulmonary vein isolation. In these patients, electrical cardioversion was performed followed by left atrial linear lesion ablation. In the remaining 25 cases (6.5%), AF terminated into AT. A detailed treatment flowchart is presented in Fig. 1. The patients’ baseline and echocardiographic and electrophysiologic parameters are shown in Table 1.
3.2 Recurrence of arrhythmia after the first procedure
A second procedure was performed in 178 (46%) patients due to AR. One patient underwent AV node ablation and was excluded from further analysis. Of the remaining 177 patients, 96 (54.2%) patients suffered from AT and 81 (45.8%) from AF. Patients with AF and AT recurrences occurring after the blanking period were considered to be AF recurrence patients. The mean time to AR was 8.5 ± 7.9 months.
3.3 Analysis of PV and line recovery in the second procedure
PV recovery was observed in 112 (63.3%) of the 177 patients with AR. At the end of the second procedure, all PVs were successfully re-isolated.
Re-conduction of previously ablated linear lesions was found in 79 (44.6%) patients. Amongst 81 (45.8%) patients with mitral isthmus block during the index ablation, conduction recovery was seen in 28 (34.6%) patients. Re-ablation resulted in bidirectional block in 22 patients (78.6%) of these cases. Additionally, de novo mitral isthmus ablation was performed in 17 patients during the second procedure. Thus, 110 patients (62.1%) had a bidirectional blocked mitral isthmus line at the end of the second procedure.
The roof line showed re-conduction in 18 (22.2%) of 81 patients with bidirectional block after the index ablation procedure. In 16 (88.8%) patients, roof line block was again achieved during the second procedure. Notably, roof line block evaluation was not possible in 9 patients (11.1%) due to the absence of electrical potentials at or isolation of the posterior wall. De novo roof line ablation was performed in 19 patients, resulting in an overall number of 98 patients (55.4%) with roof line ablation at the end of the second procedure.
3.4 Type of arrhythmia recurrence in the second procedure in relation to PV recovery
To evaluate the role of PV re-conduction in the type of AR, the number of recovered PVs at the beginning of the second procedure was compared with the occurrence of either AT or AF during follow-up.
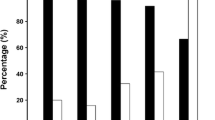
In patients with 1 or 2 recovered PVs, no statistical differences were found with regard to the type of clinical arrhythmia recurrence. However, uni- and multivariate analysis revealed that recovery of more than 2 PVs was predictive for the recurrence of AF rather than AT (e.g., 4 recovered PV, AF vs. AT, 15 (18.5%) vs. 6 (6.3%) patients; P = 0.01). On the other hand, persistent PV isolation of all PVs was significantly associated with AT rather than AF recurrence (AF vs. AT, 20 (24.7%) vs. 45 (46.9%); P < 0.01). This analysis is demonstrated in Table 2 and Fig. 2B.
Influence of PV recovery on type of arrhythmia recurrences (AR) in the second procedure. A Adjusted ROC curve (with 95% confidence corridor) of recovered PVs in relation to the type of AR (AT or AF). PV isolation and left atrial (LA) linear ablation are indicated in blue. PVI, electrogram-guided ablation, and LA linear ablation are shown in red. The two groups differ only in age and gender in baseline characteristics and are therefore adjusted. B shows the distribution of AT and AF recurrence in relation to the numbers of recovered PVs. ROC, receiver operating characteristics; AUC, area under the curve; AF, atrial fibrillation; AT, atrial tachycardia; PV, pulmonary vein. PVI, pulmonary vein isolation
The extent of substrate modification was not predictive for the type of AR in uni- and multivariate analysis (AT, 72.5 ± 27.7, vs. AF, 66.2 ± 32.1 kJ; P = 0.17) (Tables 1 and 2). The adjusted ROC analysis of PV recovery in dependence of the extent of substrate modification revealed this finding (Fig. 2A).
Furthermore, the odds of PV reconnection (at least one or more PVs) for patients presenting with AF is 2.24 (95% CI, 1.19 − 4.25; P = 0.002) compared to patients presenting with AT (Fig. 3).
3.5 Line recovery and arrhythmia recurrences
There was an association of electrical conduction recovery of LA linear lesion with the corresponding clinical AT before or during the redo procedure but not for CTI recovery. In 21 patients with mitral isthmus recovery and AT recurrences, peri-mitral flutter was observed in 11 (52.4%) patients. LA roof-dependent AT was observed in 4 (44.4%) of 9 patients with AT recurrence and roof line recovery. However, only 4 (20.0%) of 20 patients with recurrent AT with CTI recovery had common atrial flutter.
Notably, 8 patients (4.5%) presented with persistently isolated PVs and persistent bidirectional block of the mitral isthmus and the roof line at the second procedure. Of them, 6 suffered from AT and 2 from AF (P = 0.001).
3.6 Uni- and multivariate analysis of predictors of AT vs. AF recurrences
The cumulative hazard of AF recurrences was significantly higher in patients with PV reconnection (Fig. 4). In univariate analysis, spontaneous SR at the beginning of the second procedure, amiodarone use at baseline, LA diameter, electrogram-guided ablation in the first procedure, and PV recovery were significant predictors for AF rather than AT recurrences. Multivariate logistic regression analysis showed that spontaneous SR at the beginning of the procedure, left atrial diameter, and PV recovery were independent predictors for AF rather than AT recurrence, whereas electrogram-guided ablation was not (Table 2).
3.7 Outcome after the second procedure
After a follow-up of 27.6 ± 16.4 months, 105 (59.3%) patients were free of AR after the second procedure. Of them, 36 patients (32.4%) had PV reconnection, and 69 (65.7%) patients did not have PV reconnection after the index ablation (P = 0.3).
Patients with AT recurrence after index ablation were significantly more often free of AR than patients with AF as the recurrent arrhythmia (68.4% vs. 48.2%, P < 0.01) (Table 3).
4 Discussion
4.1 Main findings
The presented study revealed the following important key observations: first, durable PV isolation after complex ablation for persistent AF determines recurrence of AT rather than AF. Second, the extent of substrate modification beyond PV isolation did not correlate with the type of AR. Third, only recovery of left but not right linear lesions was associated with recurrence of its corresponding type of macro-reentrant AT during follow-up. And finally, recurrence of AT was associated with better long-term outcome than AF recurrences after the index ablation.
4.2 Prevalence of pulmonary vein recovery
Recovery of PVs is still a common observation during redo ablation procedures. However, Jing and co-workers evaluated PV conduction after 1 year after initial PV isolation for paroxysmal AF irrespective to the occurrence of clinical AR [12]. Interestingly, PV re-conduction of at least 1 PV was observed in > 90 % of the patients. In a study cohort of patients with paroxysmal (54 %) and persistent AF (46 %), Wasmer and co-workers reported that PV reconnection of at least 1 PV was observed in all patients during the first redo procedure [13]. Moreover, no differences were found in the number of recovered PVs between paroxysmal AF and persistent AF patients. Tilz et al. reported a PV reconnection rate of > 80 % of at least 1 PV in patients with long-standing persistent AF [14]. In their study, the vast majority of patients received PV isolation only while 14 % of the patients underwent additional electrogram-guided ablation during the index procedure. Of note, the predominant type of recurrence there was AF rather than AT.
4.3 Type of recurrence after complex catheter ablation for persistent atrial fibrillation
The data of the presented study revealed that durable PV isolation is predictive for the absence of AF recurrences after complex ablation and, vice versa, AT recurrence predicts durable isolation of all or almost all PVs.
While recurrent AF was significantly more often observed with an increasing number of PVs recovered, the predominant type of AR was AT in patients without PV conduction recovery.
Previous studies have shown that an increasing extent of substrate ablation was associated with AT rather than AF recurrences [9, 15]. Furthermore, electrical conduction gaps in previously ablated LA linear lesions were associated with AT recurrences [16]. However, in the STAR AF II trial, the incidence of AT recurrences was rather low. The number of recurrent AT in the electrogram-guided ablation arm and the linear ablation arm were at a similar level as compared to the PVI only randomization group. In this trial, the amount of AT recurrences was between 11 and 14 % in the three different randomization arms [17]. The rate of procedural AF termination differed significantly between 8 % in the PVI only group and 45 % in the electrogram-guided ablation group with no impact on the type of AR. On the other hand, AF termination as a procedural endpoint of complex AF ablation has been demonstrated to be a predictor of AT rather than AF recurrence [10].
Yang and coworkers found that age, arterial hypertension and LA diameter were multivariate predictors of AT recurrence after AF ablation, while PV recovery was none. This finding might be driven by the fact that this study mainly included patients with paroxysmal AF with only a small minority undergoing substrate ablation (5 %) [18].
4.4 Linear ablation for persistent atrial fibrillation
In the early days of persistent AF Ablation, LA linear ablation was used to create an electrophysiologically modified atrial architecture incapable to perpetuate the arrhythmia [19, 20]. After its initial description, it rapidly became obvious that achievement of bidirectional block is difficult and could not be obtained in a substantial proportion of patients [21]. Furthermore, electrical recovery of initially blocked lines was commonly observed during redo procedures [22]. In line with the findings of our study, the site of line recovery was linked to the clinical AT occurring during follow-up [6, 13, 20]. Thus, new ablation catheters, energy sources and ablation techniques are desired to increase acute success rates and chronic durability of linear ablation. Currently available approaches consisting of the “loss-of-pace-capture” ablation [23, 24] and contiguous point-by-point ablation [25] and the use of the Ablation Index [25] are promising techniques with the potential to achieve complete and durable linear lesions in the LA. Although early studies focusing on catheter ablation for persistent AF showed a beneficial effect of linear ablation, the STAR-AF II trial did not confirm these results [7, 17]. However, recent studies again reinforced the role of linear ablation for a beneficial outcome of persistent AF ablation [26, 27] Thus, prospective randomized trials using contemporary ablation tools are desired to elaborate the impact of linear ablation for persistent AF in the current practice.
4.5 Predictors of outcome
Uni- and multivariate regression analysis revealed that PV conduction recovery independently predicts AR. Interestingly, the proportion of patients with AT recurrences was significantly higher than AF recurrences, presumably due to a high number of patients with durable PV isolation after the second procedure. Yao et al shows an increasing proportion of AT recurrences with an increasing extent of linear ablation [26]. They also observed that linear ablation was not associated with a better outcome with a single procedure. However, an incremental use of linear ablation during multiple redo procedures was ultimately associated with a beneficial outcome. This observation is in line with the findings of our study that patients with AT recurrences finally had a better long-term outcome than those with AF recurrences.
4.6 Limitations
This was a retrospective monocentric study performed with all its inherent limitations. The procedures were performed in a period where contact force sensing was not available. Thus, the use of conventional irrigated tip catheter without contact force information may have impacted the applicability of our results to the current practice. The lesion quality was assessed according to criteria such as disappearance of the local electrogram, which may represent weaker ablation characteristics as compared to more recently available lesion measures.
5 Conclusions
Durable PV isolation but not the extent of atrial substrate ablation determines the type of arrhythmia recurrence. Thus, recovered PVs do not only trigger arrhythmia recurrences, but they also may represent important perpetuators of persistent AF even in the presence of a significantly modified atrial substrate. However, the complex mechanisms of PV recovery, structural and arrhythmia substrate development, and occurrence of AT after ablation deserve further investigations.
References
Haissaguerre M, Prashanthan S, Meleze H, Yoshihide T, Martin R, Sacher F, Rostock T, et al. Catheter ablation of long-lasting persistent atrial fibrillation: critical structures for termination. J Cardiovasc Electrophysiol. 2005;16:1125–37.
Scherr D, Khairy P, Miyazaki S, Aurillac-Lavignolle V, Pascale P, Wilton SB, Ramoul K, et al. Five-year outcome of catheter ablation of persistent atrial fibrillation using termination of atrial fibrillation as a procedural endpoint. Circ Arrhythm Electrophysiol. 2015;8:18–24.
Calkins H, Kuck KH, Cappato R, Brugada J, John Camm A, Chen SA, Crijns HJG, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. J Interv Card Electrophysiol. 2012;33:171–257.
Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66.
O’Neill MD, Wright M, Knecht S, Jaïs P, Hocini M, Takahashi Y, Jönsson A, et al. Long-term follow-up of persistent atrial fibrillation ablation using termination as a procedural endpoint. Eur Heart J. 2009;30:1105–12.
Haïssaguerre M, Hocini M, Sanders P, Sacher F, Rotter M, Takahashi Y, Rostock T, et al. Catheter ablation of long-lasting persistent atrial fibrillation: clinical outcome and mechanisms of subsequent arrhythmias. J Cardiovasc Electrophysiol. 2005;16:1138–47.
Knecht S, Hocini M, Wright M, Lellouche N, O’Neill MD, Matsuo S, Nault I, et al. Left atrial linear lesions are required for successful treatment of persistent atrial fibrillation. Eur Heart J. 2008;29:2359–66.
Spittler R, Bahlke F, Hoffmann BA, Theis C, Mollnau H, Marx A, Quesada B, et al. Predictors of successful complex catheter ablation for persistent atrial fibrillation despite failure of targeted procedural arrhythmia termination. J Cardiovasc Electrophysiol. 2019;30:1026–35.
O’Neill MD, Jaïs P, Takahashi Y, Jönsson A, Sacher F, Hocini M, Sanders P, et al. The stepwise ablation approach for chronic atrial fibrillation - evidence for a cumulative effect. J Interv Card Electrophysiol. 2006;16:153–67.
Rostock T, Steven D, Hoffmann B, Servatius H, Drewitz I, Sydow K, Müllerleile K, et al. Chronic atrial fibrillation is a biatrial arrhythmia: data from catheter ablation of chronic atrial fibrillation aiming arrhythmia termination using a sequential ablation approach. Circ Arrhythm Electrophysiol. 2008;1:344–53.
Jaïs P, Matsuo S, Knecht S, Weerasooriya R, Hocini M, Sacher F, Wright M, et al. A deductive mapping strategy for atrial tachycardia following atrial fibrillation ablation: importance of localized reentry. J Cardiovasc Electrophysiol. 2009;20:480–91.
Jiang RH, Po SS, Tung R, Liu Q, Sheng X, Zhang ZW, Sun YX, et al. Incidence of pulmonary vein conduction recovery in patients without clinical recurrence after ablation of paroxysmal atrial fibrillation: mechanistic implications. Heart Rhythm. 2014;11:969–76.
Wasmer K, Dechering DG, Köbe J, Mönnig G, Pott C, Frommeyer G, Lange PS, et al. Pulmonary vein reconnection and arrhythmia progression after antral linear catheter ablation of paroxysmal and persistent atrial fibrillation. Clin Res Cardiol. 2016;105:738–43.
Tilz RR, Julian Chun KR, Schmidt B, Fuernkranz A, Wissner E, Koester I, Boczor S, et al. Catheter ablation of long-standing persistent atrial fibrillation: a lesson from circumferential pulmonary vein isolation. J Cardiovasc Electrophysiol. 2010;21:1085–93.
Sawhney N, Anousheh R, Chen W, Feld GK. Circumferential pulmonary vein ablation with additional linear ablation results in an increased incidence of left atrial flutter compared with segmental pulmonary vein isolation as an initial approach to ablation of paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:243–8.
Pappone C, Manguso F, Vicedomini G, Gugliotta F, Santinelli O, Ferro A, Gulletta S, et al. Prevention of iatrogenic atrial tachycardia after ablation of atrial fibrillation a prospective randomized study comparing circumferential pulmonary vein ablation with a modified approach. Circulation. 2004;110:3036–42.
Verma A, Jiang C, Betts TR, Chen J, Deisenhofer I, Mantovan R, Macle L, et al. Approaches to catheter ablation for persistent atrial fibrillation (STAR AF II). N Engl J Med. 2015;372:1812–22.
Yang PS, Park YA, Kim TH, Uhm JS, Joung B, Lee MH, Pak HN. Which patients recur as atrial tachycardia rather than atrial fibrillation after catheter ablation of atrial fibrillation? PLoS One. 2017;12:e0188326.
Jaïs P, Hocini M, Hsu LF, Sanders P, Scavee C, Weerasooriya R, Macle L, et al. Technique and results of linear ablation at the mitral isthmus. Circulation. 2004;110:2996–3002.
Hocini M, Jaïs P, Sanders P, Takahashi Y, Rotter M, Rostock T, Hsu LF, et al. Techniques, evaluation, and consequences of linear block at the left atrial roof in paroxysmal atrial fibrillation: a prospective randomized study. Circulation. 2005;112:3688–96.
Willems S, Klemm H, Rostock T, Brandstrup B, Ventura R, Steven D, Risius T, et al. Substrate modification combined with pulmonary vein isolation improves outcome of catheter ablation in patients with persistent atrial fibrillation: a prospective randomized comparison. Eur Heart J. 2006;27:2871–8.
Rostock T, O’Neill MD, Sanders P, Rotter M, Jaïs P, Hocini M, Takahashi Y, et al. Characterization of conduction recovery across left atrial linear lesions in patients with paroxysmal and persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2006;17:1106–11.
Steven D, Sultan A, Reddy V, Luker J, Altenburg M, Hoffmann B, Rostock T, et al. Benefit of pulmonary vein isolation guided by loss of pace capture on the ablation line: results from a prospective 2-center randomized trial. J Am Coll Cardiol. 2013;62:44–50.
Kosmidou I, Houde-Walter H, Foley L, Michaud G. Loss of pace capture after radiofrequency application predicts the formation of uniform transmural lesions. Europace. 2013;15:601–6.
Wolf M, El HM, Fedida J, Taghji P, Van BK, Strisciuglio T, De PJ, et al. Evaluation of left atrial linear ablation using contiguous and optimized radiofrequency lesions: the ALINE study. Europace. 2018;20:f401–9.
Yao Y, Hu F, Du Z, He J, Shi H, Zhang J, Cai H, et al. The value of extensive catheter linear ablation on persistent atrial fibrillation (the CLEAR-AF Study). Int J Cardiol. 2020;316:125–9.
Inoue K, Hikoso S, Masuda M, Furukawa Y, Hirata A, Egami Y, Watanabe T, et al. Pulmonary vein isolation alone vs . more extensive ablation with defragmentation and linear ablation of persistent atrial fibrillation : the EARNEST -PVI trial. Europace. 2021;23:565–74.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Spittler, R., Bahlke, F., Hoffmann, B.A. et al. Durable pulmonary vein isolation but not complex substrate ablation determines the type of arrhythmia recurrence after persistent atrial fibrillation ablation. J Interv Card Electrophysiol 64, 417–426 (2022). https://doi.org/10.1007/s10840-021-01048-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-021-01048-1