Abstract
Application of crop residues and its biochar produced through slow pyrolysis can potentially increase carbon (C) sequestration in agricultural production systems. The impact of crop residue and its biochar addition on greenhouse gas emission rates and the associated changes of soil gross N transformation rates in agricultural soils are poorly understood. We evaluated the effect of wheat straw and its biochar applied to a Black Chernozemic soil planted to barley, two growing seasons or 15 months (at the full-bloom stage of barley in the second growing season) after their field application, on CO2 and N2O emission rates, soil inorganic N and soil gross N transformation rates in a laboratory incubation experiment. Gross N transformation rates were studied using the 15N isotope pool dilution method. The field experiment included four treatments: control, addition of wheat straw (30 t ha−1), addition of biochar pyrolyzed from wheat straw (20 t ha−1), and addition of wheat straw plus its biochar (30 t ha−1 wheat straw + 20 t ha−1 biochar). Fifteen months after their application, wheat straw and its biochar addition increased soil total organic C concentrations (p = 0.039 and <0.001, respectively) but did not affect soil dissolved organic C, total N and NH4 +-N concentrations, and soil pH. Biochar addition increased soil NO3 −-N concentrations (p = 0.004). Soil CO2 and N2O emission rates were increased by 40 (p < 0.001) and 17 % (p = 0.03), respectively, after wheat straw addition, but were not affected by biochar application. Straw and its biochar addition did not affect gross and net N mineralization rates or net nitrification rates. However, biochar addition doubled gross nitrification rates relative to the control (p < 0.001). Our results suggest that land application of biochar, as opposed to the application of the raw wheat straw, could suppress CO2 and N2O emissions and enhance soil C sequestration. However, the implications of the increased soil gross nitrification rate and NO3 −-N in the biochar addition treatment for long-term NO3 −-N dynamics and N2O emissions need to be further studied.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Incorporation of crop residue in agricultural soils is one of the main management approaches to provide multiple ecosystem services, e.g., sustaining soil organic matter content and nutrient cycling, controlling water and wind erosion, and increasing crop production (Ocio et al. 1991; Blanco-Canqui 2013). However, incorporation of crop residues in the soil can increase CO2 emission due to decomposition of crop residues and the positive priming effect on the decomposition of native soil organic carbon (SOC) (Muhammad et al. 2007). Moreover, crop residue addition may increase soil nitrogen (N) loss resulting from enhanced N2O emission and nitrate leaching due to changes in nitrification and denitrification processes in the soil (Mitchell et al. 2000; Miller et al. 2008; Begum et al. 2014), depending on soil aeration status and the supply of inorganic N and labile organic C.
The application of biochar, a recalcitrant C-rich by-product of biomass pyrolysis under high temperature and low oxygen conditions, has recently generated much attention as a means to mitigate the aforementioned potential C and N losses from crop residues incorporated into the soil. Biochar applied to agricultural lands has been promoted as a means of improving soil quality and sequestering C, considering that biochar has condensed aromatic structures and can stabilize native soil C (Lehmann and Joseph 2009; Zwieten et al. 2013). However, contradictory results have been reported on changes in soil C and N cycling as affected by biochar application, which were related to the characteristics and quantity of the biochar applied, soil conditions, and time since biochar application (Castaldi et al. 2011; Zimmerman et al. 2011; Wang et al. 2013; Yu et al. 2013; Prayogo et al. 2014). In some cases, short-term increases in soil CO2 release have been observed and linked to a priming effect from soluble biochar C on native SOC decomposition (Luo et al. 2011; Jones et al. 2011). In contrast, others have found a suppression effect of biochar addition on soil CO2 production when CO2 produced from biochar was subtracted (Spokas et al. 2009) and a diminished priming effect after long-term incubation (Smith et al. 2010; Castaldi et al. 2011; Jones et al. 2012). Similarly, biochar addition has been reported to suppress (e.g., Spokas et al. 2009; Wang et al. 2013; Wu et al. 2013; Cayuela et al. 2014) or stimulate or have no impact on N2O emissions (e.g., Castaldi et al. 2011; Cheng et al. 2012; Verhoeven and Six 2014).
It is necessary to study the changes in soil microbial activities and soil C and N transformation processes, e.g., mineralization, nitrification, denitrification, and immobilization processes, induced by biochar application because soil greenhouse gas emissions and soil C and N availability and N transformation rates are strongly linked (Berglund et al. 2004; Nelissen et al. 2012; Cayuela et al. 2014; Prayogo et al. 2014). Anderson et al. (2011) and Song et al. (2014) reported that soil inorganic N concentrations were decreased by biochar addition because of its high surface area. However, limited effects of biochar addition on the turnover of SOC, dissolved organic C (DOC), and N (DON) and lack of long-term effects on N mineralization, NH3 volatilization, denitrification, and NH4 + sorption were reported in a 3-year field trial (Jones et al. 2012). In addition to the many studies on greenhouse gas emissions, studies on the impact of biochar application on soil C and N transformation processes are needed. It is particularly important to measure gross N transformation rates to determine the real rates of N production and consumption in soils (Deluca et al. 2006; Cheng et al. 2012; Nelissen et al. 2012).
The impact of biochar application on soil C and N cycling in field conditions must be better understood before biochar is promoted for large-scale field application. However, most previous studies were conducted in laboratory conditions with a short-term nature (i.e., several weeks to a few months). Moreover, the impact of biochar addition on soil C and N cycling were different between short-term and long-term field experiments (Castaldi et al. 2011; Jones et al. 2012). Lack of long-term field studies makes it difficult to conclude the long-term effects of biochar on soil C and N cycling (Castaldi et al. 2011; Jones et al. 2012; Verhoeven and Six 2014), except that a few studies investigated the impact of charcoal as a natural product of forest wildfires (Deluca et al. 2006; Ball et al. 2010).
In this study, we determined soil total organic C, DOC, and soil inorganic N concentrations 15 months after wheat straw and its biochar addition to a Black Chernozemic soil planted to barley and evaluated the changes in soil CO2 and N2O emission rates and net and gross N transformation rates in a laboratory incubation experiment. Our objective was thus to examine the impact of wheat straw and its biochar 15 months after application to an agricultural field on soil CO2 and N2O emissions, net and gross N mineralization and nitrification rates, and soil NH4 +-N and NO3 −-N consumption rates.
Materials and methods
Site characteristics and experimental design
The study site was located at the University of Alberta experimental farm on South Campus (53° 48′ N, 113° 32′ W), in Edmonton, Canada. The climate of the study area belongs to a humid continental climate according to Köppen’s climatic classification, with mean monthly temperature ranging from −11.7 °C in January to a summer peak of 17.7 °C in July and mean annual precipitation of 477 mm with 366 mm of rainfall and 111 mm of snowfall between 1971 and 2000 (Environment Canada 2010). The soil was a Black Chernozem based on the Canadian system of soil classification, with a silty clay loam texture (18.8 % sand, 46.4 % silt, and 34.8 % clay). Soil total C concentration was 6.95 %, total N concentration was 0.64 %, and soil pH was 5.52.
A 2 × 2 factorial design was used in this experiment. One factor studied was wheat straw addition, and the other was biochar addition; both factors had two levels. Thus, four treatment combinations were set up: control (CK), addition of wheat straw (S), addition of biochar made from the wheat straw (B), and addition of wheat straw plus its biochar (SB). Chemical and physical characteristics of the wheat straw and biochar were shown in Table 1. In May 2010, 16 plots of 5 × 5 m in size were established on the experimental farm that had been planted to barley for several years before our study and were planted to barley during our study. Fertilizer was not applied during our experiment in order not to confound the effects of wheat straw and its biochar addition. Each treatment with four replications was completely randomly set up, and there was a 5-m buffer zone between adjacent plots. The biochar was produced by Alberta Innovates Technology Futures (Vegreville, Alberta, Canada) using the same wheat straw (cut to 2 cm before pyrolysis) in a fast pyrolysis process (450 °C). The cut wheat straw (about 2 cm) and its biochar were applied to the surface of the plots at 30 and 20 t ha−1, respectively, and they were manually mixed into the plough layer (0–15 cm). Thus, the amount of added C was 0, 13.8, 13.8, and 27.6 t C ha−1 in the CK, S, B, and SB treatments, respectively.
Soil sampling and C, N, and pH analyses
In August 2011, at the full-bloom stage of barley growth, five soil cores were taken from each plot using a stainless auger (2.5-cm diameter) from the plough layer (0–15 cm) and thoroughly mixed to form a composite sample. The moist soil samples were sieved through a 2-mm mesh, and the visible crop residues were removed by hand. Each soil sample was divided into two subsamples, with one subsample air-dried at room temperature for total organic C (TOC), total N (TN), and soil pH analyses. The other subsample was stored in polyethylene bags at 4 °C for about 2 weeks prior to measurement of CO2 and N2O emission rates and gross and net N transformation rates.
The air-dried soil samples were sieved (<2 mm) and used to determine soil pH at a soil/water ratio of 1:2.5 (weight/volume) by a DMP-2 mV/pH meter. For determination of TOC and TN concentrations, the air-dried soil samples were ground with a ball mill and analyzed with a Carlo Erba NA 1500 elemental analyzer (Carlo Erba Instruments, Milano, Italy).
Laboratory incubations and soil C and N transformations
Four fresh soil samples (30 g oven-dry weight basis) from each plot were weighed into a 300-mL flask, and the moisture content of soils was adjusted to 60 % water holding capacity using distilled water. Then, the flasks were sealed with silicone rubber stoppers and incubated in the dark at 25 °C for 24 h to allow equilibration. To determine CO2 and N2O emission rates, the flasks were firstly flushed with fresh air and then sealed with silicone rubber stoppers and incubated for 1 h in the dark at 25 °C. The CO2 and N2O concentrations in the headspace were determined by sampling the headspace gas and injecting this sample into a gas chromatograph (Varian CP-3800 GC, Conquer Scientific, San Diego, USA).
After the determination of soil CO2 and N2O emission rates, soil gross N mineralization and NH4 +-N consumption rates were determined by the 15NH4 + isotopic dilution method, and gross rates of nitrification and NO3 −-N consumption were determined by the 15NO3 − isotopic dilution method (Hart et al. 1994). Firstly, ammonium nitrate with either ammonium (15NH4NO3) or nitrate (NH4 15NO3) labeled with 15N at 5 atom% excess was applied evenly over the soil surface in solution (2 mL), at a rate of 20 mg N kg−1 soil (Cheng et al. 2012). Subsequently, half of the four flasks was extracted with 2 M KCl solution after 15 min (t 0), and the remaining flasks were extracted after 24 h of incubation (t 1) in the dark at 25 °C. The soil samples (1:5 weight/volume ratio of soil to solution) were shaken at 250 rpm on a mechanical shaker for 1 h, and then, the extracts were filtered through Whatman No. 42 filters. A portion of the KCl extract was steam distilled with MgO on a steam distillation system (Vapodest 20, C. Gerhardt, Königswinter, Germany); thereafter, the sample was distilled again after the addition of Devarda’s alloy. The liberated NH3 was collected in 5-mL 0.05 M H2SO4 solutions in a beaker (Keeney and Nelson 1982). To prevent isotopic cross-contamination between samples, a 20 mL reagent-grade ethanol was added to the distillation flask and steam distilled for 3 min between sample distillations (Hauck 1982). The H2SO4 solution containing NH4 + was then evaporated to dryness at 65 °C in an oven after adjustment of the solution to pH 3 using 0.05 M H2SO4. In order to get the obvious samples after drying, a 5-mL 0.2 M Na2SO4 solution were added to each beaker. The abundance of 15N in the dried samples was determined using a CN analyzer (NA Series 2, CE Instruments, Italy) linked to a stable isotope ratio mass spectrometer (Optima-EA; Micromass, Crewe, UK) at the Lethbridge Research Centre of Agriculture and Agri-Food Canada.
Gross N transformation rates were calculated from changes in atom percentage of 15N excesses (APE) and N pool size of soils at t 0 and t 1 using the following equations (Kirkham and Bartholomew 1954; Hart et al. 1994):
where m is gross N mineralization rate (mg kg−1 day−1), c is NH4 +-N consumption rate (mg kg−1 day−1), t is time (1 day), APE is atom percent 15N excesses of NH4 + pool, and [NH4 +] is total NH4 +-N concentration (mg kg−1). Gross nitrification and NO3 − consumption rates were calculated in a similar manner by substituting NO3 − concentrations and atom percent 15N excesses of NO3 − in the above equations.
Another portion of the KCl extract was used to determine soil NH4 +-N (Keeney and Nelson 1982) and NO3 −-N concentrations by a colorimetric method (Miranda et al. 2001). Soil net N mineralization and nitrification rates were calculated from the differences of soil (NH4 + + NO3 −)-N and NO3 −-N pool sizes after and before incubation. For the determination of soil DOC, a portion of KCl extracts before incubation was diluted with deionized water and determined using a TOC-VCSN total organic C analyzer (Shimadzu, Kyoto, Japan).
Statistical analysis
A general linear model (GLM) was used to analyze for treatment effects (SPSS 19.0, IBM Corp., Armonk, NY, US). All data were tested for homogeneity of variance and normality of distribution. Soil NO3 −-N concentration and gross nitrification rate were log transformed, and soil gross NO3 −-N consumption rate was sin transformed to meet homogeneity of variance assumptions. Back-transformed data were then reported. A two-way ANOVA was performed to test for effects of factors (i.e., wheat straw and biochar additions) and their interactions on each measured parameter. When an interaction effect was present; a one-way ANOVA followed by the Tukey’s test was used for multiple comparison among the four treatments (i.e., CK, B, S, and BS). Pearson’s correlation analysis was used to assess relationships between soil N transformation rates and the soil chemical properties across all data of four treatments. Statistical significance was set at α = 0.05.
Results
Soil pH, DOC, TOC, TN, and C/N ratio
There were no significant interactions between wheat straw and its biochar additions on soil pH, DOC, TOC, TN, and C/N ratio. Soil pH, DOC, and TN did not change after wheat straw and/or its biochar applications (Table 2). However, TOC increased by 4 (p = 0.039) and 16 % (p < 0.001) after wheat straw and its biochar additions, respectively. Soil C/N ratio was increased by biochar (p = 0.01), but not by wheat straw addition.
Soil NH4 +-N and NO3 −-N concentrations
Soil NO3 −-N was the main form of soil inorganic N in this study, and NO3 −-N (ranging from 17.24 to 53.08 mg kg−1) was markedly higher than NH4 +-N (ranging from 1.80 to 3.87 mg kg−1). There were no significant interactions between wheat straw and its biochar additions on soil NH4 +-N and NO3 −-N (Fig. 1). Wheat straw and/or its biochar did not change NH4 +-N 15 months after their additions (Fig. 1a). However, application of biochar (p = 0.004), but not wheat straw (p > 0.05), increased NO3 −-N (Fig. 1b).
Effects of wheat straw and its biochar on soil a NH4 +-N and b NO3 −-N concentrations 15 months after their addition to a Black Chernozemic soil planted to barley. Treatment codes of CK, S, B, and SB refer to control, wheat straw addition, biochar addition, and wheat straw plus its biochar addition, respectively. Error bars represent standard errors (n = 4). ns and asterisk indicate t test results of p > 0.05 and p < 0.05, respectively
Soil CO2 and N2O emission rates
There were no significant interactions between wheat straw and its biochar additions on CO2 and N2O emission rates (Fig. 2). Wheat straw addition increased CO2 and N2O emission rates by 40 (p < 0.001) and 17 % (p = 0.03), respectively. However, biochar addition did not affect CO2 and N2O emission rates (Fig. 2).
Effects of wheat straw and its biochar on a soil CO2 and b N2O emission rates 15 months after their addition to a Black Chernozemic soil planted to barley. Treatment codes of CK, S, B, and SB refer to control, wheat straw addition, biochar addition, and wheat straw plus its biochar addition, respectively. Error bars represent standard errors (n = 4). ns, single asterisk, and triple asterisks indicate t test results of p > 0.05, p < 0.05, and p < 0.001, respectively
Gross and net N transformation rates
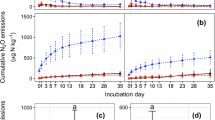
No significant interactions between wheat straw and its biochar additions on gross and net N transformation rates were observed. Soil gross N mineralization and gross nitrification rates ranged from 2.16 to 5.36 mg N kg−1 day−1 and 6.99 to 30.66 mg N kg−1 day−1, respectively. Wheat straw and/or its biochar addition did not change gross N mineralization and NH4 +-N consumption rates (Fig. 3a, c). However, gross nitrification rates were doubled after biochar application (p < 0.001) (Fig. 3b), while gross nitrification rates were not affected by wheat straw addition. Similarly, gross NO3 −-N consumption rates were increased by biochar addition (p < 0.05), but not by wheat straw addition (Fig. 3d). Net N mineralization and net nitrification rates were not affected by wheat straw and/or its biochar additions or their interactions (Fig. 3e, f).
Effects of wheat straw and its biochar on a soil gross N mineralization, b gross nitrification, c soil NH4 +-N and d NO3 −-N consumption rates, e soil net N mineralization, and f net nitrification rates 15 months after their addition to a Black Chernozemic soil planted to barley. Treatment codes of CK, S, B, and SB refer to control, wheat straw addition, biochar addition, and wheat straw plus its biochar addition, respectively. Error bars represent standard errors (n = 4). ns, single asterisk, and triple asterisks indicate t test results of p > 0.05, p < 0.05, and p < 0.001, respectively
Gross N mineralization and NH4 +-N consumption rates were positively correlated with TN, DOC, and pH (Table 3). Gross nitrification rates were positively correlated with TOC, C/N ratio, and nitrate concentration. There were positive correlations between net N mineralization and soil pH and NO3 −-N, and between net nitrification and soil pH.
Discussion
It has been suggested that the residence time of biochar in the soil may be on decadal to centennial scales, depending on the feedstock and the process used for producing the biochar (Hockaday et al. 2006; Steinbeiss et al. 2009). As a result of that, biochar application has been proposed as a potential means to offset CO2 emissions derived from fossil fuel use (Reijnders 2009). In this study, biochar application increased TOC but did not change soil CO2 emission. The small or complete lack of effects of biochar on soil C mineralization rates was also observed in some short-term studies (Smith et al. 2010; Bell and Worrall 2011; Wu et al. 2013) and a year-long study (Jones et al. 2012). In contrast, soil CO2 emission was enhanced by the raw wheat straw application in this laboratory incubation experiment and under field conditions where soil CO2 emission rates were determined weekly during 2010 and 2011 in the same study site (data not show). Cheng et al. (2012) also found that wheat straw rather than its biochar stimulated CO2 emission in a Black Chernozemic soil over 12-day incubation. Considering the increase in soil C storage after biochar application and the enhanced CO2 release by wheat straw application, biochar application should provide substantial benefit to C sequestration in soils as compared with the application of the raw straw (Jones et al. 2011). Substrate inputs from wheat straw and biochar and soil physical conditions (e.g., soil moisture content and pH) are main controlling factors on soil C mineralization (Aciego Pietri and Brookes 2009; Yu et al. 2013). However, there were no significant differences in soil DOC and pH between the CK and treated soils 15 months after addition (Table 2), reflecting changes in native and recently added C mineralization, but no longer the increases of soil active C inputs from wheat straw or biochar. Thus, the increased CO2 emission after wheat straw addition was likely related to changes in soil microbial biomass and community structure (Aciego Pietri and Brookes 2009).
Straw application not only increased the rate of emission of CO2, but also N2O, which has a greater global warming potential. Stimulated N2O emission after plant residue addition has frequently been reported (Chantigny et al. 2001; Miller et al. 2008; Begum et al. 2014), indicating increased microbial denitrification and nitrification processes responsible for N2O emissions in agricultural soils (Firestone and Davidson 1989). In this study, wheat straw addition did not affect gross nitrification rates. Thus we infer that wheat straw application increased soil denitrification, contributing to the enhanced N2O emission rate in that treatment. Li and Lang (2014) found that the contribution of denitrification to N2O emission was 32.1 % of the total N2O emission in an uncultivated soil and 43.2 % in a cultivated soil. Microbial activity and O2 consumption can be increased because of a higher available C from plant residue addition, which might lead to conditions favorable for denitrification (Miller et al. 2008).
On the other hand, N2O emission rates did not increase 15 months after biochar application. Similarly, the lack of treatment effect of biochar addition on soil N2O emission was also observed in a 2-week laboratory incubation experiment where biochar was added to a Black Chernozemic soil (Cheng et al. 2012), and the suppressing effects of biochar addition on N2O emission were found as compared with the control under the field condition in the same experiment site in two growing seasons (data not shown). Zwieten et al. (2013) also found that poultry litter biochar reduced N2O emission compared with the raw poultry litter addition. A meta-analysis conducted by Cayuela et al. (2014) suggested that biochar could reduce soil N2O emission by 54 % in laboratory and field studies. However, Verhoeven and Six (2014) observed that annual cumulative N2O emissions of biochar treatment were significantly higher than the control treatment in a 2-year field experiment. The biochar application rate, pyrolysis conditions, and C/N ratio were shown to be key factors influencing soil N2O emission (Cayuela et al. 2014). Many studies have indicated improved soil aeration leading to a reduction in denitrification rates, which is a mechanism behind reduced N2O emissions after biochar addition (Spokas et al. 2009; Cayuela et al. 2014). The abundance of Bradyrhizobiaceae and Hyphomicrobiaceae involved in denitrification had been found to decrease after biochar addition (Anderson et al. 2011). Although nitrification increased in the biochar addition treatment, the reduction of denitrification after biochar addition might offset the increased N2O emission caused by increased gross nitrification (Wang et al. 2013).
Total gross N production and consumption rates are commonly calculated with analytical equations based on incubation periods of 1–2 days, assuming that remineralization of added 15N is negligible (Hart et al. 1994) or with a 15N tracing model (Müller et al. 2004). In this study, we calculated soil gross N mineralization, nitrification, and consumption rates of soil NH4 +-N and NO3 −-N using the theoretical equation of Kirkham and Bartholomew (1954). The increased gross nitrification rate 15 months after biochar addition was consistent with Nelissen et al. (2012) who found that gross nitrification was stimulated by maize biochar addition to an agricultural soil in a short-term laboratory study. Deluca et al. (2006) and Ball et al. (2010) all found that the presence of charcoal in forest soils following wildfire increased gross nitrification mediated by ammonia-oxidizing bacteria (AOB) in both laboratory and field studies. However, Cheng et al. (2012) reported that biochar had no effect on gross rate of nitrification while wheat straw depressed gross nitrification rate by 32.2 % at early stage in a Black Chernozemic soil. This suggests that the impact of biochar addition on soil nitrification rate was different between the early and later stage. Moreover, soil characteristics (e.g., soil pH and nitrifier activity) and pyrolysis conditions and application rates of biochar have been demonstrated influence gross nitrification after biochar addition (Deluca et al. 2006; Nelissen et al. 2012).
Several mechanisms have been proposed to explain the increase in gross nitrification after biochar application. Biochar application could decrease the concentrations of plant secondary compounds and thus alleviate their inhibitory effect on the activity of nitrifying microbial community and increase gross nitrification (DeLuca et al. 2006). Furthermore, Ball et al. (2010) and Song et al. (2014) showed that charcoal or biochar could increase the abundance and activities of AOB responsible for nitrification. Nitrifiers could aggregate around biochar particles for food source due to their adsorption of various soluble C substances and produce a biofilm structure essential for nitrification (Berglund et al. 2004).
We found that soil NO3 −-N increased 15 months after biochar addition. Stimulation of soil gross nitrification rate with biochar might account for the changes in soil NO3 −-N. Moreover, higher amino acid and NO3 −-N contents in the burial aged biochar than in the fresh biochar have been reported (Jones et al. 2012), though several studies did not observe the significant differences of soil NO3 −-N with the year-long amendment of biochar (Castaldi et al. 2011; Verhoeven and Six 2014). Given the increase of soil NO3 −-N and gross nitrification rates in our experiment, N loss potential through leaching might be increased by biochar application in the agricultural soil. If the increased soil NO3 −-N can be taken up by crop plants and the N use efficiency improved with biochar addition as was observed in a greenhouse experiment in Prendergast-Miller et al. (2011), then the increased NO3 −-N in our laboratory incubation samples may not be a concern. This illustrates the need to study the impact of biochar application on soil N leaching and N uptake by crop plants in long-term field experiments.
Conclusions
We conclude that soil C and N cycling processes were changed by wheat straw and its biochar application in a Black Chernozemic soil planted to barley. Addition of biochar instead of wheat straw to agricultural soils has the potential to reduce CO2 and N2O emission rates and increase soil C sequestration; thus, biochar application should be encouraged in agricultural practices. However, gross nitrification rate and NO3 −-N increased after biochar application, which might lead to a potential risk of N leaching loss in agricultural soils. As such, we have guided readers with suggestions to address specific soil C and N cycling processes associated with greenhouse gas emissions before large-scale application of biochar in agricultural soils. In particular, the implication of the increased gross nitrification rate and NO3 −-N in the biochar addition treatment for soil NO3 −-N dynamics and N2O emissions needs to be further studied.
References
Aciego Pietri JC, Brookes PC (2009) Substrate inputs and pH as factors controlling microbial biomass, activity and community structure in an arable soil. Soil Biol Biochem 41:1396–1405
Anderson CR, Condron LM, Clough TJ, Fiers M, Stewart A, Hill RA, Sherlock RR (2011) Biochar induced soil microbial community change: implications for biogeochemical cycling of carbon, nitrogen and phosphorus. Pedobiologia 54:309–320
Ball RN, MacKenzie MD, DeLuca TH (2010) Wildfire and charcoal enhance nitrification and ammonium-oxidizing bacterial abundance in dry montane forest soils. J Environ Qual 39:1243–1253
Begum N, Guppy C, Herridge D, Schwenke G (2014) Influence of source and quality of plant residues on emissions of N2O and CO2 from a fertile, acidic Black Vertisol. Biol Fertil Soils 50:499–506
Bell MJ, Worrall F (2011) Charcoal addition to soils in NE England: a carbon sink with environmental co-benefits? Sci Total Environ 409:1704–1714
Berglund L, DeLuca TH, Zackrisson O (2004) Activated carbon amendments to soil alters nitrification rates in Scots pine forests. Soil Biol Biochem 36:2067–2073
Blanco-Canqui H (2013) Crop residue removal for bioenergy reduces soil carbon pools: how can we offset carbon losses? Bioenergy Res 6:358–371
Castaldi S, Riondino M, Baronti S, Esposito FR, Marzaioli R, Rutigliano FA, Vaccari FP, Miglietta F (2011) Impact of biochar application to a Mediterranean wheat crop on soil microbial activity and greenhouse gas fluxes. Chemosphere 85:1464–1471
Cayuela ML, Zwieten L, Singh BP, Jeffery AR, Sánchez-Monedero MA (2014) Biochar’s role in mitigating soil nitrous oxide emissions: a review and meta-analysis. Agric Ecosyst Environ 191:5–16
Chantigny MH, Rochette P, Angers DA (2001) Short-term C and N dynamics in a soil amended with pig slurry and barley straw: a field experiment. Can J Soil Sci 81:131–137
Cheng Y, Cai Z, Chang SX, Wang J (2012) Wheat straw and its biochar have contrasting effects on inorganic N retention and N2O production in a cultivated Black Chernozem. Biol Fertil Soils 48:941–946
DeLuca TH, MacKenzie MD, Gundale MJ, Holben WE (2006) Wildfire-produced charcoal directly influences nitrogen cycling in ponderosa pine forests. Soil Sci Soc Am J 70:448–453
Environment Canada (2010) National climate data and information archive. Available from http://climate.weatheroffice.gc.ca/climate_normals/
Firestone MK, Davidson EA (1989) Microbiological basis of NO and N2O production and consumption in soil. In: Andreae MO, Schimel DS (eds) Exchange of trace gases between terrestrial ecosystems and the atmosphere. Wiley, New York, pp 7–21
Hart SC, Staik JM, Davidson EA, Firestone MK (1994) Dynamics of gross nitrogen transformations in an old-growth forest: the carbon connection. Ecology 75:880–891
Hauck RD (1982) Nitrogen-isotope ratio analysis. In: Page AL (ed) Methods of soil analysis. Part 2. Chemical and microbiological properties. Soil Science Society of America, Madison, pp 735–779
Hockaday WC, Grannas AM, Kim S, Hatcher PG (2006) Direct molecular evidence for the degradation and mobility of black carbon in soils form ultrahigh-resolution mass spectral analysis of dissolved organic matter from a fire-impacted forest soil. Org Geochem 37:501–510
Jones DL, Murphy DV, Khalid M, Ahmad W, Edwards-Jones G, Deluca TH (2011) Short-term biochar-induced increase in soil CO2 release is both biotically and abiotically mediated. Soil Biol Biochem 43:1723–1731
Jones DL, Rousk J, Edwards-Jones G, Deluca TH, Murphy DV (2012) Biochar-mediated changes in soil quality and plant growth in a three year field trial. Soil Biol Biochem 45:113–124
Keeney DR, Nelson DW (1982) Nitrogen-inorganic forms. In: Page AL (ed) Methods of soil analysis. Part 2. Chemical and microbiological properties. Soil Science Society of America, Madison, pp 643–698
Kirkham D, Bartholomew WV (1954) Equations for following nutrient transformations in soil utilizing tracer data. Soil Sci Soc Am Proc 18:33–34
Lehmann J, Joseph S (2009) Biochar for environmental management: science and technology. Earthscan, London
Li P, Lang M (2014) Gross nitrogen transformations and related N2O emissions in uncultivated and cultivated black soil. Biol Fertil Soils 50:197–206
Luo Y, Durenkamp M, De Nobili M, Lin Q, Brookes PC (2011) Short term soil priming effects and the mineralization of biochar following its incorporation to soils of different pH. Soil Biol Biochem 43:2304–2314
Miller MN, Zebarth BJ, Dandie CE, Burton DL, Goyera C, Trevors JT (2008) Crop residue influence on denitrification, N2O emissions and denitrifier community abundance in soil. Soil Biol Biochem 40:2553–2562
Miranda KM, Espey MG, Wink DA (2001) A rapid, simple spectrophotometric method for simultaneous determination of nitrate and nitrite. Nitric Oxide Boil Chem 5:62–71
Mitchell RDJ, Harrison R, Russell KJ, Webb J (2000) The effect of crop residue incorporation date on soil inorganic nitrogen, nitrate leaching and nitrogen mineralization. Biol Fertil Soils 32:294–301
Muhammad S, Joergensen RG, Mueller T, Muhammad TS (2007) Priming mechanism: soil amended with crop residue. Pak J Bot 39:1155–1160
Müller C, Stevens RJ, Laughlin RJ (2004) A 15N tracing model to analyse N transformations in old grassland soil. Soil Biol Biochem 36:619–632
Nelissen V, Rütting T, Huygens D, Staelens J, Ruysschaert G (2012) Maize biochars accelerate short-term soil nitrogen dynamics in a loamy sand soil. Soil Biol Biochem 55:20–27
Ocio JA, Brookes PC, Jenkinson DS (1991) Field incorporation of straw and its effects on soil microbial biomass and soil inorganic N. Soil Biol Biochem 23:171–176
Prayogo C, Jones JE, Baeyens J, Bending GD (2014) Impact of biochar on mineralization of C and N from soil and willow litter and its relationship with microbial community biomass and structure. Biol Fertil Soils 50:695–702
Prendergast-Miller MT, Duvall M, Sohi SP (2011) Localisation of nitrate in the rhizosphere of biochar-amend soils. Soil Biol Biochem 43:2243–2246
Reijnders L (2009) Are forestation, bio-char and landfilled biomass adequate offsets for the climate effects of burning fossil fuels? Energ Policy 37:2839–2841
Smith JL, Collins HP, Bailey VL (2010) The effect of young biochar on soil respiration. Soil Biol Biochem 42:2345–2347
Song Y, Zhang X, Ma B, Chang SX, Gong J (2014) Biochar addition affected the dynamics of ammonia oxidizers and nitrification in microcosms of a coastal alkaline soil. Biol Fertil Soils 50:321–332
Spokas KA, Koskinen WC, Baker JM, Reicosky DC (2009) Impacts of woodchip biochar additions on greenhouse gas production and sorption/degradation of two herbicides in a Minnesota soil. Chemosphere 77:574–581
Steinbeiss S, Gleixner G, Antonietti M (2009) Effect of biochar amendment on soil carbon balance and soil microbial activity. Soil Biol Biochem 41:1301–1310
Verhoeven E, Six J (2014) Biochar does not mitigate field-scale N2O emissions in a Northern California vineyard: an assessment across two years. Agric Ecosyst Environ. doi:10.1016/j.agee.2014.03.008
Wang Z, Zheng H, Luo Y, Deng X, Herbert S, Xing B (2013) Characterization and influence of biochars on nitrous oxide emission from agricultural soil. Environ Pollut 174:289–296
Wu F, Jia Z, Wang S, Chang SX, Startsev A (2013) Contrasting effects of wheat straw and its biochar on greenhouse gas emissions and enzyme activities in a Chernozemic soil. Biol Fertil Soils 49:555–565
Yu L, Tang J, Zhang R, Wu Q, Gong M (2013) Effects of biochar application on soil methane emission at different soil moisture levels. Biol Fertil Soils 49:119–128
Zimmerman AR, Gao B, Ahn MY (2011) Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol Biochem 43:1169–1179
Zwieten LV, Kimber SWL, Morris SG, Singh BP, Grace PR, Scheer S, Rust J, Downie AE, Cowie AL (2013) Pyrolysing poultry litter reduces N2O and CO2 fluxes. Sci Total Environ 465:279–287
Acknowledgments
This study was supported by the Natural Science and Engineering Research Council of Canada (NSERC) and Natural Science Foundation of China (41271318). We thank Drs. Sunguo Wang and Kangho Jung, and Mr. Raza Purvez and Zheng Shi for their considerable help in conducting the field work. We would also like to thank Dr. Yi Cheng for help in the 15N tracing method and Mr. Clarence Gilbertson at the Agriculture and Agri-Food Canada Lethbridge Research Centre for 15N isotope analysis. We thank Tim Anderson, Jin Tak, and Rob Hughes at Alberta Innovates Technology Futures for providing the biochar used in this experiment. We gratefully acknowledge the helpful comments from two anonymous reviewers that improved an earlier version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, YL., Wu, FP., Zeng, DH. et al. Wheat straw and its biochar had contrasting effects on soil C and N cycling two growing seasons after addition to a Black Chernozemic soil planted to barley. Biol Fertil Soils 50, 1291–1299 (2014). https://doi.org/10.1007/s00374-014-0943-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-014-0943-6