Abstract
Arbuscular mycorrhizae (AM) are thought to improve crop growth by enhancing phosphorus (P) uptake via scavenging and enhancing dissolution. However, AM-mediated crop growth responses to P forms of varying solubility are often crop-species and soil-context dependent. The relative importance of AM associations and P source solubility on crop growth is not conclusively understood, and requires controlled factorial experiments to test their relative and interactive effects. We conducted a meta-analysis to evaluate how AM impact crop growth responses to rock phosphate relative to soluble phosphates across diverse crop species and soil characteristics. A total of 83 observations utilizing a 2 × 2 factorial design of relative presence or absence of AM and fertilization with rock phosphate vs. soluble phosphates were identified. We found that AM similarly improved crop growth with rock phosphate and soluble phosphates. A distinguishable crop growth benefit from AM coupled with rock phosphate was observed for soils with a low degree of weathering, at soil pH < 6.5 and > 7.5, and when soils were heat-sterilized prior to inoculation with AM. Shoot biomass of legumes was uniquely greater than non-legumes with rock phosphates and AM as compared to soluble phosphates and AM. However, crop growth under rock phosphate fertilization relative to soluble phosphates was still lower irrespective of AM. This meta-analysis reveals that crop growth is more dependent on P fertilizer solubility than AM. Moreover, AM do not appear to close the solubility gap of rock phosphate vs. soluble phosphate fertilizers to support similar crop growth under rock phosphate relative to soluble phosphates. Studies assessing crop growth responses to AM-crop associations effect on contrasting solubility P fertilizers should expand to the field, and greenhouse experiments should be conducted under realistic field growing conditions, such as agronomically appropriate P application rates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arbuscular mycorrhizae (AM) are known to improve phosphorus (P) uptake by host crop species in agroecosystems (Ferrol et al. 2019; Jansa et al. 2011). At least four major mechanisms have been hypothesized: (i) physical exploration of soil through fungal hyphae, which serve as root extensions to access distal P and increase the surface area of P uptake, (ii) P exchange among plants through common mycorrhizal networks, (iii) solubilization of insoluble inorganic P forms, and (iv) secretion of extracellular phosphatases to mineralize organic P (Bücking et al. 2016; Fellbaum et al. 2014; Jansa et al. 2011). Of these, solubilization is often assumed to be the chief means through which AM or the AM-hyphosphere-associated microbiome (Faghihinia et al. 2022; Zhang et al. 2022) aids in crop utilization of insoluble or low solubility P forms such as rock phosphates (RP), presumably through AM-mediated secretion of organic acids (e.g., maleic, citric, acetic acid) (Andrino et al. 2021; Tawaraya et al. 2006; Zhang et al. 2022). However, reports of P solubilization from insoluble inorganic P forms have been largely limited to ectomycorrhiza and ericoid mycorrhiza species (Blum et al. 2002; Landeweert et al. 2001; Smits et al. 2012; Van Schöll et al. 2006), rather than for AM that are more common mutualists with crop species (Plassard and Dell 2010). In some cases, crop utilization of RP is not improved by AM as compared to soluble phosphates (SP) such as superphosphate (e.g., Dann et al. 1996). Despite this, many studies continue to propose or assume, but do not test, the hypothesized benefit of AM-acquisition of P from low solubility sources such as RP over SP (Bolan et al. 1987; Klugh-Stewart and Cumming 2009; Parfitt 1979; Yao et al. 2001).
In cases where AM may improve crop P uptake from low solubility forms such as RP, the greater dissolution rate and thus crop availability of SP may outweigh the enhanced dissolution of low solubility P forms, leading to greater net crop P availability (Barrow et al. 1977; Pairunan et al. 1980; Thirkell et al. 2017). In P-deficient soils, crop yields under RP fertilization are generally lower compared to SP (Malhi et al. 2014; Mnkeni et al. 1991; Wahid et al. 2016). AM-driven dissolution of P from RP could close the gap of crop growth under RP fertilization compared to SP, conceptualized as the “solubility gap”. For example, in acidic soils, crop yields under RP can be comparable to those with SP due to abiotic dissolution of P from RP acting as an additive benefit to AM-driven dissolution (Chien and Menon 1995; Ellis et al. 1955; Mutuo et al. 1999). Nevertheless, AM could improve crop growth even under SP fertilization. For example, more than 50% of P uptake in wheat (Triticum aestivum) can be via AM hyphae, even with SP fertilizers such as ammonium polyphosphate and sodium phosphate (Li et al. 2006). Even if AM-driven chemical dissolution of inorganic P from SP is negligible, AM access to distal P up to several centimeters beyond the root (Jansa et al. 2003) can increase crop P uptake, especially in the early stages of plant growth when nascent root systems access a limited portion of bulk soil P (Barber 1995; Grant et al. 2001) and in fields with broadcast application of P fertilizers (Bolan et al. 1987; Shen et al. 2012).
Reported benefits of AM for crop growth have been critiqued for overemphasizing AM effects in pot-scale greenhouse studies (Ryan and Graham 2018; Thirkell et al. 2017). There is a general lack of field scale evaluations that assess the impact of AM-crop associations on crop growth and yield with varying P solubility sources in biotically and abiotically complex agroecosystems (Lekberg and Helgason 2018; Thirkell et al. 2017), despite historically recognized needs for field scale evaluation of AM function in crop P acquisition (Johnson et al. 1997; Read 1991). Field studies provide evaluation of the impact of AM associations on crop growth across management practices such as tillage and a range of complex biotic and abiotic interactions not replicable at the greenhouse scale (Thirkell et al. 2017). On the other hand, the effect of AM on crop growth may be confounded in a field experiment with multiple biotic interactions and spatiotemporally variable abiotic conditions compared to a controlled greenhouse experiment. High root densities in pots could negate physical P acquisition benefits of AM, though P dissolution from RP would likely be greater at high plant root densities. Additionally, the method of inducing AM colonization differences such as soil sterilization by heat or fumigants could adversely affect the complex soil biological community, overemphasizing AM effects (Forero et al. 2019; Heinze et al. 2016; Schittko et al. 2016).
Apart from experimental methods, crop species and soil conditions can account for conflicting reports of how AM impact crop growth under contrasting P fertilizer solubilities. Differences in root densities and rhizosphere acidification of legumes vs. non-legumes could potentially impact AM-driven crop growth response with different solubility P fertilizers (Bekele et al. 1983; Truog 1916). Legumes generally have greater rhizosphere acidification than non-legumes by greater exudation of organic acids and phosphatases (Shen et al. 2012) that could enhance dissolution of RP. Relative effects of AM on RP versus SP are likely to be influenced by pH and soil P fixation (Blal et al. 1990; Powell and Daniel 1978), which in part reflects the degree of soil weathering, as pH influences fertilizer dissolution and P fixation can mitigate greater availability of SP-derived P. In highly weathered soils (e.g., Oxisols) and many Andisols that are highly P-fixing, greater rate of orthophosphate release from SP will entail a higher rate of P-fixation, hence reduced labile P pools relative to gradual AM-driven RP dissolution. Another soil property that potentially influences RP dissolution is soil organic matter (SOM) content, with higher organic matter content driving RP dissolution by serving as a sink (via complexation) of Ca+2 (Chien and Menon 1995). Thus, accounting for crop species and soil properties can help disentangle agroecosystem-specific effects of AM on crop response to RP vs. SP.
To reconcile conflicting arguments about the relative importance of P source solubility and AM associations for crop growth, controlled factorial experiments that test relative and interactive effects of P solubility and AM associations are needed. Given agroecosystem-specific manifestation of AM-driven crop growth responses, meta-analysis affords quantitative assessment of P solubility and AM associations across agroecosystem contexts hypothesized to influence the relative importance of these two factors. We conducted a systematic literature review and meta-analysis to assess AM impact on host crop biomass and root colonization from RP and SP. Specific objectives were to assess AM-crop symbiosis impact on crop growth response when fertilized with (i) RP or (ii) SP, and to evaluate the effect of RP relative to SP on crop growth (iii) with or (iv) without relative presence of AM (expressed as AM-enrichment vs. AM non-enrichment). We hypothesized that AM-enrichment would increase crop biomass (total, shoot, root) and root colonization for both RP and SP relative to AM non-enrichment. Additionally, we expected crop biomass and root colonization would be improved with RP fertilization relative to SP only with AM-enrichment. For each of the response variables, we tested the effect of methodological and contextual variables that could influence interactions of AM and P solubility on crop growth. We hypothesized an increase in crop growth with RP relative to SP with AM-enrichment with a (i) greater degree of soil weathering, (ii) higher soil pH, (iii) low soil P availability as assessed by soil test P values, and (iv) in non-legumes vs. leguminous crops. We anticipated these responses to vary by study scale, crop type, and method of soil sterilization. We also analyzed methodological parameters and contextual variables to identify gaps in study design for evaluating AM benefits hypothesized for host crops for P sources of varying solubility.
Methods
Literature search and review
A comprehensive literature search was conducted on Web of Science on September 24, 2020, using the keywords: mycorrhiza/mycorrhizae AND phosphate rock within “topic” in “all databases” for the years 1864–2020. This resulted in 739 search results. The full text of initial results was surveyed to select peer-review publications evaluating AM by removing ecto- and ericoid/orchidaceous mycorrhiza studies. A subsequent search with the keywords mycorrhiza/mycorrhizae, arbuscular AND phosphate rock within the original search constraints was also performed to identify potential additional publications, none of which were found. The resulting 229 studies were then screened for robustness of experimental design. To test the effect of relative presence or absence of AM on crop growth responses when fertilized with RP or SP, only studies reporting a full 2 × 2 factorial of relative presence or absence of AM and fertilization with RP vs. SP were retained. Importantly, studies were selected only if a non-AM control for both RP and SP was included. The non-AM control is referred to as AM non-enrichment describing relative absence of AM. We use this notation because AM uninoculated or even genotypically non-AM plants have reduced AM presence, as opposed to no AM colonization (Bodenhausen et al. 2021; Nair and Bhargava 2012; Shi et al. 2013). Many agronomic evaluations of AM and RP vs. SP had a 2 (± AM) × 1 (RP) factorial design, which are unable to test AM-enrichment vs. non-enrichment for SP. Such observations were excluded from the meta-analysis because these observations do not permit a comprehensive comparison of AM effects for both RP and SP. Publications were rejected for inclusion in the meta-analysis dataset based on confounded P rates and/or sources, use of non-soil growth media (e.g., tin tailings), and incomplete methods, analysis, or missing experimental design details. This resulted in a final set of 19 peer-reviewed studies yielding n = 83 observations. Two studies by Lin and Fox (1987; 1992) were counted as one source, since they report data separately from the same experimental evaluation.
Data extraction
Reference descriptors, descriptive variables, and response variables were extracted from tables or from figures using DataThief software (Tummers 2006). Descriptive variables comprised of study scale (field vs. greenhouse), method of inducing AM colonization (sterilization only, inoculation only, sterilization + inoculation, genotype), geographical region (UN region classification), crop species, crop class (legume vs. non-legume), tillage (conventional tillage vs. no-tillage) and irrigation (irrigated vs. non-irrigated), soil order (USDA Soil Taxonomy), degree of soil weathering (low, intermediate, high), soil particle size fractions (sand, silt, clay; %), soil textural class, soil organic matter (%), soil pH, and available or extractable soil P concentration (mg kg−1 soil), measurement method (Mehlich III, Bray I, Bray II, Olsen, Resin) and method of quantification (colorimetric vs. ICP).
Phosphate rock characterization variables extracted were ore source, fineness, calcium carbonate equivalent (CCE), total P (%), and soluble P (%). Soluble phosphate characterization variables extracted were fertilizer type (e.g., ammonium phosphate, superphosphate, potassium dihydrogen phosphate), total P (%), and soluble P (%). Fertilizer P application rates were extracted as mg kg−1 for greenhouse studies and kg ha−1 for field studies. Extracted mycorrhizal descriptors were type of inoculum (external inoculum vs. native), species composition of inoculum (single vs. mixed species), AM species, confirmation of colonization by the study (yes vs. no), and method to determine colonization (root staining, root staining and spore count, root staining and PCR).
Extracted response variables were AM root colonization counts (%), shoot (aboveground), root (belowground), and total biomass (g plant−1 for greenhouse study and t ha−1 for field study), P concentration in aboveground, belowground, and total biomass fractions (%) and P uptake in the three biomass fractions (t ha−1 for field studies and mg plant−1 for greenhouse studies).
Data consolidation
Extracted data were consolidated to be in common units. For unreported data which could not be assigned a value even after assumptions and conversions, NA (not available) was assigned. Crops were classified as legume or non-legume. Percent sand as a soil particle size fraction was calculated from coarse sand and fine sand fractions (%) if only these sub-fractions were reported. Soil textural class was assigned according to USDA classification, and if unreported, was calculated based on particle size fraction (%). Reported SOM values were converted to %, and soil organic carbon (%) was converted to SOM (%) assuming that SOM is 58.1% organic carbon by mass (Van Bemmelen 1890; Wolff 1864). Degree of soil weathering was classified as low, intermediate, and high according to Brady and Weil (2016); Margalef et al. (2017); and Nakayama et al. (2021). Soil pH was classified as acidic, circumneutral, and alkaline (< 6.5, 6.5–7.5, and > 7.5) based on USDA NRCS guidelines (Burt 2014; Staff 2014). Available soil P was based on extractable or “soil test” P (STP) on an Olsen basis, though it was originally developed to predict crop yield response and was classified based on agronomic recommendations (Hertzberger et al. 2020; Mallarino and Atia 2005; Mallarino et al. 2013). There were no or low observations for total soil P (n = 0 studies), soil organic P (n = 0 studies) or available soil inorganic N (n = 4 studies).
Phosphate rock and SP fertilizer characteristics were extracted to interpret the effect sizes of P source solubility and AM associations on crop growth. Phosphate rock solubility was assessed by 2% citric acid or formic acid soluble P and consolidated as solubility (% of total P). Phosphate rock fineness and CCE (%) were removed due to large variability in method of reporting and absence of data, respectively. Unreported total P content (%) for SP was assumed based on commercial fertilizer grades and converted from P2O5 to P, as follows: diammonium phosphate (DAP) — 20.1% P; triple superphosphate (TSP) — 19.2% P; KH2PO4 — 22.8% P; tricalcium phosphate (TCP) — 20.0% P; monocalcium phosphate (MCP) — 24.6% P; calcium-superphosphate (same as single superphosphate) — 7.9% P; superphosphate — 7.9% P. For P application rates (mg kg−1 soil and kg ha−1), conversions from reported data were performed assuming 15 cm soil depth and 1.3 g cm−3 bulk density.
Response variables were used to gauge plant responses to P source solubility × AM presence. Aboveground biomass was the most abundant response and was used as a direct measure of plant response to treatments. Biomass values reported as kg fed−1 were converted to t ha−1 using 1 fed = 0.42 ha (Mahmoud et al. 2013). Biomass fraction P uptake was calculated as follows:
Equation 1.
To facilitate testing effect sizes as response ratios, the final dataset was organized into factorial sets each comprised of the 2 × 2 factorial of relative presence or absence of AM and P source solubility. This allows testing crop response to RP relative to SP in the relative presence or absence of AM, which we refer to as AM-enrichment vs. AM non-enrichment. At the end of data consolidation, only factorial sets were retained which were field and greenhouse studies with at least above ground biomass as a response, for a final dataset of n = 83 factorial sets from 19 studies.
Calculation of response ratios (RRs) and statistical analysis
The response ratio (RR), referring to effect sizes, is the ratio of mean outcome in the treatment group to that in the control group (Hedges et al. 1999). It indicates treatment response relative to the control. In this case, RR refers to the ratio of mean crop response with RP vs. SP under AM-enrichment or not. Response ratios were calculated as:
The four RRs in this evaluation were (i) AM effect on RP, (ii) AM effect on SP, (iii) RP vs. SP (+ AM), and (iv) RP vs. SP (− AM). 95% confidence intervals (CIs) were used to determine significance and directionality of treatment effects. A positive treatment effect was inferred if RR was greater than 1 and CI did not overlap with the 1:1 line on the x-axis. A negative treatment effect was inferred if RR was less than 1. An absence of treatment effect was inferred if CI overlapped with the 1:1 line on the x-axis (Akobeng 2005; Egger et al. 1997). RRs with overlapping 95% CIs were considered statistically similar (Zlowodzki et al. 2007). Summary statistics, analyses, and data visualization were conducted in Microsoft Excel, SigmaPlot, and RStudio (Team 2015) using psych (Revelle 2020), FSA (Ogle et al. 2023), ggplot2 (Wickham 2016), and ggpubr (Kassambara 2020) packages using summary, summarize, ggscatter, and ggplot functions.
Results
Approaches to assessing arbuscular mycorrhizal × P source solubility
Most observations of AM and P source solubility effects on plant growth response were inoculation-based greenhouse studies for non-legume species in acidic, P-responsive soils, but with very high field-equivalent P application rates. Geographical distribution of the observations was not uniform across regions, with more than half of the observations from Oceania (n = 42, 50.6%). Africa (n = 11, 13.3%), Latin America and the Caribbean (n = 10, 12.0%), Asia (n = 8, 9.6%), Europe (n = 6, 7.2%), and North America (n = 6, 7.2%) each contributed less than 15% of total observations. Greenhouse studies were more common (n = 78, 94%) than field studies (n = 5, 6%), the majority of which were tilled (n = 4, 80%) and irrigated (n = 5, 100%). The predominance of greenhouse studies in the metadataset, which is comprised of experiments that use a full 2 × 2 factorial design to test AM-enrichment or non-enrichment and fertilization with RP vs. SP, represents a critical literature gap of a lack of full factorial field scale evaluations needed to test the AM effect on P sources of varying solubility.
Soils with an intermediate degree of weathering constituted the majority (85%) of observations, with 10% and 5% of observations derived using soils with high and low degrees of weathering (Table 1). Approximately two-thirds of intermediately weathered soils (68%) were from a single soil type (Andisol) from one study in New Zealand. The majority of the studies did not report soil particle size fractions (89%) nor SOM content (72%). Soil particle size fractions varied widely, with a mean of approximately 41% sand, 26% silt, and 33% clay, and textural class of clay loam (Table 2). The sand, silt, and clay content (%) varied by a factor of ≈ 5, 8.5, and 11, respectively. Soil organic matter varied by 257-fold from 0.07 to 18%, with a mean of 4.2%. Soil pH, reported for 95% of observations, varied from acidic (4.1) to alkaline pH (8.2) with a mean of 6.1 (Table 2). The majority (75%) of the observations from studies reporting initial soil pH values were from acidic soils (n = 59), and circumneutral (n = 12) and alkaline soils (n = 8) accounted for approximately 15% and 10% of observations, respectively. Available soil P (Olsen) varied by 20-fold from 0.9 to 18.2 mg kg−1 (mean 8.1 mg kg−1), and three-fourths of observations were from soils below the threshold of 10–13 mg kg−1 considered to merit P fertilization for most crops (Mallarino and Atia 2005; Mallarino et al. 2013) (Table 3). Of the available soil P values reported (87% of observations), most indicated low (40%) or very low (approximately 35%) STP soils that would be expected to have a crop response to P fertilization. Soil pH and STP were positively related (R = 0.31; p = 1.2e − 07), and low and very low STP soils largely (83%) occurred in acidic soils (pH < 6.5). High STP soils (n = 13) were associated with low (pH < 6.5) to circumneutral pH (6.5–7.5) soils. Optimal STP soils were found in low pH (< 6.5) (n = 4) and circumneutral (pH 6.5–7.5) (n = 1) soils. Acidic soils occurred for all STP classifications (low to high), but alkaline soils (pH > 7.5) only had very low STP soils.
Soil P application rates varied by a factor of 1170-fold from 0.4 to 480 mg kg−1 soil. Only 29% of the observations used P applications at a field-equivalent realistic rate of 0–30 mg kg−1 (Table 4). In most soils (71%), P was applied at a rate exceeding harvest removal and thus recommended P application rates for grain crops such as maize (Zea mays), soybean (Glycine max), and wheat in low STP soils (Fernández and Hoeft 2009; Kaiser et al. 2011; Mallarino and Atia 2005). Soil P application rate was unrelated to STP of the soil used for the study (R = − 0.13, p = 0.025) (Fig. S1). In already high STP soils, P was applied at rates exceeding 120 mg P kg−1 (Table 5). In contrast, P was applied at less than 30 mg P kg−1 in most soils with optimal STP (80%). Distribution of soil P application rates was more varied in low and very low STP soils, but in at least half of the very low (60%) and low (70%) soils, P rate was still greater than 120 mg kg−1. Field-equivalent realistic P rates were only observed in 21% of low and 32% of very low STP soils.
Approximately one-fifth of crop species were legumes, comprised of lentil (Lens culinaris), fava bean (Vicia faba), soybean, alfalfa (Medicago sativa), and subterranean clover (Trifolium subterraneum) (Table 6). The 82% of observations that were non-legumes were largely grasses (66%) such as wheat, tall fescue (Festuca arundinacea), maize, sugarcane (Saccharum officinarum), and perennial ryegrass (Lolium perenne); vegetable crops (7%) such as Tabasco peppers (Capsicum frutescens); fruits (≈ 5%) such as tomato (Solanum lycopersicum); and herbs (2%) such as fennel (Foeniculum vulgare). Nearly half of the total observations (47%) were from perennial ryegrass.
All studies used AM inoculum to induce AM but approximately one-fourth of observations first sterilized soils before inoculation (Table S1). Use of external inoculum was more common (75%) than relying on soil native AM species. No study used sterilization alone or genotypic models to induce AM. Most (87%) inoculations were performed with a single AM species (Table S2), most commonly Gigaspora margarita (24%), Funneliformis mosseae (17%), and Planticonsortium tenue (22%). The majority of studies and thus observations (95%) confirmed colonization, largely by root staining. Less than 10% observations were from studies that confirmed colonization by either spore count (n = 5, 6.0%) or PCR (n = 2, 2.4%) in addition to root staining.
Overall effects of AM-crop symbiosis and P solubility sources on crop biomass
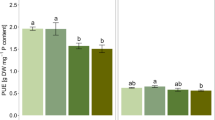
Shoot biomass increased with AM-enrichment and when fertilized with either RP or SP but did not increase with RP relative to SP irrespective of AM colonization (Fig. 1a). The increase in shoot biomass response was similar by P source (AM effect on RP and SP).
(a) Effect of arbuscular mycorrhizae (AM)-crop symbiosis and P solubility sources on shoot biomass response in the complete metadataset (n = 83) comprising of a 2 × 2 factorial of relative presence or absence of AM and fertilization with rock phosphate vs. soluble phosphates. (b) Impact of study scale on AM-crop symbiosis and P solubility sources on shoot biomass response in the complete metadataset (nfield = 5; nGH = 78) comprising of a 2 × 2 factorial of relative presence or absence of AM and fertilization with rock phosphate vs. soluble phosphates
Greenhouse studies (94% of observations) reflected the trends in overall shoot biomass responses (Fig. 1b). No differences in shoot biomass by P solubility (AM effect on RP and SP) or AM (RP vs. SP [± AM]) were observed for field studies (6% of observations). Biomass responses for n = 11 observations that measured total biomass as shoot (aboveground) and root (belowground) fractions showed distinct responses to AM and P solubility compared to shoot-only biomass observations (n = 83) (Fig. 2a–c). Total, shoot, and root biomass increased with RP but not SP fertilization under AM-enrichment. However, shoot biomass but not root biomass, as a proportion of total biomass, did not follow the same trend as the absolute biomass values (Fig. 3a, b). No differences in shoot biomass as a proportion of total biomass by P solubility or AM were observed. Root biomass increased most (54%) under AM-enrichment and RP fertilization, followed by total (30%), and shoot (27%) biomass. Root but not shoot or total biomass increased with RP fertilization relative to SP fertilization under AM-enrichment (45%).
Effect of arbuscular mycorrhizae (AM)-crop symbiosis and P solubility sources on (a) total (aboveground and belowground) shoot biomass response, (b) shoot (aboveground) biomass response, and (c) root (belowground) biomass response in a subset of (n = 11) containing observations for total, shoot, and root biomass response
Influence of soil properties and P application rates
The overall crop shoot biomass responses varied by soil classification and properties of degree of weathering, pH, and STP (Fig. 4a–c) and by P application rates (Fig. 4d). Only in low weathered soils did AM-enrichment entail 32% greater shoot biomass for RP relative to SP (Fig. 4a). In highly weathered soils, shoot biomass decreased compared to low and intermediately weathered soils, regardless of P source solubility or AM-enrichment, and decreases in shoot biomass with RP relative to SP (45%) were nearly double in magnitude compared to shoot biomass responses across soils (≈20%). In intermediately weathered soils, which accounted for over 80% of observations, shoot biomass was lower with RP than SP irrespective of AM-enrichment but the decrease was similar to the mean response across soils. However, in contrast to highly weathered soils, shoot biomass increased with AM-enrichment regardless of P source solubility. In low weathered soils, shoot biomass increased under AM-enrichment with RP but not with SP, and the increase was 33% in contrast to a 14% increase in intermediately weathered soils.
Impact of soil properties and P application rates on arbuscular mycorrhizae (AM)-crop symbiosis and P solubility sources on shoot biomass response in the complete metadataset comprising of a 2 × 2 factorial of relative presence or absence of AM and fertilization with rock phosphate vs. soluble phosphates. (a) Impact of degree of soil weathering which were high (n = 6), intermediate (n = 50), and low (n = 3); (b) Impact of soil pH (acidic (n = 59), circumneutral (n = 12), and alkaline (n = 8)); (c) Impact of soil test phosphorus (STP) (high (n = 13), optimal (n = 5), low (n = 29), and very low (n = 25); (d) Impact of P application rate, expressed as mg P kg.±1 (n[0–30] = 24; n(30–120] = 6; n(120–150] = 40; n(150–480] = 13)
Related to but separate from degree of weathering, soil pH also modulated shoot biomass response to P source solubility and AM (Fig. 4b). Shoot biomass increased under AM-enrichment and RP fertilization in tandem with soil pH, albeit inconsistently. Shoot biomass increase was ≈ 1.8- or 1.5-fold higher for RP or SP in circumneutral soils as compared to mean responses across soils (1.3- and 1.1-fold increase). In acidic and alkaline soils, shoot biomass increased with RP but not with SP under AM-enrichment. In contrast, shoot biomass increased regardless of P source solubility under AM-enrichment in circumneutral soils by + 77%, which was greater than acidic soils (+ 15%) and similar to alkaline soils (+ 29%). Similar to mean responses across soils, shoot biomass did not increase with RP relative to SP irrespective of AM-enrichment. In contrast to highly variable shoot biomass responses with soil pH, shoot biomass increases under AM-enrichment was similar across STP classes, but only for RP (Fig. 4c). Shoot biomass responses with SP fluctuated across STP classes with an increased shoot biomass only observed in low STP soils. Similar to mean responses across soils, across STP classes, shoot biomass did not increase with RP relative to SP irrespective of AM-enrichment.
At P application rates that are agronomically typical (< 30 mg kg−1 or < 60 kg ha−1), shoot biomass responses were similar between P solubility sources under AM-enrichment (Fig. 4d). At P application rates greater than 30 mg kg−1, shoot biomass varied inconsistently by P source solubility and was not necessarily proportionate to the application rate. At 30–120 mg kg−1 P application rate, shoot biomass increased with RP but not SP fertilization under AM-enrichment. In contrast, at 120–150 mg kg−1 application rate, shoot biomass did not increase with either P solubility source regardless of AM-enrichment. At P application rates exceeding 150 mg kg−1, shoot biomass increased with both RP and SP only under AM-enrichment. Across P application rates, shoot biomass was similar regardless of P source solubility and AM-enrichment. However, at the highest P application rates (> 150 mg P kg−1), shoot biomass was lower with RP relative to SP under AM-enrichment compared to the lowest application rates (< 30 mg P kg−1). Even without AM-enrichment, shoot biomass with RP relative to SP fertilization was greater at the lowest P application rate (< 30 mg kg−1) than at the highest P rates (> 150 mg kg−1), but an increase with RP compared to SP was not observed.
Influence of crops and method of AM-enrichment
Crop shoot biomass responses varied across P solubility sources depending on the crop species and how AM-enrichment was induced (Figs. 5 and 6). Legume shoot biomass increased under AM-enrichment for RP but not SP (Fig. 5). Legume shoot biomass increased by approximately 50% for RP under AM-enrichment compared to approximately 25% across all crop classes. In contrast, non-legume shoot biomass increased under AM-enrichment regardless of P solubility sources, and the increase was similar to the mean response across crops. Shoot biomass of legumes and non-legumes did not increase with RP relative to SP, irrespective of AM-enrichment.
Impact of crop class on arbuscular mycorrhizae (AM)-crop symbiosis and P solubility sources on shoot biomass response in the complete metadataset (nlegume = 15; nnon-legume = 68) comprising of a 2 × 2 factorial of relative presence or absence of AM and fertilization with rock phosphate vs. soluble phosphates
(a) Impact of method of arbuscular mycorrhizae (AM) colonization induction on AM-crop symbiosis and P solubility sources on shoot biomass response in the complete metadataset (nsterilization+inoculation = 18; ninoculation only = 65) comprising of a 2 × 2 factorial of relative presence or absence of AM and fertilization with rock phosphate vs. soluble phosphates; (b) Impact of method of AM colonization induction on AM-crop symbiosis and P solubility sources on shoot biomass response in the complete metadataset (nheat sterilization = 11; nchemical sterilization = 7; ninoculation only = 65) comprising of a 2 × 2 factorial of relative presence or absence of AM and fertilization with rock phosphate vs. soluble phosphates
Though all soils generated AM contrasts though AM inoculation, soils that were first sterilized had a mean + 54% greater shoot biomass with RP than the mean response (26%) across soils, and + 76% if heat sterilized or + 19% if chemically sterilized (Fig. 6a). In contrast, inoculating without sterilization led to largely similar shoot biomass with AM-enrichment for both RP and SP. In chemically sterilized soils, shoot biomass responses under AM-enrichment across P solubility sources were similar to the mean responses in inoculated soils as well as across soils (Fig. 6b). However, in heat-sterilized soils, shoot biomass increased under AM-enrichment only for RP but not SP. Regardless of how AM-enrichment differences were induced, shoot biomass was consistently lower with RP relative to SP. When reported, root colonization rates were elevated by approximately 3-fold for RP and SP fertilization under AM-enrichment (Fig. 7). Under AM-enrichment, root colonization rates for plants fertilized with RP varied from 16% lower to 69% higher than those fertilized with SP. Without AM enrichment, root colonization rates varied from 49% lower to 3-fold higher for RP relative to SP.
Effect of arbuscular mycorrhizae (AM) enrichment and P solubility sources on crop root colonization rate response (from n = 18 observations that reported root colonization %) from a metadataset comprising of a 2 × 2 factorial of relative presence or absence of AM and fertilization with rock phosphate vs. soluble phosphates
Discussion
AM improve crop growth with both RP and SP, but not for RP relative to SP
Similar increases in shoot biomass with AM regardless of P solubility indicate enhanced crop P acquisition from RP and SP, though potentially by different mechanisms. Whereas AM can increase crop P acquisition from RP by P dissolution and/or P scavenging, the high water solubility of SP means that AM could improve crop growth with SP fertilizers primarily via scavenging. However, lower shoot biomass with RP relative to SP irrespective of AM-enrichment was opposite to our hypothesis. These results demonstrate that AM-enrichment impacts on P availability — proxied by crop growth in these largely P-limited soils — when fertilized with RP are secondary in importance to inherent differences in fertilizer solubility. More broadly, this suggests that AM alone cannot fully close the gap in P availability between RP and conventional soluble fertilizers as has been often proposed (e.g., Covacevich et al. 2006; Murdoch et al. 1967). Though AM-enrichment may increase P availability from low solubility P sources such as RP, the often-asserted AM dissolution mechanism of P access from RP may not operate at sufficient magnitude to compensate for lower solubility, which is 5.6 × 105 to 1.4 × 109 fold lower than superphosphates (66% of observations) (Wei et al. 2013). The absence of studies reporting RP fineness, which affects RP dissolution rates, is a critical research gap in evaluating AM impact on RP dissolution.
For limited studies that reported belowground biomass, we identified differential responses to AM than aboveground biomass that are consistent with previous evaluations of impacts of this mutualism on biomass allocation of the host plant. The distinct increase in absolute biomass of roots but not shoots with RP relative to SP as well as the root biomass as a proportion of total biomass suggests greater root growth to increase P acquisition with AM associations. An increase in plant root biomass with RP fertilization in conjunction with AM species such as Rhizophagus fasciculatus and Rhizophagus irregularis and P solubilizing microorganisms such as Bacillus cereus, Bacillus subtilis, and Pseudomonas striata has been observed in sesame (Sesamum indicum L.), mung bean (Vigna radiata), and maize (Duponnois et al. 2005; Gaind and Gaur 1991; Sabannavar and Lakshman 2009; Singh and Reddy 2011; Yasmeen et al. 2022). We can hypothesize that under potential P starvation induced by RP fertilization, an increase in plant root: shoot ratio could have led to an increase in the root biomass (Hetrick 1991; Powell 1974). However, the limited number (12%) of total observations reporting available soil P at the end of the experiment (12%) and the very few studies reporting root biomass (13%) prevents evaluation of this potential mechanism that could mediate in part effect of AM on crop growth.
That P source did not affect root colonization ensured that comparisons of AM effects on crop growth and P uptake between RP and SP were not confounded. The lack of root colonization suppression by SP compared to RP also indicates that AM colonization of plant roots is unaffected by the relatively solubility and consequent P availability of P sources. Though reduction in AM root colonization with increasing KH2PO4 application has been reported (Thomson et al. 1986; 1991), this occurred at very high application rates (up to 280 mg P kg−1). Moreover, plant P status (Menge et al. 1978; Sanders 1975) and specifically the root P concentration in the colonized root tissues (Lu et al. 1994) regulate AM colonization, rather than soil P availability and thus application rate or P fertilizer solubility necessarily (Lu et al. 1994; Sobat and Whalen 2022). Thus, differing solubilities of SP and RP would not affect AM root colonization rates if P concentration in colonized root tissues was similar. Though the absence of reported data on root colonization and colonized root tissue P concentration differences between P sources precludes testing this mechanism, Lin and Fox (1992) found a 43% increase in root P concentration in AM vs. non-AM roots in unfertilized soil, but similar root P % between RP and TSP. Greater variability in root colonization rates with and without AM enrichment than by P source could suggest that P source solubility does not affect AM root colonization.
Influence of experimental scale
Crop shoot biomass response to AM and P source varied by whether experiments were conduct at the greenhouse or field scale. The minority of field studies (6% of total observations) — a key limitation of current evaluations — means that crop responses largely reflected greenhouse studies. Shoot biomass was unresponsive to RP or SP under AM-enrichment in the field scale evaluations, but this could be partly attributable to the limited number of field scale observations with subsequent high variability (CV of shoot biomass in field scale observations = 33%). Higher crop biomass response to AM in greenhouse studies could be due to reduced biotic and abiotic interactions expected at the field-scale, such as native AM species and variable weather (Ryan and Graham 2018). A possible reason for limited number of field studies comparing AM effects on crops growth could be the difficulty in furnishing AM treatment differences by sterilization. However, the results of this meta-analysis indicate that inoculation alone appears to be sufficient to generate AM treatment differences for crop shoot biomass response to P solubility sources — based on largely greenhouse studies. Soil sterilization can eliminate non-AM microbiota and have considerable microbial necromass for heterotrophic survival (Kästner et al. 2021), which can confound observed plant responses. Moreover, sterilizing soil is not necessary for generating AM presence or absence contrasts in field studies if these can be achieved by genotypic models (Ruzicka et al. 2012). For example, plant genotypic models such as the mycorrhiza-defective tomato mutant (rmc) and its mycorrhizal wild-type progenitor (76R MYC +) (Barker et al. 1998) avoid the confounding factors due to soil sterilization. The tomato AM genotypes have similar growth under various environmental conditions including non-AM conditions (Cavagnaro et al. 2008) and have been used both in field and greenhouse studies to study AM effect on plant P uptake and growth (e.g., Di Tomassi et al. 2021; Poulsen et al. 2005).
Influence of soil properties
Soil pH and P availability as determined by soil tests largely impacted AM-driven crop response under different solubility P fertilizers. Lower shoot biomass with a greater degree of soil weathering regardless of AM-enrichment under RP fertilization is inconsistent with P limitation effects in highly weathered soils. Though highly weathered and acidic soils (median soil pH = 6.3) in our metadataset were severely P limited with Olsen P equivalent ≤ 2.5 mg kg−1 (median 1 mg kg−1) well below critical values of 12–15 mg kg−1 (Mallarino et al. 2013), shoot biomass was not responsive to RP under AM. The absence of an AM effect for RP in these highly weathered and P-deficient soils could be due to the high P application rates (all > 30 mg kg−1 and ~ 80% > 150 mg kg−1) leading to high soil P availability. Even under AM non-enrichment, crop shoot biomasses were statistically similar to AM-driven biomass response under RP, suggesting that the crop response was determined by fertilizer P addition and not AM in these highly P limited soils. In long-term experiments in severely P limited (1.2 mg kg−1 Bray P equivalent) Oxisols in Western Kenya, application of P as either Minjingu RP or TSP at 50 kg P ha−1 similarly increased maize and soybean yield and biomass, which were both significantly higher compared to the P-unfertilized control (Savini et al. 2016).
In the acidic soils that dominated this metadataset, higher shoot biomass for RP but not SP under AM-enrichment signifies abiotic dissolution via soil acidity, which could occur independently of hyphal-driven dissolution. Bolan and Hedley (1990) observed an increase in North Carolina RP dissolution from 30 to 83% and Nauru RP dissolution from 12 to 60% as soil pH decreased from 6.5 to 3.9. However, it is not possible to assess the magnitude of AM and abiotic dissolution mechanism without quantifying dissolution or derivation of P from P sources. This would require mass-based dissolution data (e.g., Di Tomassi et al. 2021) or direct dissolution measured by radiolabeling (e.g., Cruz-Paredes and Gavito 2020). The distinct shoot biomass increase with RP but not with SP also in alkaline soils supports hypothesized AM-driven RP dissolution. Since abiotic dissolution of RP is low to negligible in alkaline soils, our finding that AM support relatively higher crop biomass with RP in alkaline soils points to a soil-specific benefit of AM for low solubility P forms. Finally, we note that this metadataset had a geographical skewness towards observations from Oceania, which were primarily Andisols, and a comparatively lower representation of observations from primary global consumers of P and RP (i.e., North America, Asia). This reflects the reality of where research on comparing AM effects on RP and SP are being performed, presenting a gap in full factorial evaluation of AM effect on P solubility in regions of major consumption of P and RP.
Influence of P application rate
That the large proportion of soils in our meta-dataset were fertilized at agronomically unrealistic P rates challenges evaluation of crop growth response to AM and P source solubility. As argued by Thirkell et al. (2017), under high nutrient availability due to high rates of chemical fertilizer addition in intensive agriculture systems, AM may act as neutral mutualists or even parasites. AM benefits to crop P uptake tend to be reduced under high soil P availability because host plants tend to reduce carbon allocation to roots and AM (Johnson 2010; Johnson et al. 2003). However, within this metadataset, soil P application rate was not correlated (r = − 0.1) with AM colonization of crop roots, contradicting the hypothesized reduction in host carbon allocation to AM under high P application rate. Several studies in the metadataset published before 1990 (e.g., Badr El-Din and Moawad 1988) used the root slide technique (Phillips and Hayman 1970) to estimate colonization counts, and more recent studies in the 2000s (e.g., Rubio et al. 2003; Zarei et al. 2006) used the gridline intersect method (Giovannetti and Mosse 1980). The absolute colonization counts from both these methods are not comparable given the potential overestimation or underestimation of AM colonization by the root slide technique (McGonigle et al. 1990). However, the observations used in the correlation that report the use of specific methods are limited: n = 8 observations report root slide technique and n = 16 observations report gridline intersect method, with n = 48 observations that do not report the method of colonization count estimation. Additionally, due to the weak correlation (r = − 0.1) between P application rate and AM root colonization, the differences in absolute colonization counts of the two methods are expected to not have impacted the correlation. At high P application rates, the same proportion but a greater net amount of RP would dissolve to generate a crop growth response similar to SP. However, shoot biomass was lower with RP relative to SP irrespective of AM enrichment. Contrary to the plant biomass responses with AM and P source solubility across P rates, the reduced plant biomasses with both RP and SP at the 120–150 mg P kg−1 rate is unexpected, and not fully explained.
The absence of studies reporting RP CCE, which can be of significant magnitude (59–63%) (Sikora 2002), underscores liming effects of RP, particularly at high P application rates as observed for many studies. The absence of CCE data being reported is critical since the appreciable CCE of RP makes it a liming source along with a P fertilizer, potentially confounding comparisons between RP and SP. For example, a P application rate of 150 mg P kg−1 is equivalent to adding ~ 750 mg RP [Ca3(PO4)2] kg−1 soil, which assuming a conservative CCE of 50% entails the addition of 375 mg CaCO3 kg−1 soil or approximately 0.75 Mg CaCO3 ha−1 soil assuming incorporation into the 0–15 cm depth. The liming effect of addition of high quantities of RP will increase soil pH and potentially influence the AM effect on RP. Hence, any direct comparisons of RP and SP as P fertilizers would require a reporting of CCE values of the corresponding RP.
Influence of crop species
The distinct increase in legume shoot biomass with RP but not SP under AM-enrichment could reflect generally greater rhizosphere acidification due to proton release during nitrogen fixation of legumes (Hinsinger et al. 2003). However, if RP dissolution limited P availability and thus legume shoot biomass, legume rhizosphere acidification would mute the beneficial effect of AM for legumes fertilized with RP – yet, the opposite was observed. Legumes generally have higher root acid phosphomonoesterase activity relative to non-legumes (Lambers et al. 1998; Richardson 2001) particularly under conditions of low P availability (Olde Venterink 2011). Such low soil P availability conditions could occur under fertilization with low solubility fertilizers such as RP, which could favor production of extracellular phosphomonoesterases by legume roots to acquire P from organic matter via mineralization. In the case of potential P limitation with RP fertilization, legume root phosphatases would be expected to mineralize more organic P than without P limitation, as with SP fertilization. Increased P availability from organic P mineralization may have resulted in greater shoot biomass under RP fertilization compared to SP. However, shoot biomass with RP relative to SP under AM non-enrichment was not greater in legumes compared to non-legumes. This suggests that increased legume shoot biomass with RP but not SP under AM was not due to increased organic P mineralization caused by root phosphatases production under P limitation.
A possible confounding effect on crop response to P source solubility and AM could be soil P availability. Since majority of the non-legumes (78%, Fig. S2) as well as legumes (63%, Fig. S2) in the metadataset were grown in P-limited soils, non-legumes benefitted from AM for both RP and SP. Legume shoot biomass did not increase with SP due to possibly a competition effect for photosynthate between rhizobia and AM (Kiers and Denison 2008). Competition for photosynthate between rhizobia and AM in legumes could explain the legume-specific reduction in biomass with SP. With SP fertilization, a flush of readily available orthophosphates is available soon after application, potentially negating the AM dependance for P dissolution, unlike RP. Since colonization was similar between legumes and non-legumes and across P sources, the photosynthate sink to AM in legumes could have led to reduced shoot biomass compared to non-legumes. Finally, while other possible comparisons of AM effect across crop classifications such as annuals vs. perennials, monocots vs. dicots could be performed, we focused on the legume vs. non-legume comparison because of known rhizobia-AM interactions and their effects on crop growth.
How AM contrasts are established influence crop response to P source
Increased shoot biomass with RP alone when soils were heat sterilized before inoculation could reflect increases in soil N and P availability due to pyromineralization of organic N and P (Giardina et al. 2000; Nye and Greenland 1961; Raison 1979). Higher relative plant response to AM inoculation in sterilized soils has been observed under low P or P-deficient soils than when soil P is high or under high P application rates (Ortas 2003). For example, Ortas (2003) observed AM dependency values up to 81% with Funneliformis mosseae and Claroideoglomus etunicatum with zero P addition to sterile soil and 38% and 21% with 125 mg P kg−1 soil application. Arbuscular mycorrhizal dependence is defined as a plant’s dependency on AM to generate its maximum yield or growth at a given soil fertility level (Gerdemann 1975) and more recently has been modified as inoculation effectiveness, the ratio of yield (or another plant growth metric) differences between inoculated and non-inoculated plants to the yield of inoculated plants (Ortas 2012). Plant P limitation under RP fertilization, especially in early crop growth stages and in P limited soils, would be expected to induce a higher mycorrhizal colonization in response to RP. Since available soil P pools were not reported, we were unable to test how P solubility and AM enrichment may have affected soil P availability.
Reduced shoot biomass in soils fertilized with SP that were heat sterilized compared to chemically sterilized or non-sterilized is unexpected but could be due to increased soil P availability. Extreme sterilization treatments like autoclaving may release large amount of P that mask benefits of AM (Jakobsen and Andersen 1982), though often studies are incubated for a sufficient period of time for re-fixation or immobilization of nutrients before use. Since the majority studies in this metadataset (65%) were steam sterilized, at a temperature of at least 100 °C (40%), with most studies not reporting if the heat-sterilized soils were incubated before use, AM benefits may have been muted in the heat-sterilized soils. In non-sterilized soils, inoculated AM could be acting synergistically with native AM and other microorganisms (Requena et al. 1997). However, both AM inoculation and sterilization produced similar AM root colonization differences between the AM-enrichment and non-enrichment in the metadataset. Nonetheless, similar crop responses to AM treatments after chemical sterilization and non-sterilization suggest that the reduced shoot biomass with SP in heat-sterilized soils is not due a biotic effect of soil sterilization. None of the studies in the metadataset, including the studies employing mixed AM inoculum, confirmed which species of AM colonized plant roots despite AM species being functionally diverse. Additionally, due to limited number of observations using mixed inoculum (n = 4), the comparison of crop growth responses to single vs. mixed AM inoculum was not possible. Thus, future evaluations of AM impacts on P acquisition by host crop species should consider resolving colonizing AM species, particularly with multi species inoculum.
Conclusion
This meta-analysis assessed the relative importance of P source solubility and AM associations for crop growth and the agroecosystem context dependency of this relationship under factorial experiments that tested both relative and interactive effects of P solubility and AM-crop associations. In the largely P-limited soils in the metadataset, AM-driven crop shoot biomass increased similarly under RP and SP, but crop biomass was lower under RP relative to SP fertilization irrespective of AM-enrichment. Thus, though AM-enrichment appears to increase P availability from low solubility P sources such as RP, this appears insufficient in magnitude to compensate for lower solubility relative to SP. In the largely acidic soils that dominated this metadataset, shoot biomass increased for RP but not SP under AM-enrichment suggestive of a strong effect of abiotic dissolution via soil acidity that reduces AM benefits for RP in acidic soils. The distinct shoot biomass increase with RP but not with SP in alkaline soils could point to AM-driven RP dissolution, but studies are needed to quantify dissolution. Crop biomass did not increase for RP relative to SP in highly weathered soils, rejecting the hypothesized benefit of RP over rapidly solubilizing P fertilizers in strongly P-fixing soils. Moreover, that SP did not suppress root colonization compared to RP indicates that AM colonization of plant roots is unaffected by the relative solubility and subsequent P availability of the RP and soluble P fertilizers evaluated for the largely P-deficient, slightly acidic soils used in extracted studies. Arbuscular mycorrhizae therefore appear to improve crop growth response to RP and SP alike under these edaphic conditions. To elucidate the realistic potential of AM-crop associations to improve crop P use efficiency, future evaluations should be conducted under field or greenhouse conditions that reflect realistic field-growing conditions and agronomically sound P application rates.
Data availability
The metadataset used in the study is provided as an Excel spreadsheet along with other supplementary information.
Abbreviations
- AM:
-
Arbuscular mycorrhiza
- RP:
-
Rock phosphate
- SP:
-
Soluble phosphate
- PSM:
-
Phosphorus solubilizing microbes
- CCE:
-
Calcium carbonate equivalent
- SOM:
-
Soil organic matter
- SOC:
-
Soil organic carbon
- STP:
-
Soil test phosphorus
- PCR:
-
Polymerase chain reaction
- TSP:
-
Triple superphosphate
- TCP:
-
Tricalcium phosphate
- MCP:
-
Monocalcium phosphate
- USDA-NRCS:
-
United States Department of Agriculture-Natural Resources Conservation Service
- RR:
-
Response ratio
- CI:
-
Confidence interval
- CV:
-
Coefficient of variation
References
Akobeng AK (2005) Understanding randomised controlled trials. Arch Dis Child 90:840–844. https://doi.org/10.1136/adc.2004.058222
Andrino A, Guggenberger G, Kernchen S, Mikutta R, Sauheitl L, Boy J (2021) Production of organic zcids by arbuscular mycorrhizal fungi and their contribution in the mobilization of phosphorus bound to iron oxides. Front Plant Sci 12:1–13. https://doi.org/10.3389/fpls.2021.661842
Badr El-Din SMS, Moawad H (1988) Enhancement of nitrogen fixation in lentil, faba bean, and soybean by dual inoculation with Rhizobia and mycorrhizae. Plant Soil 108:117–123. https://doi.org/10.1007/BF02370106
Barber SA (1995) Nutrient acquisition by plant roots. In: Soil nutrient bioavailability: a mechanistic approach, 2nd edn. John Wiley & Sons, New York, pp 49–87
Barker S, Stummer B, Gao L, Dispain I, O’connor P, Smith S (1998) A mutant in Lycopersicon esculentum Mill. with highly reduced VA mycorrhizal colonization: isolation and preliminary characterisation. Plant J 15:791–797
Barrow NJ, Malajcazuk N, Shaw TC (1977) A direct test of the ability of vesicular-arbuscular mycorrhiza to help plants take up fixed soil phosphate. New Phytol 78:269–276. https://doi.org/10.1111/j.1469-8137.1977.tb04830.x
Bekele T, Cino B, Ehlert P, Van der Maas A, Van Diest A (1983) An evaluation of plant-borne factors promoting the solubilization of alkaline rock phosphates. Plant Soil 75:361–378
Blal B, Morel C, Gianinazzi-Pearson V, Fardeau JC, Gianinazzi S (1990) Influence of vesicular-arbuscular mycorrhizae on phosphate fertilizer efficiency in two tropical acid soils planted with micropropagated oil palm (Elaeis guineensis jacq.). Biol Fertil Soils 9:43–48. https://doi.org/10.1007/BF00335860
Blum JD, Klaue A, Nezat CA, Driscoll CT, Johnson CE, Siccama TG, Eagar C, Fahey TJ, Likens GE (2002) Mycorrhizal weathering of apatite as an important calcium source in base-poor forest ecosystems. Nature 417:729–731
Bodenhausen N, Deslandes-Hérold G, Waelchli J, Held A, van der Heijden MGA, Schlaeppi K (2021) Relative qPCR to quantify colonization of plant roots by arbuscular mycorrhizal fungi. Mycorrhiza 31:137–148. https://doi.org/10.1007/s00572-020-01014-1
Bolan NS, Hedley MJ (1990) Dissolution of phosphate rocks in soils. 2. Effect of pH on the dissolution and plant availability of phosphate rock in soil with pH dependent charge. Fertil Res 24:125–134. https://doi.org/10.1007/BF01073580
Bolan NS, Robson AD, Barrow NJ (1987) Effects of vesicular-arbuscular mycorrhiza on the availability of iron phosphates to plants. Plant Soil 99:401–410. https://doi.org/10.1007/BF02370885
Brady N, Weil R (2016) Soil classification. In: Fox D (ed) The nature and properties of soil, 15th edn. Pearson, New York, pp 83–128
Bücking H, Mensah JA, Fellbaum CR (2016) Common mycorrhizal networks and their effect on the bargaining power of the fungal partner in the arbuscular mycorrhizal symbiosis. Commun Integr Biol 9:e1107684. https://doi.org/10.1080/19420889.2015.1107684
Burt R (2014) Soil survey field and laboratory methods manual. In: Burt R, Soil Survey Staff (eds) Soil survey investigations report no. 51, version 2.0. United States Department of Agriculture, Natural Resources Conservation Service, Washington DC, pp 27–241
Cavagnaro TR, Langley AJ, Jackson LE, Smukler SM, Koch GW (2008) Growth, nutrition, and soil respiration of a mycorrhiza-defective tomato mutant and its mycorrhizal wild-type progenitor. Funct Plant Biol 35:228–235
Chien SH, Menon RG (1995) Factors affecting the agronomic effectiveness of phosphate rock for direct application. Fertil Res 41:227–234. https://doi.org/10.1007/bf00748312
Covacevich F, Marino MA, Echeverría HE (2006) The phosphorus source determines the arbuscular mycorrhizal potential and the native mycorrhizal colonization of tall fescue and wheatgrass. Eur J Soil Biol 42:127–138. https://doi.org/10.1016/j.ejsobi.2005.12.002
Cruz-Paredes C, Gavito ME (2020) Isotope labeling to study phosphorus uptake in the arbuscular mycorrhizal symbiosis. Methods Mol Biol 2146:213–222. https://doi.org/10.1007/978-1-0716-0603-2_16
Dann P, Derrick J, Dumaresq D, Ryan M (1996) The response of organic and conventionally grown wheat to superphosphate and reactive phosphate rock. Aust J Exp Agric 36:71–78. https://doi.org/10.1071/EA9960071
Di Tomassi I, Chatterjee N, Barrios-Masias FH, Zhou Q, Gu C, Margenot AJ (2021) Arbuscular mycorrhizae increase biomass and nutrient uptake of tomato fertilized with struvite compared to monoammonium phosphate. Plant Soil 464:321–333. https://doi.org/10.1007/s11104-021-04957-2
Duponnois R, Colombet A, Hien V, Thioulouse J (2005) The mycorrhizal fungus Glomus intraradices and rock phosphate amendment influence plant growth and microbial activity in the rhizosphere of Acacia holosericea. Soil Biol Biochem 37:1460–1468
Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634. https://doi.org/10.1136/bmj.315.7109.629
Ellis R Jr, Quader MA, Truog E (1955) Rock phosphate availability as influenced by soil pH. Soil Sci Soc Am J 19:484–487. https://doi.org/10.2136/sssaj1955.03615995001900040025x
Faghihinia M, Jansa J, Halverson L, Staddon P (2022) Hyphosphere microbiome of arbuscular mycorrhizal fungi: a realm of unknowns. Biol Fertil Soils 59:17–34. https://doi.org/10.1007/s00374-022-01683-4
Fellbaum CR, Mensah JA, Cloos AJ, Strahan GE, Pfeffer PE, Kiers ET, Bücking H (2014) Fungal nutrient allocation in common mycorrhizal networks is regulated by the carbon source strength of individual host plants. New Phytol 203:646–656. https://doi.org/10.1111/nph.12827
Fernández F, Hoeft R (2009) Managing soil pH and crop nutrients. In: Illinois agronomy handbook, 24th edn. College of Agriculture, University of Illinois Urbana-Champaign, Illinois, Co-operative Extension Service, pp 91–112
Ferrol N, Azcon-Aguilar C, Perez-Tienda J (2019) Review: Arbuscular mycorrhizas as key players in sustainable plant phosphorus acquisition: an overview on the mechanisms involved. Plant Sci 280:441–447. https://doi.org/10.1016/j.plantsci.2018.11.011
Forero LE, Grenzer J, Heinze J, Schittko C, Kulmatiski A (2019) Greenhouse- and field-measured plant-soil feedbacks are not correlated. Front Environ Sci 7:1–8. https://doi.org/10.3389/fenvs.2019.00184
Gaind S, Gaur A (1991) Thermotolerant phosphate solubilizing microorganisms and their interaction with mung bean. Plant Soil 133:141–149
Gerdemann J (1975) Vesicular-arbuscular mycorrhizae. In: Torrey J, Clarkson D (eds) The development and function of roots. Academic Press, London, pp 575–591
Giardina C, Sanford R, Døckersmith I (2000) Changes in soil phosphorus and nitrogen during slash-and-burn clearing of a dry tropical forest. Soil Sci Soc Am J 64:399–405
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84:489–500
Grant C, Flaten D, Tomasiewicz D, Sheppard S (2001) The importance of early season phosphorus nutrition. Can J Plant Sci 81:211–224
Hedges LV, Gurevitch J, Curtis PS (1999) The meta-analysis of response ratios in experimental ecology. Ecology 80:1150–1156. https://doi.org/10.1890/0012-9658(1999)080[1150:TMAORR]2.0.CO;2
Heinze J, Sitte M, Schindhelm A, Wright J, Joshi J (2016) Plant-soil feedbacks: a comparative study on the relative importance of soil feedbacks in the greenhouse versus the field. Oecologia 181:559–569
Hertzberger AJ, Cusick RD, Margenot AJ (2020) A review and meta-analysis of the agricultural potential of struvite as a phosphorus fertilizer. Soil Sci Soc Am J 84:653–671. https://doi.org/10.1002/saj2.20065
Hetrick BAD (1991) Mycorrhizas and root architecture. Experientia 47:355–362. https://doi.org/10.1007/BF01972077
Hinsinger P, Plassard C, Tang C, Jaillard B (2003) Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: a review. Plant Soil 248:43–59. https://doi.org/10.1023/A:1022371130939
Jakobsen I, Andersen A (1982) Vesicular-arbuscular mycorrhiza and growth in barley: effects of irradiation and heating of soil. Soil Biol Biochem 14:171–178
Jansa J, Mozafar A, Frossard E (2003) Long-distance transport of P and Zn through the hyphae of an arbuscular mycorrhizal fungus in symbiosis with maize. Agronomie 23:481–488
Jansa J, Finlay R, Wallander H, Smith FA, Smith SE (2011) Role of mycorrhizal symbioses in phosphorus cycling. In: Bünemann E, Oberson A, Frossard E (eds) Phosphorus in action. Springer, Berlin, Heidelberg, pp 137–168
Johnson NC (2010) Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol 185:631–647. https://doi.org/10.1111/j.1469-8137.2009.03110.x
Johnson NC, Graham JH, Smith FA (1997) Functioning of mycorrhizal associations along the mutualism–parasitism continuum. New Phytol 135:575–585. https://doi.org/10.1046/j.1469-8137.1997.00729.x
Johnson NC, Rowland DL, Corkidi L, Egerton-Warburton LM, Allen EB (2003) Nitrogen enrichment alters mycorrhizal allocation at five mesic to semiarid grasslands. Ecology 84:1895–1908. https://doi.org/10.1890/0012-9658(2003)084[1895:NEAMAA]2.0.CO;2
Kaiser DEL, John A, Eliason R (2011) Fertilizer guidelines for agronomic crops in Minnesota. University of Minnesota Extension, Minnesota
Kassambara A (2020) ggpubr: ‘ggplot2’ based publication ready plots. version 0.4.0 edn
Kästner M, Miltner A, Thiele-Bruhn S, Liang C (2021) Microbial necromass in soils—linking microbes to soil processes and carbon turnover. Front Environ Sci 9:597
Kiers ET, Denison RF (2008) Sanctions, cooperation, and the stability of plant-rhizosphere mutualisms. Annu Rev Ecol Evol Syst 39:215–236
Klugh-Stewart K, Cumming JR (2009) Organic acid exudation by mycorrhizal Andropogon virginicus L. (broomsedge) roots in response to aluminum. Soil Biol Biochem 41:367–373
Lambers H, Chapin Iii FS, Pons T (1998) Mineral nutrition. In: Plant physiological ecology, 1st edn. Springer, New York, pp 270–282
Landeweert R, Hoffland E, Finlay RD, Kuyper TW, van Breemen N (2001) Linking plants to rocks: ectomycorrhizal fungi mobilize nutrients from minerals. Trends Ecol Evol 16:248–254
Lekberg Y, Helgason T (2018) In situ mycorrhizal function - knowledge gaps and future directions. New Phytol 220:957–962. https://doi.org/10.1111/nph.15064
Li H, Smith SE, Holloway RE, Zhu Y, Smith FA (2006) Arbuscular mycorrhizal fungi contribute to phosphorus uptake by wheat grown in a phosphorus-fixing soil even in the absence of positive growth responses. New Phytol 172:536–543. https://doi.org/10.1111/j.1469-8137.2006.01846.x
Lin ML, Fox RL (1987) External and internal p requirements of mycorrhizal and non-mycorrhizal banana plants. J Plant Nutr 10:1341–1348. https://doi.org/10.1080/01904168709363666
Lin ML, Fox RL (1992) The comparative agronomic effectiveness of rock phosphate and superphosphate for banana. Fertil Res 31:131–135
Lu S, Braunberger P, Miller M (1994) Response of vesicular-arbuscular mycorrhizas of maize to various rates of P addition to different rooting zones. Plant Soil 158:119–128
Mahmoud EA, Ramadan BS, Bekheet MA, Gomaa MA (2013) Effect of nitrogen fertilization and plant density on productivity and quality of sweet sorghum. Am-Eurasia J Agric Environ Sci 13:654–659
Malhi SS, Vera CL, Brandt SA (2014) Feasibility of rock phosphate and other amendments in preventing P deficiency in barley on a P-deficient soil in northeastern Saskatchewan. Agric Sci 5:1491
Mallarino AP, Atia AM (2005) Correlation of a resin membrane soil phosphorus test with corn yield and routine soil tests. Soil Sci Soc Am J 69:266–272
Mallarino AP, Sawyer JE, Barnhart S (2013) A general guide for crop nutrient and limestone recommendations in Iowa. Iowa State University Extension and Outreach, Iowa
Margalef O, Sardans J, Fernández-Martínez M, Molowny-Horas R, Janssens IA, Ciais P, Goll D, Richter A, Obersteiner M, Asensio D, Peñuelas J (2017) Global patterns of phosphatase activity in natural soils. Sci Rep 7:1337. https://doi.org/10.1038/s41598-017-01418-8
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular—arbuscular mycorrhizal fungi. New Phytol 115:495–501. https://doi.org/10.1111/j.1469-8137.1990.tb00476.x
Menge J, Steirle D, Bagyaraj D, Johnson E, Leonard R (1978) Phosphorus concentrations in plants responsible for inhibition of mycorrhizal infection. New Phytol 80:575–578
Mnkeni P, Semoka J, Buganga J (1991) Effectiveness of Minjingu phosphate rock as a source of phosphorus for maize in some soils of Morogoro, Tanzania. Zimbabwe J Agric Res (zimbabwe) 29:27–37
Murdoch CL, Jackobs JA, Gerdemann JW (1967) Utilization of phosphorus sources of different availability by mycorrhizal and non-mycorrhizal maize. Plant Soil 27:329–334. https://doi.org/10.1007/BF01376326
Mutuo PK, Smithson PC, Buresh RJ, Okalebo RJ (1999) Comparison of phosphate rock and triple superphosphate on a phosphorus-deficient Kenyan soil. Commun Soil Sci Plant Anal 30:1091–1103. https://doi.org/10.1080/00103629909370270
Nair A, Bhargava S (2012) Reduced mycorrhizal colonization (rmc) tomato mutant lacks expression of SymRK signaling pathway genes. Plant Signal Behav 7:1578–1583. https://doi.org/10.4161/psb.20156
Nakayama Y, Wade J, Margenot AJ (2021) Does soil phosphomonoesterase activity reflect phosphorus pools estimated by Hedley phosphorus fractionation? Geoderma 401:115279. https://doi.org/10.1016/j.geoderma.2021.115279
Nye PH, Greenland DJ (1961) The soil under shifting cultivation. Soil Sci 92:354
Ogle D, Doll J, Wheeler AP, Dinno A (2023) FSA: simple fisheries stock assessment methods. version 0.9.4 edn
Olde Venterink H (2011) Legumes have a higher root phosphatase activity than other forbs, particularly under low inorganic P and N supply. Plant Soil 347:137–146. https://doi.org/10.1007/s11104-011-0834-7
Ortas I (2003) Effect of selected mycorrhizal inoculation on phosphorus sustainability in sterile and non-sterile soils in the Harran plain in South Anatolia. J Plant Nutri 26:1–17. https://doi.org/10.1081/PLN-120016494
Ortas I (2012) The effect of mycorrhizal fungal inoculation on plant yield, nutrient uptake and inoculation effectiveness under long-term field conditions. Field Crops Res 125:35–48. https://doi.org/10.1016/j.fcr.2011.08.005
Pairunan AK, Robson AD, Abbott LK (1980) The effectiveness of vesicular-arbuscular mycorrhizas in increasing growth and phosphorus uptake of subterranean clover from phosphorus sources of different solubilities. New Phytol 84:327–338. https://doi.org/10.1111/j.1469-8137.1980.tb04433.x
Parfitt RL (1979) The availability of P from phosphate geothite bridging complexes. desorption and uptake by ryegrass. Plant Soil 53:55–65
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158-IN118. https://doi.org/10.1016/S0007-1536(70)80110-3
Plassard C, Dell B (2010) Phosphorus nutrition of mycorrhizal trees. Tree Physiol 30:1129–1139. https://doi.org/10.1093/treephys/tpq063
Poulsen KH, Nagy R, Gao LL, Smith SE, Bucher M, Smith FA, Jakobsen I (2005) Physiological and molecular evidence for Pi uptake via the symbiotic pathway in a reduced mycorrhizal colonization mutant in tomato associated with a compatible fungus. New Phytol 168:445–454
Powell CL (1974) Effect of P fertilizer on root morphology and P uptake ofCarex coriacea. Plant Soil 41:661–667
Powell CL, Daniel J (1978) Mycorrhizal fungi stimulate uptake of soluble and insoluble phosphate fertilizer from a phosphate-deficient soil. New Phytol 80:351–358. https://doi.org/10.1111/j.1469-8137.1978.tb01568.x
Raison RJ (1979) Modification of the soil environment by vegetation fires, with particular reference to nitrogen transformations: a review. Plant Soil 51:73–108
Read DJ (1991) Mycorrhizas in ecosystems. Experientia 47:376–391. https://doi.org/10.1007/BF01972080
Requena N, Jimenez I, Toro M, Barea JM (1997) Interactions between plant-growth-promoting rhizobacteria (PGPR), arbuscular mycorrhizal fungi and Rhizobium spp. in the rhizosphere of Anthyllis cytisoides, a model legume for revegetation in mediterranean semi-arid ecosystems. New Phytol 136:667–677. https://doi.org/10.1046/j.1469-8137.1997.00786.x
Revelle W (2020) Procedures for Personality and Psychological Research. Version = 2.1.3 edn. Northwestern University, Evanston, Illinois, USA
Richardson AE (2001) Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Funct Plant Biol 28:897–906. https://doi.org/10.1071/PP01093
Rubio R, Borie F, Schalchli C, Castillo C, Azcón R (2003) Occurrence and effect of arbuscular mycorrhizal propagules in wheat as affected by the source and amount of phosphorus fertilizer and fungal inoculation. Appl Soil Ecol 23:245–255. https://doi.org/10.1016/S0929-1393(03)00045-3
Ruzicka DR, Hausmann NT, Barrios-Masias FH, Jackson LE, Schachtman DP (2012) Transcriptomic and metabolic responses of mycorrhizal roots to nitrogen patches under field conditions. Plant Soil 350:145–162. https://doi.org/10.1007/s11104-011-0890-z
Ryan MH, Graham JH (2018) Little evidence that farmers should consider abundance or diversity of arbuscular mycorrhizal fungi when managing crops. New Phytol 220:1092–1107. https://doi.org/10.1111/nph.15308
Sabannavar S, Lakshman H (2009) Effect of rock phosphate solubilization using mycorrhizal fungi and phosphobacteria on two high yielding varieties of Sesamum indicum L. World J Agric Sci 5:470–479
Sanders F (1975) The effect of foliar-applied phosphate on the mycorrhizal infections of onion roots. In: Sanders FE, Mosse B, Tinker PB (eds) Endomycorrhizas. Academic Press, London, New York, pp 261–276
Savini I, Kihara J, Koala S, Mukalama J, Waswa B, Bationo A (2016) Long-term effects of TSP and Minjingu phosphate rock applications on yield response of maize and soybean in a humid tropical maize–legume cropping system. Nutr Cycling Agroecosyst 104:79–91. https://doi.org/10.1007/s10705-016-9760-7
Schittko C, Runge C, Strupp M, Wolff S, Wurst S (2016) No evidence that plant–soil feedback effects of native and invasive plant species under glasshouse conditions are reflected in the field. J Ecol 104:1243–1249
Shen J, Li C, Mi G, Li L, Yuan L, Jiang R, Zhang F (2012) Maximizing root/rhizosphere efficiency to improve crop productivity and nutrient use efficiency in intensive agriculture of China. J Exp Bot 64:1181–1192. https://doi.org/10.1093/jxb/ers342
Shi A-D, Li Q, Huang J-G, Yuan L (2013) Influence of arbuscular mycorrhizal fungi on growth, mineral nutrition and chlorogenic acid Content of Lonicera confusa seedlings under field conditions. Pedosphere 23:333–339. https://doi.org/10.1016/S1002-0160(13)60024-7
Sikora FJ (2002) Evaluating and quantifying the liming potential of phosphate rocks. Nutr Cycling Agroecosyst 63:59–67. https://doi.org/10.1023/A:1020513722249
Singh H, Reddy MS (2011) Effect of inoculation with phosphate solubilizing fungus on growth and nutrient uptake of wheat and maize plants fertilized with rock phosphate in alkaline soils. Eur J Soil Biol 47:30–34
Smits M, Bonneville S, Benning LG, Banwart SA, Leake JR (2012) Plant-driven weathering of apatite–the role of an ectomycorrhizal fungus. Geobiology 10:445–456
Sobat E, Whalen JK (2022) Mycorrhizal colonization associated with roots of field-grown maize does not decline with increasing plant-available phosphorus. Soil Use Manag 38:1370–1379. https://doi.org/10.1111/sum.12786
Staff SS (2014) Soil survey field and laboratory methods manual. In: Burt R, Soil Survey Staff (eds) Soil survey investigations report no 51, version 2.0. U.S. Department of Agriculture, Natural Resources Conservation Service, Washington DC, pp 227–241
Tawaraya K, Naito M, Wagatsuma T (2006) Solubilization of insoluble inorganic phosphate by hyphal exudates of arbuscular mycorrhizal fungi. J Plant Nutri 29:657–665. https://doi.org/10.1080/01904160600564428
Team R (2015) RStudio: integrated development for R. RStudio, Inc, Boston, MA. http://www.rstudio.com 42: 84. Accessed 20 Feb 2021
Thirkell TJ, Charters MD, Elliott AJ, Sait SM, Field KJ (2017) Are mycorrhizal fungi our sustainable saviours? Considerations for achieving food security. J Ecol 105:921–929. https://doi.org/10.1111/1365-2745.12788
Thomson B, Robson A, Abbott L (1986) Effects of phosphorus on the formation of mycorrhizas by Gigaspora calospora and Glomus fasciculatum in relation to root carbohydrates. New Phytol 103:751–765
Thomson BD, Robson AD, Abbott LK (1991) Soil mediated effects of phosphorus supply on the formation of mycorrhizas by Scutellispora calospora (Nicol. & Gerd.) Walker & Sanders on subterranean clover. New Phytol 118:463–469. https://doi.org/10.1111/j.1469-8137.1991.tb00028.x
Truog E (1916) The utilization of phosphates by agricultural crops, including a new theory regarding the feeding power of plants. Agricultural Experiment Station of the University of Wisconsin
Tummers B (2006) DataThief III. https://datathief.org/. Accessed 26 Oct 2020
Van Bemmelen J (1890) Über die Bestimmung des Wassers, des Humus, des Schwefels, der in den colloïdalen Silikaten gebundenen Kieselsäure, des Mangans usw im Ackerboden. Die Landwirthschaftlichen Versuchs-Stationen 37:e290
Van Schöll L, Hoffland E, Van Breemen N (2006) Organic anion exudation by ectomycorrhizal fungi and Pinus sylvestris in response to nutrient deficiencies. New Phytol 170:153–163
Wahid F, Sharif M, Steinkellner S, Khan MA, Marwat K, Khan S (2016) Inoculation of arbuscular mycorrhizal fungi and phosphate solubilizing bacteria in the presence of rock phosphate improves phosphorus uptake and growth of maize. Pak J Bot 48:739–747
Wei C, Zhu Y, Yang F, Li J, Zhu Z, Zhu H (2013) Dissolution and solubility of hydroxylapatite and fluorapatite at 25°C at different pH. Res J Chem Environ 17:57–61
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer-Verlag, New York
Wolff E (1864) Entwurf zur bodenanalyse. Z Anal Chem 3:85–115
Yao Q, Li X, Feng G, Christie P (2001) Mobilization of sparingly soluble inorganic phosphates by the external mycelium of an abuscular mycorrhizal fungus. Plant Soil 230:279–285. https://doi.org/10.1023/A:1010367501363
Yasmeen T, Arif MS, Shahzad SM, Riaz M, Tufail MA, Mubarik MS, Ahmad A, Ali S, Albasher G, Shakoor A (2022) Abandoned agriculture soil can be recultivated by promoting biological phosphorus fertility when amended with nano-rock phosphate and suitable bacterial inoculant. Ecotoxicol Environ Saf 234:113385. https://doi.org/10.1016/j.ecoenv.2022.113385
Zarei M, Saleh-Rastin N, Alikhani HA, Aliasgharzadeh N (2006) Responses of lentil to co-inoculation with phosphate-solubilizing rhizobial strains and arbuscular mycorrhizal fungi. J Plant Nutr 29:1509–1522. https://doi.org/10.1080/01904160600837667
Zhang L, Zhou J, George TS, Limpens E, Feng G (2022) Arbuscular mycorrhizal fungi conducting the hyphosphere bacterial orchestra. Trends Plant Sci 27:402–411. https://doi.org/10.1016/j.tplants.2021.10.008
Zlowodzki M, Poolman RW, Kerkhoffs GM, Tornetta P 3rd, Bhandari M (2007) How to interpret a meta-analysis and judge its value as a guide for clinical practice. Acta Orthop 78:598–609. https://doi.org/10.1080/17453670710014284
Acknowledgements
Jeonggu Lee helped make final figures for the manuscript. We thank the editor and two anonymous reviewers for their constructive feedback that helped in improving the manuscript.
Funding
Financial support was in part provided by the Illinois Nutrient Research and Education Council (NREC) award #2018–4–360731–385 (A.J.M.). Additional financial support was provided by the Brockson fellowship and Illinois Corn Marketing Growers fellowship to N.C. from the Department of Crop Sciences, University of Illinois Urbana-Champaign.
Author information
Authors and Affiliations
Contributions
Both authors contributed to the study conception and design. Data collection and analysis were performed by Neha Chatterjee. The first draft of the manuscript was written by Neha Chatterjee and both authors commented and reviewed previous versions. Both authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• AM-driven crop shoot biomass increased similarly under RP and SP.
• Crop biomass was lower under RP relative to SP fertilization irrespective of AM-enrichment.
• In soil pH < 6.5 and > 7.5, shoot biomass increased with RP but not SP under AM.
• Legume shoot biomass increased two-fold more than all crops for RP with AM.
• Root colonization when fertilized with SP was not lower than RP.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chatterjee, N., Margenot, A.J. Crop growth is increased by arbuscular mycorrhizae for both phosphate rock and soluble phosphorus fertilizers, but fertilizer solubility primarily determines crop growth. Biol Fertil Soils 59, 843–862 (2023). https://doi.org/10.1007/s00374-023-01751-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-023-01751-3