Abstract
The effect of biochar and nitrapyrin (a nitrification inhibitor) applications on nitrous oxide (N2O) emissions from a cropland soil was studied in a 35-day incubation experiment. The biochars were produced using canola (Brassica napus L.) straw under two pyrolysis temperatures: 300 (BC300) and 700 °C (BC700). Biochars (20 g kg−1 soil) and nitrapyrin (80 mg kg−1 soil) were applied alone or in combination. The cumulative N2O emissions were affected by both biochar and nitrapyrin applications (p < 0.05, same below) but not by their interaction. Cumulative N2O emissions were not affected by BC700, but were increased by BC300, as compared with the CK treatment (no biochar addition). Nitrapyrin significantly decreased cumulative N2O emissions by inhibiting nitrification, whether biochar was applied or not. There were positive relationships (p < 0.05) between cumulative N2O emissions and soil microbial biomass carbon to nitrogen ratio, nitrate and dissolved organic nitrogen concentrations, and net nitrification rates. Our results show that biochars need to be appropriately selected (such as the use of BC700) that do not increase N2O emissions, while the effectiveness of nitrapyrin in reducing N2O emissions was not affected by the co-application of biochars. We conclude that the co-application of biochar and nitrapyrin may be able to both increase soil C sequestration by the addition of stable C contained in the biochar and reduce N2O emissions from agricultural production systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrous oxide (N2O), one of the most critical greenhouse gases, was estimated to have 265 times the global warming potential to that of CO2 on a 100-year time frame (Stocker et al. 2013) and has also been implicated in destroying the atmospheric ozone layer (Ravishankara et al. 2009). Land-use activities such as intensively managed agriculture and forestry are the primary cause of increased anthropogenic N2O emissions (Jia et al. 2019). Emissions of N2O from anthropogenic sources, dominantly caused by fertilizer nitrogen (N) application in agriculture, have recently been reported to exceed the highest emission scenarios previously projected (Tian et al. 2020). Thus, reducing N2O emissions from agricultural production systems is an urgent matter (Baah-Acheamfour et al. 2016; Omonode et al. 2017; Smith 2017; Tian et al. 2020).
Biochar, an organic material derived from biomass through pyrolysis without or with limited oxygen, has been shown to potentially reduce greenhouse gas emissions when applied to the soil (Lehmann and Joseph 2015). The effect of biochar application on N2O emissions is often variable. Some meta-analysis studies showed that biochar addition reduced N2O emissions by 12 to 54% (Cayuela et al. 2014, 2015; Liu et al. 2018). However, some have shown that N2O emissions were increased by biochar application. For instance, in a 12-day incubation study, the application of a biochar produced by the pyrolysis of green waste at 550 °C increased N2O emissions by 54% in agricultural soils (Sánchez-García et al. 2014), as a result of enhanced nitrification. Similarly, a biochar made from spruce woodchips at ~ 450 °C increased N2O emissions from an abandoned grassland soil indirectly through increasing soil water content and reducing plant N uptake (Saarnio et al. 2013).
Many abiotic and biotic mechanisms have been proposed to explain biochar effects on N2O production (Kammann et al. 2017); most of these mechanisms are dependent on soil and biochar properties (Saarnio et al. 2013; Lehmann and Joseph 2015; Levesque et al. 2020). Pyrolysis temperature is one of the primary determinants of biochar characteristics (Clough et al. 2013; Lehmann and Joseph 2015). Biochars produced under high-pyrolysis temperatures usually have a greater surface area and higher organic C adsorption capacity that have a greater ability to adsorb nitrate (NO3−) and other nutrients as compared with biochars produced under low-pyrolysis temperatures (Mukherjee and Zimmerman 2013). Many have studied the effect of biochars made from different feedstocks or under different pyrolysis conditions on N2O emissions; however, studies on the effect of canola (Brassica napus L.) straw biochar on N2O emissions are rare, especially canola straw biochars pyrolyzed under different temperatures, while canola is a major agricultural crop and the proper disposal of its residue will benefit the agriculture sector. A better understanding of the effect of biochars made from canola straw under different pyrolysis temperatures on N2O production from the soil is needed.
Another widely used method to mitigate soil N2O emissions is the application of nitrification inhibitors; nitrification is one of the main processes responsible for N2O emissions from agricultural soils. The product (NO3−) from nitrification is also subject to losses through denitrification (which is another process that produces N2O) and leaching. Among the nitrification inhibitors, nitrapyrin is widely used and can effectively decrease N2O emissions by inhibiting the activities of the bacteria Nitrosomonas (Dittmar et al. 2009).
Applying biochar and nitrification inhibitors together has been proposed to be a viable approach to enhance biochar’s benefit as a soil amendment (Chen et al. 2019). The combined application of biochar and 3,4-dimethylpyrazole phosphate has been shown to decrease N2O emissions more than biochar application alone (Chen et al. 2019). However, Li et al. (2015) demonstrated that applying wheat straw biochar and nitrapyrin together increased N2O emissions by 9% in a managed vegetable field compared with nitrapyrin application alone. In contrast, there were no significant differences between combined or individual application of nitrapyrin and maize straw biochar in N2O emissions (Niu et al. 2018). Biochar may adsorb nitrification inhibitors when they are applied together and reduce the effectiveness of the nitrification inhibitor (Li et al. 2020). To the best of our knowledge, no one has tested the interaction of canola straw biochars that are produced at different pyrolysis temperatures and nitrapyrin on soil N2O emissions. We were interested in understanding if the co-application of canola straw biochar would reduce the effectiveness of nitrification inhibitors and if canola straw biochars made under different pyrolysis temperatures behave differently when co-applied with nitrification inhibitors. In this study, we conducted a 35-day laboratory incubation experiment with two specific objectives: (1) to investigate the interactive effects of biochars produced under different pyrolysis temperatures and nitrapyrin on soil N2O emissions, and (2) to examine the relationships between soil characters and cumulative N2O emissions.
Materials and methods
Soil sampling and biochar production
Five canola fields near Edmonton (53° 32′ 0″ N, 113° 30′ 0″ W), Alberta, Canada, were selected as the soil sampling sites (each site represents a block in our experiment). The sampling sites were located more than 20 km from each other. The cropland was canola rotated with wheat or fallow. The soils at those sampling sites were classified as Black Chernozems based on the Soil Classification Working Group (1998). One composite surface mineral soil sample (0–10 cm) was collected from each of these five sampling sites. Each composite sample consists of ten core samples collected 5 m from each other using a 5-cm diameter auger to obtain a representative sample for each site. Each composite sample was treated as a replicate (block) in the lab incubation experiment. The soil samples were kept fresh and gently sieved through a 2-mm sieve after litter and plant roots were removed. The samples were stored in a refrigerator under − 20 °C for no more than 2 weeks until further analysis. The soil pH ranged from 4.3 to 6.5, total C from 20.6 to 74.7 g kg−1, total N from 2.0 to 7.0 g kg−1, exchangeable NH4+ from 0.36 to 6.27 mg kg−1, and NO3− from 1.4 to 35.4 mg kg−1 (see below for the methods of analysis of those soil properties).
Biochars were produced in a limited oxygen environment using a slow-pyrolysis process (Kwak et al. 2019). Before pyrolysis, the canola straw was chopped to pieces less than 10 mm long and oven-dried at 60 °C for 24 h. The biochars were then produced by heating the canola straw at different temperatures, with a heating rate of 10 °C min−1. For producing low- and high-pyrolysis temperature biochars, the final temperature was set at 300 (BC300) and 700 °C (BC700), respectively. The chemical and physical properties of the biochars can be found in Supplementary materials (Table S1).
Incubation procedure
In the laboratory incubation experiment, we used a completely randomized block design with biochar applied at three levels: no biochar addition (CK), the addition of biochar produced at 300 °C (BC300), and biochar produced at 700 °C (BC700) using canola straw as the feedstock, and nitrapyrin applied at two levels: with and without nitrapyrin addition, as the treatments. Biochar was applied at 2% (w/w; oven-dry weight basis, equivalent to 4.5 Mg ha−1) (Pokharel et al. 2018), and nitrapyrin was applied at 80 mg kg−1 soil (w/w, equivalent to 180 kg ha−1) (Islam et al. 2007). Two parallel incubation experiments were conducted. The first one was conducted in 500-mL mason jars using 100-g (oven-dry equivalent) fresh soil for determining N2O emission rates. The second set was conducted in 1.5-L mason jars using 300-g (oven-dry equivalent) fresh soil for measuring the changes in soil chemical and microbial properties over time in the incubation experiment. The biochar and/or nitrapyrin was thoroughly mixed with the soil before placing into the mason jars. The soils in the mason jars were adjusted to 40% water holding capacity (WHC) using deionized water and were pre-incubated for 3 days at 25 °C in the dark to stabilize microbial activities. After the 3-day pre-incubation, the water content was adjusted to 60% WHC, which was maintained for the 35-day incubation period by adding water every 2 days based on weight loss.
Gas sampling and measurement
N2O flux measurements were conducted on days 0 (after pre-incubation), 1, 3, 5, 7, 10, 13, 18, 23, 28, and 35. On each of those sampling days, the 500-mL mason jars were sealed tightly with lids that come with silicone pads for N2O flux measurement. Once the mason jars were sealed, they were pumped with 10-mL syringes three times to make the air in the headspace uniform, and a 5-mL gas sample was taken using a 10-mL syringe. The gas sampling was repeated three times every 8 h. On non-sampling days, the 500-mL mason jars were covered with aluminum foil punched with a few small holes to minimize water loss from the jars but allow air in the headspace to be exchanged with the atmosphere. At each gas sampling, the 5-mL gas sample was injected into a pre-evacuated 3-mL glass container to create a positive pressure for the N2O measurement. A gas chromatograph (Varian CP-3800, Mississauga, Ontario, Canada) that had an electron capture detector (detection limit: 5–20 μg L−1) was used to analyze the N2O concentrations in the collected gas samples. Daily N2O fluxes were then calculated. The N2O emissions on days not measured were calculated by linear interpolation. The cumulative N2O emissions were summed from daily N2O emissions over the incubation period (Yu et al. 2019).
Soil analysis
The soil was destructively sampled using a spatula on days 1, 7, 14, 21, 28, and 35 from the 1.5-L mason jars. For measuring the dissolved organic C (DOC) and N (DON) concentrations, 10 g of moist soil samples was extracted using 50 mL K2SO4 solution (0.5 mol L−1) by shaking at 250 rpm for 30 min and then filtered through Whatman No. 42 filter papers. The C and N concentrations in the extracts were determined using a TOC-TNM1 analyzer (Shimadzu Corporation, Kyoto, Japan). Soil exchangeable NH4+–N and NO3−–N in the extracts described above were analyzed colorimetrically. The NH4+–N in the extracts was determined using the indophenol blue method (Keeney and Nelson 1982). Briefly, a 0.5-mL aliquot of the filtered extract was incubated in a 40 °C water bath for 30 min, then reacted with 0.2 mL phenol-nitroprusside and 1.7 mL of diluted hypochlorite reagent to form indophenol-blue in a test tube. The absorbance was read at 636 nm on a spectrophotometer (Genesys 10-S, Thermo Scientific Inc., Rochester, NY); a 0.5 M K2SO4 was used as the blank (Keeney and Nelson 1982). The NO3−–N concentration in the extract was determined using the vanadium oxidation method (Miranda et al. 2001). First, a 20-μL aliquot of the filtered extract was transferred into a 2.0-mL microcentrifuge tube, then 1.0 mL vanadium regent was added into the microcentrifuge tube. The microcentrifuge tubes were placed in the dark at room temperature (25 °C) for 24 h, after which the absorbance was read at 540 nm on the spectrophotometer described earlier against the absorbance of a blank (0.5 mol L−1 K2SO4) (Miranda et al. 2001). The net nitrification rates (NNR) were calculated from the changes in the NO3−–N pool size between two incubation intervals (Han et al. 2012).
Soil pH was measured using a Thermo Scientific pH meter (710A, Beverly, MA) in a 1:5 soil:water (w:v) suspension. We used the chloroform fumigation-extraction method to determine soil microbial biomass C (MBC) and N (MBN) (Brookes et al. 1985). Briefly, both fumigated (fumigated in the dark, 24 h, at room temperature) and non-fumigated fresh samples were extracted using 1:5 soil: 0.5 mol L−1K2SO4 solution (w:v). Carbon and N concentrations in the extracts were measured using the TOC-TNM1 analyzer described above to calculate MBC and MBN using an extraction coefficient of 0.45 (Jenkinson et al. 2004).
Data analysis
All data analyses were carried out using R (R Core Team 2018). Assumptions of normality of distribution were evaluated by the Shapiro-Wilk’s test, and homogeneity of variance was tested using the Levene’s test, before further data analysis. The effects of fixed factors (nitrapyrin and biochar) on cumulative N2O emissions were tested using the linear mixed-effects model (LMM), with sampling site or replication as random factors. Natural logarithmic transformations were applied to the N2O data before data analysis (to make the distribution of the residuals normal), while the untransformed N2O data are presented in this paper. Other data were not transformed as they met the normality of distribution and heterogeneity of variance assumptions. For the repeated measurements of soil pH, NO3−, exchangeable NH4+, DOC, DON, MBC, and MBN, the LMM was used with nitrapyrin and biochar as the fixed effects, and sampling site (as a random effect) was nested in sampling day. The calculated net nitrification rate was analyzed using the LMM with nitrapyrin and biochar as the fixed effects and sampling site as the random effect. When the analysis of variance (ANOVA) showed significant effects, the least square means were compared with the ‘lsmeans’ function in the emmeans package at p ≤ 0.05. Relationships between soil properties and cumulative N2O emissions were explored using Pearson correlation analysis. Soil chemical properties significantly correlated with cumulative N2O emissions were analyzed with linear regression to quantify the response of cumulative N2O emissions to each chemical variable. All statistical significance was set at α = 0.05 unless otherwise stated.
Results
Effects of biochar and nitrapyrin on N2O emissions
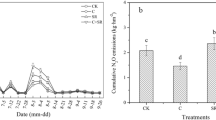
The highest daily N2O emission rates were observed on either day 0 (BC300 addition, regardless of the nitrapyrin treatment) or day 1 (CK and BC700, regardless of the nitrapyrin treatment); the rate then sharply decreased until day 10, and then remained stable until day 35 (Fig. 1(a)). At the later stage of the incubation, soil N2O emission rates were low and not different between treatments regardless of treatment (nitrapyrin and/or biochar addition; Fig. 1(a)). No interaction between the biochar and nitrapyrin treatments was observed on cumulative N2O emissions (Fig. 1(b) and Table S2, F = 0.23, p = 0.80), which was affected by biochar (F = 37.46, p < 0.001) and nitrapyrin (F = 10.91, p = 0.004) application independently. The BC300 treatment increased cumulative N2O emissions by 5.4 and 8.4 times compared to the CK and BC700 treatments, respectively (Fig. 1(c), in the original scale). Nitrapyrin addition reduced cumulative N2O emissions by 42% compared to no nitrapyrin addition (Fig. 1(d)).
(a) Daily N2O emissions (mean ± SE; same below), (b) cumulative N2O emissions, (c) the main effect of biochar on cumulative N2O emissions, (d) the main effect of nitrapyrin on cumulative N2O emissions from a cropland soil. Different lowercase letters in (c) and (d) indicate significant differences between biochar/nitrapyrin treatments (P = 0.05; Tukey’s test). CK, no biochar addition; BC300, the addition of biochar produced under low-pyrolysis temperature (300 °C); BC700, the addition of biochar produced under high-pyrolysis temperature (700 °C)
Effects of biochar and nitrapyrin on soil chemical and microbial properties
Repeated measures ANOVA showed that incubation time, biochar, nitrapyrin, and/or their interaction influenced soil pH, exchangeable NH4+, DOC, DON, MBC, MBN, and MBC:MBN ratio (Table S3). More specifically, on day 1, soil pH was higher in biochar addition (BC300 and BC700) than in the CK. The highest soil DOC was found in BC700, which was higher than that in CK and BC300. The highest soil MBC and MBC:MBN ratio were found in BC300, and were greater in BC300 than in CK and BC700. Biochar and nitrapyrin interacted to affect soil exchangeable NH4+, DON, and MBN. Soil NO3− concentration was not different across all treatments on day 1 (Table 1).
Soil pH and NO3− were affected by biochar and nitrapyrin when measured on day 35 (Table 2). Among the treatments, soil pH was the highest, while NO3− concentration was the lowest in the BC700 treatment. Similarly, soil pH was higher, and NO3− was lower with nitrapyrin than without nitrapyrin addition. Nitrapyrin application increased soil exchangeable NH4+ but decreased MBC:MBN ratio. The biochar affected DOC, MBC, and MBN. The highest soil DOC concentration was found in BC300, which was higher than that in CK and BC700. Both BC300 and BC700 increased soil MBC, while BC700 also increased soil MBN. Biochar and nitrapyrin interacted to affect soil DON: without nitrapyrin addition, soil DON was lowest in BC700 as compared with CK and BC300, while with nitrapyrin addition, soil DON was not affected by biochar addition (Table 2). In addition, without nitrapyrin addition, NO3− concentration increased over time (Fig. 2(a)), while with nitrapyrin addition, exchangeable NH4+ concentration increased over time (Fig. 2(b)).
Effects of biochar and nitrapyrin on soil net nitrification rates
Soil net nitrification rate (NNR) was affected by biochar (F = 20.27, p < 0.01) and nitrapyrin additions (F = 63.86, p < 0.01), but not by their interaction (F = 1.96, p = 0.17) (Fig. 3). The addition of BC700 significantly reduced soil NNR (− 0.23 mg N kg−1 day−1), as compared with CK and BC300 addition (Fig. 3).
Effects of biochar and nitrapyrin on net nitrification rates. The black dot represents an outlier. Different lowercase letters indicate significant differences between biochar addition treatments regardless of the nitrapyrin treatment (P = 0.05; Tukey’s test). CK, no biochar addition; BC300, the addition of biochar produced under low-pyrolysis temperature (300 °C); BC700, the addition of biochar produced under high-pyrolysis temperature (700 °C)
Relationships between soil properties and cumulative N2O emissions
Cumulative N2O emissions were positively related to soil NO3− concentration, DON, MBC:MBN ratio, and NNR; however, no relationships were found between N2O emissions and soil pH, MBC, and MBN (Fig. 4). In addition, soil NNR was negatively related with soil exchangeable NH4+ while positively with NO3− (Fig. 4). The MBC:MBN ratio, NO3−, DON, and NNR explained 26.5, 19.8, 11.4, and 11.3%, respectively, of the variation in cumulative N2O emissions (Table 3).
Pearson correlation of soil properties and cumulative N2O emissions in a 35-day laboratory incubation experiment. White circle indicates the correlations is not significant (P > 0.05). Color scheme with values indicates the Pearson correlation coefficient. DOC and N, dissolved organic C and N, respectively; MBC and N, microbial biomass C and N, respectively; NNR, net nitrification rates
Discussion
Our results show that the cumulative N2O emissions were not interactively affected by biochar and nitrapyrin applications, indicating that the co-application of biochars produced at different pyrolysis temperatures and nitrapyrin will not reduce the effectiveness of nitrapyrin in reducing nitrification rates and N2O emissions. Many researchers found that amending soil with biochar can reduce soil N2O emissions (Borchard et al. 2019; Duan et al. 2020; Wu et al. 2013). However, our results show that the pyrolysis temperature used for biochar production significantly influenced cumulative N2O emissions; the addition of BC300, but not BC700, increased cumulative N2O emissions compared to the CK.
The main difference in N2O emissions between BC300 and BC700 occurred in the first few days of the incubation. Similar to that reported in Ameloot et al. (2013), the increased N2O emission rate in the early few days in the BC300 treatment might have been caused by the higher volatile matter content in the BC300 biochar. Biochars produced at a low pyrolysis temperature usually have a higher volatile matter content than those produced at a high pyrolysis temperature (Lehmann et al. 2011; Mukherjee and Zimmerman 2013). Biochar addition can affect the function and abundance of N2O-producing nitrifiers and denitrifies by altering soil chemical properties (Braker and Conrad 2011). The BC300 treatment markedly increased soil MBC and the MBC:MBN ratio on day 1 compared with the CK and BC700 treatments (Table 1), indicating that the soluble C introduced by BC300 addition might have caused a short-term positive priming effect, resulting in the enhancement of microbial growth (Tan and Chang 2007) and the concurrent immobilization of soil mineral N (Deenik et al. 2010), and eventually increasing N2O fluxes in the early stage of the incubation experiment. This finding is consistent with Nelissen et al. (2012) where they found that NH4+ consumption by microbial populations was higher in the soil applied with biochar produced under a low pyrolysis temperature (350 °C) as compared to that applied with biochar produced under a high pyrolysis temperature (550 °C) or no biochar addition. On the other hand, the BC700 in this study had a large surface area (Table S1) and aromatic-C that would result in a high adsorption capacity, which might decrease the availability of substrates for microbial populations (Clough et al. 2013; Lehmann et al. 2011). Furthermore, the addition of BC700 might have increased soil aeration, which would reduce denitrification rates, as increased oxygen concentration in the soil inhibits denitrifying microbial activities (Van Zwieten et al. 2010).
Soil pH was less than 7 throughout the incubation in all treatments (Table 2). However, with a higher pH of the BC700 biochar than the BC300 biochar (Table S1), soil amended with BC700 had the highest pH (Table 2). An increase in soil pH might increase the N2O-reductase (denitrifies) activities, and eventually, increase N2 formation from N2O and decrease the N2O to N2 ratio (Singh et al. 2010).
The lower net nitrification rates in the BC700 treatment compared to the CK and BC300 treatments are consistent with the lack of BC700 treatment effect on N2O emissions (Figs. 2 and 3 and Table 2); the lower net nitrification rates means that BC700 suppressed nitrification rates and reduced N2O emissions, which counteracts the potential contribution of the volatile matter contained in the biochar that may enhance N2O emissions. Furthermore, under 60% WHC, nitrification would be the dominant process contributing to N2O production (Inubushi et al. 1996). The strong relationship between net nitrification rates and cumulative N2O emissions (Fig. 4 and Table 3) illustrates the contribution of nitrification to N2O emissions in the 35-day incubation. However, larger N2O emissions occurred after the application of BC300 in the earlier stage of the incubation, and there were no significant differences in net nitrification rates between the BC300 and CK treatments, suggesting that other processes such as denitrification were enhanced by the priming effect and contributed to N2O emissions. Biochar application can alter the activities of a range of enzymes related to C and N cycling (Pakheral et al. 2020) and genes that regulate N transformations in the soil (Ji et al. 2020), thereby influencing N2O emissions. The closure of the mason jars for measuring N2O emissions and the high greenhouse gas (such as CO2) concentrations in the BC300 applied soils during the earlier incubation stage might have created a temporary anaerobic condition that enhanced the activities of denitrifiers (Sherlock and Goh 1983).
Another strong predictor for N2O emission rates could be the H:C molar ratio of biochar. According to Cayuela et al. (2015), biochars with a H:C ratio < 0.3 have the ability to suppress N2O emissions, and those with a H:C ratio > 0.5 are not effective in suppressing N2O emissions. In our study, the H:C values of BC700 and BC300 were 0.26 and 0.95, respectively (Table S1), and the application of BC300 (biochar with a high H:C ratio) increased N2O emissions. The lower H:C ratios imply higher redox activities and sorption abilities, such biochars can directly mediate the process of denitrification to produce more N2 rather than N2O (Klüpfel et al. 2014; Qiu et al. 2014; Zumft 1997).
Unlike many other studies that have shown that biochars are potentially beneficial in mitigating N2O emissions (Cayuela et al. 2014), our research shows that canola straw biochar either increased (BC300) or had no effect (BC700) on cumulative N2O emissions compared with no biochar addition. This result may be partly related to the canola straw being an N-rich feedstock that the produced biochar contained relatively high N concentration, especially when pyrolyzed at a low temperature, as compared to other feedstocks such as woodchips (Kwak et al. 2019). The N introduced by biochar application may cause a positive priming effect on soil N and increase the availability of substrates for microbes in a short period of time (Fiorentino et al. 2019). Therefore, we need to be cautious when selecting a feedstock and the pyrolysis condition for producing biochar to use as a soil amendment in order not to increase N2O emissions.
Conclusions
Cumulative N2O emissions from cropland soils were affected by biochar and nitrapyrin applications, but not their interaction. The cumulative N2O emissions were increased by the BC300 treatment but were not affected by the BC700 treatment, as compared with the CK treatment. Significantly higher NH4+ consumption rates, MBC, and MBC:MBN ratio in soils with BC300 addition in the early stage of the incubation contributed to the higher cumulative N2O emissions. The greater surface area, lower volatile matter content, and lower H:C value of the BC700 biochar suppressed net nitrification and NH4+ consumption rates, counteracting the stimulation on nitrification rates by the added dissolvable C, and eventually did not affect soil N2O emissions relative to BC300. Nitrapyrin addition was effective in reducing net nitrification rates and cumulative N2O emissions. Biochar addition did not affect the effectiveness of nitrapyrin in reducing N2O emissions in the studied soil. Biochars need to be appropriately selected (such as BC700) in order not to increase N2O emissions when applied to the soil. Since the effectiveness of nitrapyrin in reducing N2O emissions was not affected by the co-application of biochar, we suggest that the co-application of biochar and nitrapyrin will be beneficial to both increase soil C sequestration through the addition of the stable C contained in biochar, and reduce N2O emissions. Future research needs to investigate the effectiveness of biochars produced from different crop residues (feedstock type) and with different pyrolysis conditions, and their interactions with nitrification inhibitors, on reducing N2O emissions under different soil conditions in long-term field studies.
References
Ameloot N, De Neve S, Jegajeevagan K, Yildiz G, Buchan D, Funkuin YN, Prins W, Bouckaert L, Sleutel S (2013) Short-term CO2 and N2O emissions and microbial properties of biochar amended sandy loam soils. Soil Biol Biochem 57:401–410. https://doi.org/10.1016/j.soilbio.2012.10.025
Baah-Acheamfour M, Carlyle CN, Lim SS, Bork EW, Chang SX (2016) Forest and grassland cover types reduce net greenhouse gas emissions from agricultural soils. Sci Total Environ 571:1115–1127. https://doi.org/10.1016/j.scitotenv.2016.07.106
Borchard N, Schirrmann M, Cayuela ML, Kammann C, Wrage-Mönnig N, Estavillo JM, Fuertes-Mendizábal T, Sigua G, Spokas K, Ippolito JA, Novak J (2019) Biochar soil and land-use interactions that reduce nitrate leaching and N2O emissions: a meta-analysis. Sci Total Environ 651:2354–2364. https://doi.org/10.1016/j.scitotenv.2018.10.060
Braker G, Conrad R (2011) Chapter 2 - diversity structure and size of N2O-producing microbial communities in soils—what matters for their functioning? In: Laskin AI, Sariaslani S, Gadd GM (eds) Advances in Applied Microbiology. Academic Press, London, pp 33–70. https://doi.org/10.1016/B978-0-12-387046-9.00002-5
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842. https://doi.org/10.1016/0038-0717(85)90144-0
Cayuela ML, van Zwieten L, Singh BP, Jeffery S, Roig A, Sánchez-Monedero MA (2014) Biochar’s role in mitigating soil nitrous oxide emissions: a review and meta-analysis. Agric Ecosyst Environ 191:5–16. https://doi.org/10.1016/j.agee.2013.10.009
Cayuela ML, Jeffery S, van Zwieten L (2015) The molar H:Corg ratio of biochar is a key factor in mitigating N2O emissions from soil. Agric Ecosyst Environ 202:135–138. https://doi.org/10.1016/j.agee.2014.12.015
Chen H, Yin C, Fan X, Ye M, Peng H, Li T, Zhao Y, Wakelin SA, Chu G, Liang Y (2019) Reduction of N2O emission by biochar and/or 34-dimethylpyrazole phosphate (DMPP) is closely linked to soil ammonia oxidizing bacteria and nosZI-N2O reducer populations. Sci Total Environ 694:133658. https://doi.org/10.1016/j.scitotenv.2019.133658
Clough TJ, Condron LM, Kammann C, Müller C (2013) A review of biochar and soil nitrogen dynamics. Agronomy 3:275–293. https://doi.org/10.3390/agronomy3020275
Deenik JL, McClellan T, Uehara G, Antal MJ, Campbell S (2010) Charcoal volatile matter content influences plant growth and soil nitrogen transformations. Soil Sci Soc Am J 74:1259–1270. https://doi.org/10.2136/sssaj2009.0115
Dittmar H, Drach M, Vosskamp R, Trenkel M.E, Gutser R, Steffens G (2009) Fertilizers 2. Types in: Ullmann’s Encyclopedia of Industrial Chemistry. American Cancer Society. doi:https://doi.org/10.1002/14356007.n10_n01
Duan M, Wu FP, Jia ZK, Wang SG, Cai YJ, Chang SX (2020) Wheat straw and its biochar differently affect soil properties and field-based greenhouse gas emission in a Chernozemic soil. Biol Fertil Soils 56:1023–1036. https://doi.org/10.1007/s00374-020-01479-4
Fiorentino N, Sánchez-Monedero MA, Lehmann J, Enders A, Fagnano M, Cayuela ML (2019) Interactive priming of soil N transformations from combining biochar and urea inputs: a 15N isotope tracer study. Soil Biol Biochem 131:166–175. https://doi.org/10.1016/j.soilbio.2019.01.005
Han WY, Xu JM, Yi XY, Lin YD (2012) Net and gross nitrification in tea soils of varying productivity and their adjacent forest and vegetable soils. Soil Sci Plant Nutr 58:173–182. https://doi.org/10.1080/00380768.2012.664783
Inubushi K, Naganuma H, Kitahara S (1996) Contribution of denitrification and autotrophic and heterotrophic nitrification to nitrous oxide production in andosols. Biol Fertil Soils 23:292–298. https://doi.org/10.1007/BF00335957
Islam A, Chen D, White RE (2007) Developing a technique to quantify heterotrophic and autotrophic nitrification in acidic pasture soils. Commun Soil Sci Plan 38:2309–2321. https://doi.org/10.1080/00103620701588437
Jenkinson DS, Brookes PC, Powlson DS (2004) Measuring soil microbial biomass. Soil Biol Biochem 36:5–7. https://doi.org/10.1016/j.soilbio.2003.10.002
Ji C, Li SQ, Geng YJ, Miao YC, Ding Y, Liu SW, Zou JW (2020) Differential responses of soil N2O to biochar depend on the predominant microbial pathway. Appl Soil Ecol 145:103348. https://doi.org/10.1016/j.apsoil.2019.08.010
Jia G, Shevliakova E, Artaxo P, De Noblet-Ducoudré N, Houghton R, House J, Kitajima K, Lennard C, Popp A, Sirin A, Sukumar R, Verchot L (2019) Land–climate interactions. In: Shukla PR, Skea J, Calvo Buendia E, Masson-Delmotte V, Pörtner H-O, Roberts DC, Zhai P, Slade R, Connors S, van Diemen R, Ferrat M, Haughey E, Luz S, Neogi S, Pathak M, Petzold J, Portugal Pereira J, Vyas P, Huntley E, Kissick K, Belkacemi M, Malley J (eds) Climate Change and Land: an IPCC special report on climate change desertification land degradation sustainable land management food security and greenhouse gas fluxes in terrestrial ecosystems. IPCC, Geneva, pp 131–247 https://www.ipcc.ch/srccl/cite-report/
Kammann C, Ippolito J, Hagemann N, Borchard N, Cayuela ML, Estavillo JM, Fuertes-Mendizabal T, Jeffery S, Kern J, Novak J, Rasse D, Saarnio S, Schmidt HP, Spokas K, Wrage-Mönnig N (2017) Biochar as a tool to reduce the agricultural greenhouse-gas burden – knowns unknowns and future research needs. J Environ Eng Landsc 25:114–139. https://doi.org/10.3846/16486897.2017.1319375
Keeney DR, Nelson DW (1982) Nitrogen—inorganic forms. In: Page AL (ed) Methods of Soil Analysis. Agronomy Monograph 9 Part 2, 2nd edn. American Society of Agronomy, Madison, pp 643–698. https://doi.org/10.2134/agronmonogr9.2.2ed.c33.
Klüpfel L, Keiluweit M, Kleber M, Sander M (2014) Redox properties of plant biomass-derived black carbon (biochar). Environ Sci Technol 48:5601–5611. https://doi.org/10.1021/es500906d
Kwak JH, Islam MS, Wang S, Messele SA, Naeth MA, El-Din MG, Chang SX (2019) Biochar properties and lead (II) adsorption capacity depend on feedstock type pyrolysis temperature and steam activation. Chemosphere 231:393–404. https://doi.org/10.1016/j.chemosphere.2019.05.128
Lehmann J, Joseph S (2015) Biochar for environmental management: science technology and implementation. Routledge, London
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota – a review. Soil Biol Biochem 43:1812–1836. https://doi.org/10.1016/j.soilbio.2011.04.022
Levesque V, Rochette P, Hogue R, Jeanne T, Ziadi N, Chantigny MH, Dorais M, Antoun H (2020) Greenhouse gas emissions and soil bacterial community as affected by biochar amendments after periodic mineral fertilizer applications. Biol Fertil Soils 56:907–925
Li B, Fan CH, Xiong ZQ, Li QL, Zhang M (2015) The combined effects of nitrification inhibitor and biochar incorporation on yield-scaled N2O emissions from an intensively managed vegetable field in southeastern China. Biogeosciences 12:2003–2017. https://doi.org/10.5194/bg-12-2003-2015
Li S, Chen DW, Wang C, Chen D, Wang Q (2020) Reduced nitrification by biochar and/or nitrification inhibitor is closely linked with the abundance of comammox Nitrospira in a highly acidic sugarcane soil. Biol Fertil Soils 56:1219–1228
Liu Q, Zhang Y, Liu B, Amonette JE, Lin Z, Liu G, Ambus P, Xie Z (2018) How does biochar influence soil N cycle? A meta-analysis. Plant Soil 426:211–225. https://doi.org/10.1007/s11104-018-3619-4
Miranda KM, Espey MG, Wink DA (2001) A rapid simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 5:62–71. https://doi.org/10.1006/niox.2000.0319
Mukherjee A, Zimmerman AR (2013) Organic carbon and nutrient release from a range of laboratory-produced biochars and biochar–soil mixtures. Geoderma 193–194:122–130. https://doi.org/10.1016/j.geoderma.2012.10.002
Nelissen V, Rütting T, Huygens D, Staelens J, Ruysschaert G, Boeckx P (2012) Maize biochars accelerate short-term soil nitrogen dynamics in a loamy sand soil. Soil Biol Biochem 55:20–27. https://doi.org/10.1016/j.soilbio.2012.05.019
Niu Y, Luo J, Liu D, Müller C, Zaman M, Lindsey S, Ding W (2018) Effect of biochar and nitrapyrin on nitrous oxide and nitric oxide emissions from a sandy loam soil cropped to maize. Biol Fertil Soils 54:645–658. https://doi.org/10.1007/s00374-018-1289-2
Omonode RA, Halvorson AD, Gagnon B, Vyn TJ (2017) Achieving lower nitrogen balance and higher nitrogen recovery efficiency reduces nitrous oxide emissions in north America’s maize cropping systems. Front Plant Sci 8:1–16. https://doi.org/10.3389/fpls.2017.01080
Pakheral P, Ma ZL, Chang SX (2020) Biochar increases soil microbial biomass with changes in extraand intracellular enzyme activities: a global meta-analysis. Biochar 2:65–79
Pokharel P, Kwak JH, Ok YS, Chang SX (2018) Pine sawdust biochar reduces GHG emission by decreasing microbial and enzyme activities in forest and grassland soils in a laboratory experiment. Sci Total Environ 625:1247–1256. https://doi.org/10.1016/j.scitotenv.2017.12.343
Qiu M, Sun K, Jin J, Gao B, Yan Y, Han L, Wu F, Xing B (2014) Properties of the plant- and manure-derived biochars and their sorption of dibutyl phthalate and phenanthrene. Sci Rep 4:5295. https://doi.org/10.1038/srep05295
R Core Team (2018) A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. Available online at https://www.R-project.org/
Ravishankara AR, Daniel JS, Portmann RW (2009) Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science 326:123–125. https://doi.org/10.1126/science.1176985
Saarnio S, Heimonen K, Kettunen R (2013) Biochar addition indirectly affects N2O emissions via soil moisture and plant N uptake. Soil Biol Biochem 58:99–106. https://doi.org/10.1016/j.soilbio.2012.10.035
Sánchez-García M, Roig A, Sánchez-Monedero MA, Cayuela ML (2014) Biochar increases soil N2O emissions produced by nitrification-mediated pathways. Front Environ Sci 2:1–10. https://doi.org/10.3389/fenvs.2014.00025
Sherlock RR, Goh KM (1983) Initial emission of nitrous oxide from sheep urine applied to pasture soil. Soil Biol Biochem 15:615–617. https://doi.org/10.1016/0038-0717(83)90061-5
Singh BP, Hatton BJ, Singh B, Cowie AL, Kathuria A (2010) Influence of biochars on nitrous oxide emission and nitrogen leaching from two contrasting soils. J Environ Qual 39:1224. https://doi.org/10.2134/jeq2009.0138
Smith KA (2017) Changing views of nitrous oxide emissions from agricultural soil: key controlling processes and assessment at different spatial scales. Eur J Soil Sci 68:137–155. https://doi.org/10.1111/ejss.12409
Soil Classification Working Group (1998) The Canadian System of Soil Classification Publication/Canada. In: Agriculture and Agri-Food Canada. NRC Research Press, Ottawa
Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschuang J, Nauels A, Xia Y, Bex V, Midgley PM (2013) Climate change 2013: the physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, United Kingdom, p 1535
Tan X, Chang SX (2007) Soil compaction and forest litter amendment affect carbon and net nitrogen mineralization in a boreal forest soil. Soil Tillage Res 93:77–86. https://doi.org/10.1016/j.still.2006.03.017
Tian HQ, Xu RT, Canadell JG et al (2020) A comprehensive quantification of global nitrous oxide sources and sinks. Nature 586:248–256
Van Zwieten L, Kimber S, Morris S, Downie A, Berger E, Rust J, Scheer C (2010) Influence of biochars on flux of N2O and CO2 from Ferrosol. Aust J Soil Res 48:555–568
Wu FP, Jia ZK, Wang SG, Chang SX, Startsev A (2013) Contrasting effects of wheat straw and its biochar on greenhouse gas emissions and enzyme activities in a Chernozemic soil. Biol Fertil Soils 49:555–565. https://doi.org/10.1007/s00374-012-0745-7
Yu Y, Zhao C, Zheng N, Jia H, Yao H (2019) Interactive effects of soil texture and salinity on nitrous oxide emissions following crop residue amendment. Geoderma 337:1146–1154. https://doi.org/10.1016/j.geoderma.2018.11.012
Zumft WG (1997) Cell biology and molecular basis of denitrification. Microbiol Mol Biol Rev 61:533–616
Acknowledgments
We thank Cole Gross, Christopher Nzediegwu, Dauren Kaliaskar, Na Chen, Prem Pokharel, Qian Wu, Yanchen Zhang, and Zilong Ma for their assistance in the laboratory. We also thank the Editor in Chief, the Regional Editor, and two anonymous reviewers for their constructive comments that improved earlier versions of this manuscript.
Funding
We thank the China Scholarship Council (CSC) and the Natural Science and Engineering Research Council of Canada (NSERC) for funding. The first author was also supported by a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 23 kb)
Rights and permissions
About this article
Cite this article
Li, J., Kwak, JH., Chen, J. et al. Canola straw biochars produced under different pyrolysis temperatures and nitrapyrin independently affected cropland soil nitrous oxide emissions. Biol Fertil Soils 57, 319–328 (2021). https://doi.org/10.1007/s00374-020-01535-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-020-01535-z