Abstract
Many investigators have reported that the total amount of coronary calcium correlates with the overall magnitude of atherosclerotic plaque burden in the entire coronary tree and is a powerful predictor of future cardiovascular events. However, the development and spatial distribution of coronary calcifications remain unclear. We investigated the spatial distribution of calcifications throughout the coronary tree during coronary artery evaluation using coronary computed tomography angiography (CTA). A further aim was to assess the progression of existing calcifications and the development of new deposits in a follow-up study. The study population consisted of 287 patients for the cross-sectional study using CTA to evaluate the spatial distribution of calcifications by parent coronary arteries. Next, we analyzed a CTA dataset of 57 patients who had undergone two CTA examinations. In this group, the two CTA images were used for assessing the progression of existing calcifications and the development of new deposits. The coronary calcifications tended to be clustered within the proximal and middle portions. Moreover, in the proximal left anterior descending coronary artery (LAD), small calcifications were located more toward the inner pericardial side. Finally, new calcium deposits developed within the proximal and middle portions of the LAD and left circumflex coronary artery, but those in the right coronary artery were likely to appear evenly from the proximal to the distal portion. This study shows the characteristic patterns of the longitudinal and circumferential distribution of calcifications by parent coronary arteries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Atherosclerotic plaque calcification has usually been considered an indicator of long-standing advanced atherosclerotic disease. Several studies have focused on the mechanism and role of calcification in atherosclerotic plaque development. In particular, following the introduction of non-contrast electron-beam computed tomography (CT), many investigators have reported that the total amount of coronary calcium correlates with the overall magnitude of atherosclerotic plaque burden in the entire coronary tree [1–3], and is a powerful predictor of future cardiovascular events [4]. Identification and quantification of coronary calcium by non-contrast CT may well be one of the best non-invasive methods currently available to estimate the extent of coronary atherosclerotic plaque burden.
Previous pathological and intravascular ultrasound (IVUS) studies have shown that atherosclerotic plaques in patients with coronary artery disease (CAD) occur in the proximal portions of the major coronary arteries, and tend to be located along the inside of the vessel curve [5–8]. IVUS is the optimal technique to investigate the distribution of atherosclerotic plaques using direct visualization of plaque burden in the culprit vessel, but not throughout the coronary tree, including non-culprit vessels. Moreover, this modality is unsuitable for the observation of mildly stenotic lesions that have not yet caused cardiac ischemia in subjects without CAD.
Recently, coronary computed tomography angiography (CTA) has reached a spatial and temporal resolution high enough for assessment of not only coronary artery stenosis, but also atherosclerotic plaques [9]. However, the level of contrast enhancement in the coronary vessels may obscure plaque and may interfere with reliable measurements of plaque density, especially in non-calcified plaques [10]. Nevertheless, it is conceivable that owing to the relatively high density of calcified plaques compared with non-calcified plaques, the amount of coronary calcium deposits could be estimated by CTA. Only a few studies have addressed this issue previously [11, 12]. Here, we hypothesized that although coronary CTA is for evaluating coronary artery stenosis, it might allow us to assess the longitudinal and circumferential distribution patterns of calcium deposits within the entire coronary artery tree in much greater detail than non-contrast axial CT, even in patients who are suspected of having CAD but are found not to have significant coronary artery stenosis. To the best of our knowledge, there have been no previous CTA studies on the spatial distribution and progression patterns of calcium deposits by parent coronary arteries.
This study comprised 2 components. First, given the spatial distribution of calcium deposits by parent coronary arteries, we conducted a cross-sectional study. Second, we retrospectively analyzed a CTA dataset of patients who had undergone two CTA examinations to assess the progression of existing coronary calcium and the development of new deposits.
Patients and methods
Patients
First, we retrospectively analyzed CT data from 292 consecutive patients between May 2008 and March 2010 who were suspected of having CAD or who had at least one coronary risk factor and clinical indications for CTA for coronary artery evaluation (a cross-sectional study). Patients with atrial fibrillation, previous coronary artery bypass grafting, or percutaneous transluminal intervention were excluded from the study, because these factors affect coronary calcium deposits. Five of the 292 patients were excluded because the images were of insufficient quality for analysis of the distribution of coronary calcium deposits. Thus, the study population comprised 287 patients (mean age 64 years, range 33–88 years, 71 % men) for the cross-sectional study. In this group, imaging was used to evaluate the extent and spatial distribution of calcium deposits by parent coronary arteries.
Second, we analyzed the CT dataset of 57 patients (mean age 62 years, range 38–81 years, 74 % men), who had undergone two CTA examinations between May 2008 and March 2013 (a retrospective follow-up study). These patients were referred for a follow-up CTA examination on the basis of the physician’s clinical judgment because at least one parent vessel with intermediate stenosis was present. In this group of patients who underwent follow-up examinations, two CTA images were used to assess the progression of existing coronary calcium deposits and the development of new deposits.
Informed consent was obtained from all patients, and the study was approved by the hospital ethics committee.
CT image acquisition
The patients were scanned in the supine position during a single breath-hold in the craniocaudal direction using a 64-slice CT scanner (SOMATOM Sensation 64 Siemens, Germany). Patients with a heart rate of >65 beats/min received 2.5 or 5 mg bisoprolol fumarate orally 2 h before the CTA scan. In addition, all patients received 0.6 mg nitroglycerin sublingually immediately before scanning. The electrocardiographic (ECG) signal was digitally recorded during the scan and all patients included showed sinus rhythm throughout the scan.
First, to determine the calcium score, a non-contrast ECG gated scan was performed using standardized parameters (tube voltage of 120 kV, an effective tube current–time product of 100 effective mAs, collimation of 24 × 1.2 mm, and gantry rotation time of 330 ms). Then, for CTA, 50–85 mL of contrast medium (Iopamiron 370; Bayer HealthCare, Berlin, Germany) was injected through a dual-head injector at a rate of 2.5–5.0 mL/s (body weight × 0.06 mL/s/kg) into a cubital vein, followed by 30 mL of saline solution chaser. A bolus tracking technique was used to synchronize the arrival of the contrast in the coronary arteries, and the scan was started once contrast attenuation in a preselected region of interest in the ascending aorta reached a predefined threshold of +150 Hounsfield units (HU). CTA examination was performed with a tube voltage of 120 kV, an effective tube current–time product of 770 effective mAs, a collimation of 64 × 0.6 mm, a pitch of 0.2, and a gantry rotation time of 330 ms.
CT image reconstruction
First, non-contrast images for the measurement of coronary calcium scores were obtained with a slice thickness of 3 mm, with slices starting at the level of the carina and proceeding to the level of the diaphragm. Tomographic imaging was electrocardiographically triggered to 60–80 % of the R–R interval. Coronary calcification was defined as a volume of 2 consecutive pixels with a CT number of >130 HU within the distribution of a coronary artery. All sections of the CT scan obtained were then reviewed by an experienced investigator who was blinded to all clinical data. The intraobserver correlation was 0.99. The quantitative coronary calcium score was calculated according to the method described by Agatston et al. [13]. The total coronary calcium score (TCS) was defined as the sum of the scores for each lesion. Four absolute TCS categories were considered: zero score (TCS = 0), mild score (0 < TCS < 100), moderate score (100 ≤ TCS < 400), and severe score (TCS ≥ 400).
Then, to obtain nearly motion-free image quality, axial image series of the contrast-enhanced CTA datasets were reconstructed within the mid- to end-diastolic phase, using retrospective ECG gating. The first heart phase reconstruction was performed at 70 % of the R–R interval. Additional reconstructions in 5 % steps were performed if motion artifacts were present. Datasets with the least artifacts in a single coronary vessel were selected and sent to a dedicated workstation (Virtual Place; AZE, Tokyo, Japan), where subsequent evaluation was conducted with 0.75-mm effective slice thickness and 0.4-mm increment. A field of view of 180 × 180 mm2, a 512 × 512 matrix, and a medium smooth convolution kernel (B25f) were applied.
Evaluation of extent and distribution of calcium deposits
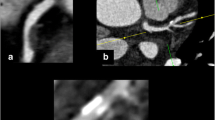
Calcium deposits within all coronary artery segments >2 mm in diameter were evaluated using curved multiplanar reconstruction (MPR) and partial width maximum intensity projection (PWMIP) images generated by Virtual Place visualization software (AZE, Tokyo, Japan). Briefly, the software extracts the centerline of coronary arteries and calculates radial basis function by coronary centerline. It makes a smooth flat surface area from the curve line through all coronary on all axial images. Then, the software adds surface thickness, which is calculated as maximum intensity projection and displayed on the viewer. Any structure on the vessel wall with a CT density above that of the contrast-enhanced coronary lumen was identified as a calcium deposit. If a possible calcium deposit was visually unclear or difficult to distinguish (CT density below that of the contrast-enhanced coronary lumen), its location was marked, and the identification was confirmed with non-contrast imaging. The area to be imaged using non-contrast axial CT was carefully matched to the corresponding marked area in the contrast axial image according to the surrounding cardiac and chest wall structures. In addition, any structure on the coronary artery wall with a CT density of >130 HU in a non-contrast image was identified as a calcium deposit (Fig. 1) [14].
Identification of the calcium deposit with low HUs. a–d Displays contrast-enhanced CTA images showing an area of potential calcium deposition. Deposition was unclear or difficult to distinguish because the structure had a CT density below that of the contrast-enhanced coronary lumen. e and f, Calcium was ascertained as a structure with a CT density of >130 HU that could be assigned to the coronary artery wall in a non-contrast image
For the first cross-sectional study, the size of calcium deposits in the proximal, middle, and distal coronary segments of the left anterior descending coronary artery (LAD), left circumflex coronary artery (LCx), right coronary artery (RCA), large side branches, and left main artery was assessed according to the modified classification of the American Heart Association [15, 16]. The proximal LAD was defined as the arterial region from the left main bifurcation to the first major septal branch. The vessel segment from the first major septal branch to the apex of the heart was divided in half, with the middle LAD comprising the partial segment closest to the branch and the distal LAD comprising the partial segment closest to the apex. The proximal LCx was defined as the main stem of the LCx from its origin off left main bifurcation to the obtuse marginal branch. The middle LCx was defined as the stem of the LCx distal to the origin of the obtuse marginal branch and running along or close to the posterior left atrioventricular groove. The vessel segment from the ostium to the acute margin of the heart was divided in half, with the proximal RCA comprising the partial segment closest to the ostium and the middle RCA comprising the partial segment closest to the heart margin. The distal RCA was defined as the region from the acute margin to the origin of the posterior descending branch. On the basis of the size of each coronary calcium deposit, each segment was classified into one of 4 groups: a no calcification segment was defined as one in which calcium was not detected, a small calcification segment was defined as one in which a calcium deposit <3 mm of longitudinal length on MPR images and occupying only 1 side on cross-sectional images was present, an intermediate calcification segment was defined as one in which a calcium deposit with a length of 3–8 mm and/or occupying more than 1 side on cross-sectional images was present, and a large calcification segment was defined as one in which the calcification was larger than 8 mm in size [17, 18]. In each segment, the largest size of the calcium deposit obtained was assigned per coronary segment and was used for final analysis.
When a segment was classified as “small calcification segment” in the parent vessels of the LAD, LCx, and RCA, the circumferential distribution of the calcium deposit within the vessel wall at that segment was visually assessed with regard to following 2 points, referred to the previous report [19]: (A) the side of the pericardium or myocardium on the curved MPR images and (B) the side of the atrium or ventricle for parent vessels of the RCA and LCx and the inner (right ventricle) or lateral side for the LAD on the PWMIP images (Fig. 2). In cases where a small calcium deposit (<3 mm in length) was present on more than 1 side, the side with the center of the calcium deposit was chosen for grouping. Calcium distribution was recorded as “unclassified” when such a small calcium deposit occupied the entire lumen because of small vessel diameter or when the center of the small calcium deposit was just on the center of the vessel. On the basis of this information, the circumferential distribution of small calcifications was divided into 8 directions (Fig. 3). In both “intermediate calcification” and “large calcification” segments, it was not possible to reliably assess the circumferential distribution of calcium deposits because of the extent of the calcium deposit. Consequently, the circumferential distribution in these segments was not assessed.
Circumferential distribution of small calcium deposits. a Small calcium deposits were detected on the inner (right ventricle) or lateral side on the PWMIP images and on the side of the pericardium or myocardium on the curved MPR images for the LAD. b and c Small calcium deposits were detected on the side of the atrium or ventricle on the PWMIP images and on the side of the pericardium or myocardium on the curved MPR images for parent vessels of the RCA and LCx
The CT image dataset was analyzed by 2 experienced cardiologists who were blinded to the clinical data. In case of disagreement, consensus was reached by an additional joint reading. The κ values for inter- and intra-observer agreements with regard to classification according to the calcium deposit size were 0.89 and 0.93, respectively, whereas κ values with regard to classification of the calcium circumferential distribution into 8 directions were 0.89 and 0.92, respectively.
Progression of existing calcium deposits and development of new deposits: serial follow-up data
We retrospectively analyzed the CT dataset of 57 patients who had undergone two CTA examinations to assess the progression of existing coronary calcium deposits and the development of new deposits. First, the size and longitudinal distribution of all calcium deposits were recorded from baseline images. Then, for follow-up images, progression in the size of previous calcium deposits and the development of new calcium deposits were observed. When the size of each calcium deposit was classified into categories for larger deposits on follow-up images, compared with the category on baseline images, that calcium deposit was described as having progressed in size. On the basis of the change in size or the development of new coronary calcium deposits between 2 CT examinations, each patient was classified into one of 4 groups: the no change group included patients in whom calcium deposits did not change in size or number; the progression group included patients in whom any calcium deposit became larger but the number of deposits did not change; the new development group included patients in whom at least 1 new calcium deposit appeared; the progression and new development group included patients in whom any calcium deposit became larger and at least 1 new calcium deposit appeared.
Statistical analysis
The results are expressed as mean ± SD. Statistical comparisons between more than 3 groups were performed using one-way analysis of variance and post hoc multiple comparison using Scheffe’s test. Categorical variables were compared using the Chi square test. All calculations were performed using JMP software (version 10, SAS Institute Inc., Cary, North Carolina, USA) and values of p < 0.05 were considered significant.
Results
Extent of calcium deposits
The clinical characteristics of all 287 patients are shown in Table 1. The 287 patients included 101 patients (35 %) with a zero TCS. In the remaining 186 (65 %) patients, 82 patients (29 %) had a mild TCS, 53 (18 %) had a moderate TCS, and 51 (18 %) had a severe TCS. Overall, calcium deposits were observed in 804 (29 %) of 2777 segments in 186 patients with mild, moderate, and severe TCS. As shown in Fig. 4a, the frequency of segments with calcium deposits for all segments was significantly different among the mild, moderate, and severe TCS groups (p < 0.0001). Calcium deposits were observed in 170 (14 %) of 1225 segments in 82 patients with a mild TCS, 213 (27 %) of 782 segments in 53 patients with a moderate TCS, and in 421 (55 %) of 770 segments in 51 patients with a severe TCS. Four hundred and eleven (51 %) of 804 segments containing calcium deposits were classified as small calcification segments, 246 (31 %) as intermediate, and 147 (18 %) as large. As shown in Fig. 4b, the frequency of each segment type according to the size of the calcium deposit (small, intermediate, or large) for all segments with calcium deposits was also significantly different among the 3 TCS groups (p < 0.0001). The frequency of small calcification segments for all segments with calcium deposits was the highest in the mild TCS group, whereas the frequency of type of segment with regard to the size of the calcium deposit in the severe TCS group was relatively even from small to large calcium deposits.
Characteristics of calcium deposits in patients with a mild, moderate, and severe TCS. a The frequency of segments with calcium deposits for all segments. b The frequency of each type of segment with regard to the size of the calcium deposit (small, intermediate, or large) for all segments with calcium deposits. Open bar small calcification segment. Striped bar intermediate calcification segment. Solid bar large calcification segment
Distribution of calcium deposits
Figure 5 shows the longitudinal distribution of 804 segments containing calcium deposits of any size among the LAD, LCx and RCA in detail. The calcium deposits were primarily located in the proximal and middle segments of the LAD and LCx and were uncommon in the distal segment. In the RCA, the calcium deposits were observed primarily in the proximal and middle segments, but the distal segment contained more calcium deposits than the distal segment of the LAD or the posterolateral left ventricular branch of the LCx.
The longitudinal distribution of 804 segments containing calcium deposits of any size among the LAD, LCx and RCA. LAD left anterior descending coronary artery, LCx left circumflex coronary artery, RCA right coronary artery, LM left main artery, D diagonal branch, PL posterolateral left ventricular branch, M obtuse marginal branch, PD posterior descending branch, AV atrioventricular branch, Pro proximal, Mid middle, Dis distal
Figure 6 shows the circumferential distribution of the small calcium deposits (n = 324) at each segment in the parent vessels of the LAD, LCx, and RCA. In the proximal and middle segments of the LAD including the left main artery, small calcium deposits were oriented more toward the inner pericardial side than the myocardial or lateral side. Only 10 LADs had small calcium deposits in their distal segments. Among these deposits, distribution was recorded as “unclassified” in 6 cases because the deposit occupied the entire lumen. Small calcifications in the proximal segment of the LCx tended to be located more toward the atrial side than the ventricular side, and those in the middle portion of the LCx were present more frequently toward the myocardial–ventricular side. Small calcium deposits from the proximal to the distal segments of the RCA were located more toward the myocardial and/or ventricular side, but there was no definite tendency.
Follow-up study for calcium deposits
Sixteen (28 %) of 57 patients who underwent 2 CT examinations had no coronary calcium deposits during the study period (mean follow-up period, 22 ± 14 months). In the remaining 41 (72 %) patients (mean follow-up period, 21 ± 11 months), 18 patients (31 %) were assigned to the no change group, 3 (5 %) to the progression group, 14 (24 %) to the new development group, and 6 (10 %) to the progression and new development group. Eighteen calcium deposits increased in size, and 45 new calcium deposits developed during the study period. Figure 7 shows the longitudinal distribution of the progression of calcium deposits and development of new calcium deposits during the follow-up period. Calcium deposits that showed progression in size tended to be clustered within the proximal portion of the parent vessels. Conversely, the development of new coronary calcium deposits occurred within the proximal and middle portions in the LAD and LCx, but those in the RCA were likely to appear evenly from the proximal to the distal portion.
The longitudinal distribution of the progression of calcium deposits (a) and development of new calcium deposits (b) during the follow-up period. For abbreviations see Fig. 5
Discussion
Even during the CTA, we found the characteristic distribution patterns of calcium deposits by parent coronary arteries. First, coronary calcium deposits were not uniformly distributed throughout the coronary tree but tended to be clustered within the proximal and middle portions, except in the case of the RCA. Second, there was a characteristic pattern of circumferential distribution of small calcium deposits according to the parent coronary arteries. Finally, this is the first study to demonstrate that calcium deposits that showed progression in size tended to be clustered within the proximal portion of the parent vessels and the development of new coronary calcium deposits also occurs within the proximal and middle portions of the LAD and LCx, but those in the RCA are likely to appear evenly from the proximal to distal portion.
Several investigators have noted the longitudinal spatial distribution of atherosclerotic plaques throughout the coronary tree using IVUS or CT [8, 16, 20]. Many studies have reported that coronary plaques are frequently located in the proximal and middle segments of the major coronary arteries. Overall, our present data, based on both quantitative and qualitative analysis of calcium deposits, are in agreement with these previous findings, although the pattern of the circumferential spatial distribution of atherosclerotic plaques within the vessel wall was not investigated in the previous studies. The total amount of coronary calcification correlates with the overall magnitude of atherosclerotic plaque burden in the entire coronary tree [1–3]. However, few clinicians consider the TCS to be a good discriminator of significant stenosis, because the specificity for detection of significant stenosis is relatively low. The location and amount of calcification are reported to be positively, but nonlinearly, associated with narrowing of the lumen of coronary vessels [3, 21, 22]. Kajinami et al. [23] compared, on a site by site basis, the morphologic features of coronary calcifications determined by electron-beam CT and coronary atherosclerosis determined by coronary angiography, and reported that the calcification morphology was associated with significant stenosis and angiographic lesion morphology. Thus, the distribution and development of calcium deposits may influence the progression of atherosclerotic plaques and plaque morphology.
Regarding the circumferential spatial distribution of atherosclerotic plaques, some IVUS studies have demonstrated that coronary atherosclerotic plaques in the very proximal LAD portion are localized on the wall opposite to the LCx takeoff and form preferentially along the inner arc of the coronary vessel [5, 6, 8]. However, these studies examined atherosclerotic plaques only in the very proximal segment of the LAD and, therefore, complete proximal, middle, and distal segments of the whole coronary tree including non-culprit vessels could not be analyzed. Preferential sites of atherosclerosis are normally branching or curved arteries, where blood flow is non-linear and slow, which exerts pro-atherogenic low, or oscillatory shear stress [24]. Low shear stress occurs in inner curvatures, such as opposite the LCx and myocardial aspect of LAD. Oscillatory shear stress involves changes in both the magnitude of shear stress and direction of blood flow, normally at branch points, bifurcations or downstream of stenosis [25]. However, due to the asymmetrical and complex structure of bifurcations and bends in the coronary artery, the exact flow patterns were highly asymmetrical and far more complex than those observed in model vessels. Therefore, these findings cannot be generalized across all cases encountered in the human coronary tree, nor used to describe the detailed characteristics of flow at each location on the arterial tree [7, 26]. Clearly, among the 3 major coronary arteries, there are differences in blood flow patterns, flow separation (side branches), and flow turbulence, which result in different wall shear stresses. As shown in the present study, it is very interesting that there was a different pattern of the circumferential distribution of small calcium deposits by parent coronary arteries.
The clinical relevance and role of calcification in plaque vulnerability in patients with acute coronary syndrome (ACS) is still controversial. Our previous quantitative and qualitative IVUS analysis of calcifications demonstrated that the culprit segments of patients with ACS were mostly characterized by the presence of spotty calcification, associated with fibrofatty plaques and positive remodeling [27]. In this study population, no one suffered from ACS and any plaque morphology except for the coronary calcium deposits was not assessed. We propose that the term “spotty calcification” be used to represent the small and discrete calcium deposits associated with fibrofatty plaques and positive remodeling in patients with ACS. In general, calcification itself is considered to be part of the process of atherosclerosis, but its process is different from the acute progression following plaque rupture. Therefore, we used the term “small calcification” on the basis of the size of each coronary calcium deposit in the present study.
The understanding of natural history of atherosclerotic plaque development will ultimately require serial observation of mildly atherosclerotic lesions that have not yet caused an acute coronary event or induced cardiac ischemia, using non-invasive modalities such as CT or magnetic resonance imaging [28]. Thus far, several studies have attempted to validate the use of CTA for measuring coronary calcium score to obviate the need for a separate non-contrast CT scan and decrease radiation dose [11, 12]. However, there have been no previous CTA studies on the spatial distribution and progression patterns of coronary calcium deposits. This CTA study, although CTA is for evaluating coronary artery stenosis, demonstrates that calcium deposits that show progression in size tend to be clustered within the proximal portion of the vessel, especially in the LAD. The relationship of the proximal LAD stenosis to sudden cardiac death has been confirmed in a general population [29]. Our results suggest that during checking for calcium deposits, particular attention should be paid to the proximal portion of the LAD. The findings of our retrospective follow-up study may seem merely to reiterate commonly accepted results from studies with other modalities instead of being of obvious significance or providing new information to improve patient care. Nevertheless, we believe that our attempt to test the conventional wisdom by retrospective analysis of routinely acquired CTA data obtained to check for coronary artery stenosis is important for educational and scientific purposes.
This study has a number of limitations. First, the biggest limitation of this study is that CTA is used to assess the coronary calcium deposits. The relatively high radiation dose and use of iodinated contrast material required by CTA should be emphasized. For these reasons, we cannot presently adopt coronary CTA as the primary method of analyzing the distribution patterns of coronary calcium deposits. Second, in this study non-calcified and mixed plaques were not assessed. CTA has the capacity to provide additional information on non-calcified and mixed plaques rather than calcified [14, 18–20]. Combining the analysis of coronary anatomy, stenosis and these complex plaques may provide incremental value into this study. Third, all analyses for the first, cross-sectional study were not per lesion but per segment, although per lesion analyses were performed for the second, retrospective follow-up study. When small and large calcium deposits existed in the same segment, the distribution of the large calcium deposit was recorded and the small one was not analyzed. We chose “per segment” analyses in the cross-sectional study because the presence of large calcium deposits can sometimes conglutinate other calcium deposits, which causes difficulty in distinction and subsequent interpretation of the data. However, this method of analysis could have masked important findings and potentially led to inaccuracies. Further studies, using “per lesion” analyses, are needed to assess more detailed distribution, progression, and development patterns of calcified and non-calcified plaques by parent coronary arteries. Fourth, the number of patients with serial follow-up data was relatively small. Essentially, our present study cohort based on physicians’ clinical judgment was not designed to assess the formation of calcium deposits. Fifth, to our regret, we did not assess the effects of medication on coronary artery calcium development and progression. However, the aim of this study is to investigate the spatial distribution of coronary calcium deposits, and their progression and the development pattern, not to assess the factors associated with these phenomena. Finally, our cohort did not include patients with ACS who may have a different spatial distribution of calcium deposits.
In conclusion, this study shows the characteristic patterns of the longitudinal and circumferential distribution of calcium deposits by parent coronary arteries. This is the first study to determine that calcium deposits that show progression in size tend to be clustered within the proximal portion of the vessel, especially in the LAD. Thus, we should pay particular attention to the proximal portion of the vessel when checking patients for calcium deposits.
Abbreviations
- CT:
-
Computed tomography
- IVUS:
-
Intravascular ultrasound
- CAD:
-
Coronary artery disease
- CTA:
-
Computed tomography angiography
- ECG:
-
Electrocardiography
- TCS:
-
Total coronary calcium score
- MPR:
-
Multiplanar reconstruction
- PWMIP:
-
Partial width maximum intensity projection
- LAD:
-
Left anterior descending coronary artery
- LCx:
-
Left circumflex coronary artery
- RCA:
-
Right coronary artery
- ACS:
-
Acute coronary syndrome
References
Simons DB, Schwartz RS, Edwards WD, Sheedy PF, Breen JF, Rumberger JA (1992) Noninvasive definition of anatomic coronary artery disease by ultrafast computed tomographic scanning: a quantitative pathologic comparison study. J Am Coll Cardiol 20:1118–1126
Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS (1995) Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation 92:2157–2162
Sangiorgi G, Rumberger JA, Severson A, Edwards WD, Gregoire J, Fitzpatrick LA, Schwartz RS (1998) Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol 31:126–133
Keelan PC, Bielak LF, Ashai K, Jamjoum LS, Denktas AE, Rumberger JA, Sheedy PF II, Peyser PA, Schwartz RS (2001) Long-term prognostic value of coronary calcification detected by electron-beam computed tomography in patients undergoing coronary angiography. Circulation 104:412–417
Kimura BJ, Russo RJ, Bhargava V, McDaniel MB, Peterson KL, DeMaria AN (1996) Atheroma morphology and distribution in proximal left descending coronary artery: in vivo observations. J Am Coll Cardiol 27:825–831
Watanabe H, Yoshida K, Akasaka T, Hozumi T, Yoshikawa J (1996) Intravascular ultrasound assessment of plaque distribution in the ostium of the left anterior descending coronary artery. Am J Cardiol 78:827–829
Badak O, Schoenhagen P, Tsunoda T, Magyar WA, Coughlin J, Kapadia S, Nissen SE, Tuzcu EM (2003) Characteristics of atherosclerotic plaque distribution in coronary artery bifurcations: an intravascular ultrasound analysis. Coron Artery Dis 14:309–316
Iwami T, Fujii T, Miura T, Otani N, Iida H, Kawamura A, Yoshitake S, Kohno M, Hisamatsu Y, Iwamoto H, Matsuzaki M (1998) Importance of left anterior descending coronary artery curvature in determining cross-sectional plaque distribution assessed by intravascular ultrasound. Am J Cardiol 82:381–384
Fujimura T, Miura T, Nao T, Yoshimura M, Nakashima Y, Okada M, Okamura T, Yamada J, Ohshita C, Wada Y, Matsunaga N, Matsuzaki M, Yano M (2014) Dual-source computed tomography coronary angiography in patients with high heart rate. Heart Vessels 29:443–448
Cademartiri F, Mollet NR, Runza G, Bruining N, Hamers R, Somers P, Knaapen M, Verheye S, Midiri M, Krestin GP, de Feyter PJ (2005) Influence of intracoronary attenuation on coronary plaque measurements using multislice computed tomography: observations in an ex vivo model of coronary computed tomography angiography. Eur Radiol 15:1426–1431
van der Bijl N, Joemai RM, Geleijns J, Bax JJ, Schuijf JD, de Roos A, Kroft LJ (2010) Assessment of Agatston coronary artery calcium score using contrast-enhance CT coronary angiography. Am J Roentgenol 195:1299–1305
Otton JM, Lønborg JT, Boshell D, Feneley M, Hayen A, Sammel N, Sesel K, Bester L, McCrohon J (2012) A method for coronary artery calcium scoring using contrast-enhanced computed tomography. J Cardiovasc Comput Tomogr 6:37–44
Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R (1990) Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 15:827–832
Kitagawa T, Yamamoto H, Ohhashi N, Okimoto T, Horiguchi J, Hirai N, Ito K, Kohno N (2007) Comprehensive evaluation of noncalcified coronary plaque characteristics detected using 64-slice computed tomography in patients with proven or suspected coronary artery disease. Am Heart J 154:1191–1198
Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, McGoon DC, Murphy ML, Roe BB (1975) A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation 51:5–40
Mollet NR, Cademartiri F, Nieman K, Saia F, Lemos PA, McFadden EP, Serruys PW, Krestin GP, de Feyter PJ (2005) Noninvasive assessment of coronary plaque burden using multislice computed tomography. Am J Cardiol 95:1165–1169
Ehara S, Kobayashi Y, Kataoka T, Yoshiyama M, Ueda M, Yoshikawa J (2007) Quantification of coronary calcification by intravascular ultrasound. Circ J 71:530–535
Motoyama S, Sarai M, Harigaya H, Anno H, Inoue K, Hara T, Naruse H, Ishii J, Hishida H, Wong ND, Virmani R, Kondo T, Ozaki Y, Narula J (2009) Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol 54:49–57
van der Giessen AG, Wentzel JJ, Meijboom WB, Mollet NR, van der Steen AF, van de Vosse FN, de Feyter PJ, Gijsen FJ (2009) Plaque and shear stress distribution in human coronary bifurcations: a multislice computed tomography study. Eurointervention 4:654–661
Kashiwagi M, Tanaka A, Shimada K, Kitabata H, Komukai K, Nishiguchi T, Ozaki Y, Tanimoto T, Kubo T, Hirata K, Mizukoshi M, Akasaka T (2013) Distribution, frequency and clinical implications of napkin-ring sign assessed by multidetector computed tomography. J Cardiol 61:399–403
Haberl R, Becker A, Leber A, Knez A, Becker C, Lang C, Brüning R, Reiser M, Steinbeck G (2001) Correlation of coronary calcification and angiographically documented stenoses in patients with suspected coronary artery disease: results of 1764 patients. J Am Coll Cardiol 37:451–457
Ho JS, Fitzgerald SJ, Stolfus LL, Wade WA, Reinhardt DB, Barlow CE, Cannaday JJ (2008) Relation of a coronary artery calcium score higher than 400 to coronary stenoses detected using multidetector computed tomography and to traditional cardiovascular risk factors. Am J Cardiol 101:1444–1447
Kajinami K, Seki H, Takekoshi N, Mabuchi H (1997) Coronary calcification and coronary atherosclerosis: site by site comparative morphologic study of electron beam computed tomography and coronary angiography. J Am Coll Cardiol 29:1549–1556
Caro CG, Fitz-Gerald JM, Schroter RC (1971) Atheroma and arterial wall shear. Observation, correlation and proposal of a shear dependent mass transfer mechanism for atherogenesis. Proc R Soc Lond B Biol Sci 177:109–159
Giannoglou GD, Antoniadis AP, Koskinas KC, Chatzizisis YS (2010) Flow and atherosclerosis in coronary bifurcations. EuroInterv Suppl 6:16–23
Asakura T, Karino T (1990) Flow patterns and spatial distribution of atherosclerotic lesions in human coronary arteries. Circ Res 66:1045–1066
Ehara S, Kobayashi Y, Yoshiyama M, Shimada K, Shimada Y, Fukuda D, Nakamura Y, Yamashita H, Yamagishi H, Takeuchi K, Naruko T, Haze K, Becker AE, Yoshikawa J, Ueda M (2004) Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction. An intravascular ultrasound study. Circulation 110:3424–3429
Ehara S, Nakamura Y, Matsumoto K, Hasegawa T, Shimada K, Takagi M, Hanatani A, Izumi Y, Terashima M, Yoshiyama M (2013) Effects of intravenous atrial natriuretic peptide and nitroglycerin on coronary vasodilation and flow velocity determined using 3 T magnetic resonance imaging in patients with nonischemic heart failure. Heart Vessels 28:596–605
Vlay SC, Burger L, Vlay LC, Yen O, Novotny H, Grimson R (1993) Prediction of sudden cardiac arrest: risk stratification by anatomic substrate. An Heart J 126:807–815
Conflict of interest
The authors declare that there are no financial or other relations that could lead to a conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ehara, S., Matsumoto, K., Hasegawa, T. et al. Characteristic patterns of the longitudinal and circumferential distribution of calcium deposits by parent coronary arteries observed from computed tomography angiography. Heart Vessels 31, 508–518 (2016). https://doi.org/10.1007/s00380-015-0652-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-015-0652-6