Abstract
Biochar produced from plant biomass through pyrolysis has been shown to be much more resistant to biodegradation in the soil as compared with the raw biomass, such as cereal straw that is routinely shredded and discharged on to farm fields in large amounts. Biochar application to soil has also been reported to decrease greenhouse gas (GHG) emissions, although the mechanisms are not fully understood. In this study, the emissions of three main GHGs (CO2, CH4, and N2O) and enzyme activities (urease, β-glycosidase, and dehydrogenase) were measured during a 100-day laboratory incubation of a Chernozemic soil amended with either straw or its biochar at rates of 0.67 and 1.68 % (based on the amount of C added) for the low and high rates, respectively. The biochar application dramatically reduced N2O emissions, but CO2 or CH4 emissions were not different, as compared with the un-amended soil. At the same C equivalent application rate, CO2 and N2O emission rates were greater while CH4 emission rates were lower in straw than in biochar application treatments. The activities of both the dehydrogenase and β-glycosidase significantly declined while that of urease significantly increased with the biochar as compared with the straw treatment. We conclude that pyrolysis of cereal straw prior to land application would significantly reduce CO2 and N2O emissions, in association with changed enzyme activities, while increasing the soil C pool through the addition of stable C in the form of biochar.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Increases in atmospheric concentrations of greenhouse gases (GHGs) such as carbon dioxide (CO2), methane (CH4), and nitrous oxide (N2O) are of major concerns as factors affecting global climate warming (Gärdenäs et al. 2011; Snyder et al. 2009). It has been reported that the global atmospheric concentrations of CO2 and CH4 have exceeded the natural range that has likely occurred over the last 650,000 years (IPCC 2007). Much of the anthropogenic C in the atmosphere is of a soil origin because the soil has lost on average over half of its C content as a result of massive expansion of arable lands and excessive use of mineral fertilizers that destabilizes the otherwise stable Ca-forms of soil organic C contained in aggregates, leading to the formation of labile humic substances (Muñoz et al. 2010; Shevtsova et al. 2003). Soils produce CO2 mostly under aerobic conditions, CH4 under anaerobic conditions, and N2O under either aerobic (nitrification) or anaerobic (denitrification) conditions. More than a third of all N2O emissions are from anthropogenic activities and primarily due to agricultural activities (IPCC 2007). Over the past few decades, the temperatures of the atmosphere and oceans have increased due to GHG emissions. The global warming potential, which is related to the catalytic destruction of stratospheric ozone, of CH4 and N2O are 23 and 296 times greater than CO2 in a 100-year period (Muñoz et al. 2010). Therefore, reducing emissions of GHGs, particularly that of N2O, can make a substantial contribution to decreasing the effect of GHG emissions on the global climate.
The production of CO2, N2O, and CH4 are affected by biophysical conditions and the incorporation/decomposition of organic residues in the soil. It is widely accepted that the addition of crop residues to the soil helps to maintain soil organic matter levels, thereby affecting soil structure, soil fertility, and crop production. Crop residue additions to the soil also have significant effects on the sustainable development of agriculture as the incorporation of crop residue alters the soil environment, physical properties, nutrient supply, and the subsequent improvement of soil productivity, which offers a way to resolving the conflict between maximizing short-term production at minimum cost versus providing sustainable health and long-term productivity of the soil. (Gao et al. 2010; Han et al. 2003; Janzen 2006; Kumar and Goh 2000) and for the reduction of N2O emissions in fertilized and irrigated soils in southern Alberta (Hao et al. 2001). However, application of crop residues may also result in the temporary immobilization of soil nutrients during their decay, delay soil warming in the spring, and increase the risk of disease, weed, and pest transfer from the previous crop (Procházková et al. 2003). The conversion of crop residues into biochar prior to soil application could present a viable alternative to eliminate these problems.
Biochar is a stable (relative to its raw biomass C form) and C rich material produced by the pyrolysis of organic material in the absence of oxygen or in a low oxygen environment (Sohi et al. 2009). In the pyrolysis process, approximately 50 % of the C contained in the biomass is immediately released as bioenergy, leaving the remaining 50 % of the C in a stable form called biochar (Day et al. 2005; Katyal et al. 2003; Lehmann et al. 2002). Un-pyrolyzed organic material decomposing in the soil initially releases C quickly and then slows over time. However, the release of C continues until less than 10–20 % of the added C remains in agricultural soils after 5–10 years depending on C quality and the environment. Thus, in the long term, biochar application ultimately leads to greater amounts of C remaining in the soil than the direct application of un-charred organic matter (Lehmann et al. 2006; Woolf et al. 2010).
The application of biochar can increase plant growth by improving soil physical, chemical, and biological properties, including soil structure, nutrient availability, and water and nutrient retention (Glaser et al. 2002; Lehmann et al. 2003; Lehmann and Rondon 2005; Sombroek et al. 1993). Biochar application has also been reported to reduce CO2 and N2O production and reduce ambient CH4 oxidation (Major et al. 2010; Spokas et al. 2009), while reduced N2O emission and no effects on CO2 emission were observed by Wang et al. (2011), indicating that biochar application has the potential to reduce GHG emissions and increase C sequestration. In biochar-amended soils with or without N fertilization, total N2O emissions decreased by 40–51 and 21–28 %, respectively, while CH4 emissions increased by 34 % with biochar addition relative to treatments without biochar and by 41 % by N fertilizer addition as compared with treatments without N fertilizer addition (Zhang et al. 2010).
Soil GHGs are mostly produced from microbiological processes, i.e., biological oxidation of organic matter in the soil (soil respiration), CH4 oxidation, and nitrification and denitrification (Brzezińska et al. 2004; Tiedje 1998). Enzyme activities are critical in maintaining nutrient availability, and their activities are “sensors” of soil microbial function and soil physicochemical conditions (Asmar et al. 1994; Baum et al. 2003; Nannipieri et al. 2003; Sparling 1997; Wick et al. 1998). For example, soil urease activity reflected N2O emission because of the close relationship between urease activity and nitrification (Bai et al. 2003), while the highest dehydrogenase activities occurred in the period of rapid depletion of CH4 and O2 and simultaneous CO2 production due to the adaption of soil methanotrophic microorganisms in this period (Brzezińska et al. 2004). The emission of N2O was found to be significantly related to dehydrogenase activities as the N2O was produced during NO3 − transformation in which dehydrogenation of the organic matter was catalyzed by dehydrogenases (Włodarczyk et al. 2002). Fluxes of N2O during the dry season were positively correlated to microbial biomass N and β-glucosidase activities as heterotrophic nitrifying and denitrifying bacteria (which can both produce N2O) require an organic form of C as energy source and may depend on β-glucosidase activities for their C supply (Wick et al. 2005). However, there have been few studies on the effects of biochar application on GHG emissions and their relationship with soil enzyme activities (Bailey et al. 2011; Lammirato et al. 2011; Paz-Ferreiro et al. 2011).
Evaluating the effects of crop residue and its biochar on soil processes is particularly interesting due to the fact that discharge of shredded straw in large amounts on to the fields during crop harvest is a common agricultural practice. The amount of crop residue returned to the soil is not based on the requirement for soil enhancement for the next crop. Indeed, excessive loading of straw can delay seed germination and lead to the oversupply of fertilizers to compensate for the N that would be locked up during the critical early summer crop growing period (Graham et al. 1986; Procházková et al. 2003).
An important anthropogenic source of GHGs was agriculture which accounts for approximately 20 % of the annual increase in radioactive forcing on climate change (Cole et al. 1997). Moreover, many of the earlier investigations only focused on the biochar effects on mitigating GHG emissions, as well as on increasing crop production and changing soil physical and chemical properties, while very few studies made comparison of the effects of biochar and its straw on GHG emissions and microbiological properties such as changes in enzyme activities that are considered as soil quality indicators that may respond to alterations in soil management on a shorter period of time than those based on physical or chemical properties (Paz-Ferreiro et al. 2011; Roldán et al. 2005). The objective of this study was to compare the effects of applying wheat straw and its biochar on GHG emissions from a Chernozemic soil as well as on enzyme activities in a laboratory incubation experiment. More specifically, we tested the hypothesis that the conversion of straw residues to biochar will suppress GHG emissions.
Materials and methods
The soil, wheat straw, and biochar
Surface soil (0–6 cm) was obtained from Flagstaff County (52°46′ N, 111°41′ W) in southeast Alberta, for the laboratory incubation experiment reported here. The soil is an Orthic Black Chernozem based on the Canadian system of soil classification with the following properties: loam texture, pH (1:2 v/v soil/water) 5.6 (see the “Analysis of soil, straw, and biochar properties” section below for the methods of analysis), cation exchange capacity (CEC) 13.5 cmol kg−1, organic C 2.41 %, total N 0.24 %, total P 597 mg kg−1, total K 5390 mg kg−1, available NO3-N 21.9 mg kg−1, available PO4-P 38.5 mg kg−1, and available K 274 mg kg−1. The soil was sieved to pass a 2-mm sieve and homogenized for the incubation experiment.
The biochar was produced from wheat straw using a fast pyrolysis process (450 °C and at a heating rate of 15 °C min−1) by Alberta Innovates—Technology Futures (AITF; Vegreville, AB, Canada), which yields approximately 30 % biochar (by weight, same below), 35 % oil, and 35 % gases. The biochar had a pH of 9.93, fixed C (or stable C, C leftover after the majority of the volatile organic matter was lost after heating) of 81 %, aeration porosity of 51 %, bulk density of 54 kg m−3, volatile matter of 5.0 %, water holding capacity of 44 %, and ash content of 13.8 %. The biochar contained 72.6 % total C, 0.32 % N, 2.8 % H, 15.4 % O, and 0.02 % S on an oven-dry weight basis (Waste Materials Engineering Lab., AITF). The wheat straw had 46 % C, 1.0 % N, 6.25 % H, 0.24 % S, and 6.78 % ash on an oven-dry basis. The wheat straw used in this experiment was the same as that used for biochar production.
Experimental design and treatments
The straw and biochar were ground to <2 mm. Three levels of soil amendments with straw and biochar were established, based on an equivalent amount of elemental C added by weight (0, 0.67, and 1.68 %). Since pyrolysis increased the C concentration in the final product, the dry weight percentages of added wheat straw and biochar were different: 0, 0.99, and 2.44 % for biochar (treatments CK, B1, and B2) and 0, 1.48, and 3.61 % for straw (treatments CK, S1, and S2). The equivalent field rates of these amendments incorporated into the 0–10-cm layer would be about 0, 10, and 25 t ha−1 of biochar and 0, 15, and 37.5 t ha−1 of straw.
For each treatment, the soil (100 g per jar) and amendments were mixed and placed into 1,000 mL sterilized glass jars and then moistened with distilled water to 80 % water filled pore space (WFPS) as calculated using the following equation (Yanai et al. 2007):
where WFPS = water fill pore space, in percent; θ m = gravimetric water content, megagrams per megagram; P B = soil bulk density, in megagrams per cubic meter; ρ H2O = density of water, 1 Mg m−3; TP = total soil porosity = 1 – P B/P P, in percent; and P P is soil particle density, megagrams per cubic meter.
Five replications were established for a 100-day incubation experiment. All 25 jars were sealed with air-tight lids and kept at 25 °C in the dark. The water content was kept constant during the incubation period by adding distilled water twice a week.
Gas sampling and analysis
Gas samples were periodically collected from the incubators for analysis: daily for the first 7 days, then once every 4 days for the next 47 days, and weekly thereafter (Cheng et al. 2012; Wang et al. 2011). Before each air sample collection, the lids were opened for half an hour to ensure thorough gas exchange between the atmosphere and the inside of the jar. The lids were then sealed before sampling. Gas samples were taken at time 0 and 1 h incubation time using a 20-mL gas-tight syringe (BD Luer-Lok™ Tip) and injected into pre-evacuated 12-mL Exetainer® vials with septa. Gas samples were analyzed on a gas chromatograph (Varian GC-3800) fitted with a COMBI PAL autosampler. The CO2, CH4, and N2O concentrations were analyzed by thermal conductivity, flame ionization, and electron capture detectors, respectively. The change in the gas concentrations was used to calculate the greenhouse gas emission rates.
Measurement of soil enzyme activities
Soil samples were collected from each jar immediately at the end of each incubation period, stored at 4 °C and processed within 24 h. Enzyme activities were determined in triplicates and expressed on an oven-dry soil basis. The experimental setup only allowed us to determine enzyme activities at the end of the incubation to evaluate the impact of the treatments on enzyme activities.
For the determination of urease activities, 5 g of moist soil (≤2 mm) was incubated at 37 °C for 5 h after adding a 5-mL (2 mg mL−1) urea solution (controls were processed by adding 5 mL of distilled water instead of a urea solution). Urea remaining after the incubation was extracted with 2 mol L−1 KCl-PMA (potassium chloride–phenylmercuric acetate) solution (Tabatabai 1994). Soil suspension was filtered under suction after the incubation. And then 30 mL of a color reagent (2.5 % diacetylmonoxime solution and 0.25 % thiosemicarbazide solution in H3PO4 and H2SO4) was added to 2 mL filtrate in 50 mL volumetric flask. The intensity of the red color developed was measured at 527 nm. The remaining urea was calculated using a calibration curve established with standards containing 0, 0.5, 1, 2, and 4 μg mL−1 of urea. Urease activity ω (N) was expressed as the reduction of milligrams of urea-N per kilogram of dry soil per hour.
where: m 1 = urea content in soil samples, in micrograms; V 1 = total volume of extraction solution, in milliliter; V 2 = volume of extraction used to determine urea concentration, in milliliter; 0.466 = N content (as a fraction) in urea; m 2 = the amount of soil used for the incubation, in grams; and t = incubation time, in hours.
The method used to determine β-glucosidase activity was based on the colorimetric determination of p-nitrophenyl released by β-glucosidase when 1 g of soil was incubated for 1 h at 37 °C with a buffered PNG (p-nitrophenyl-β-d-glucoside) solution (pH 6.0) and toluene (Tabatabai 1994). The amount of p-nitrophenyl released from the substrate was determined by measuring the yellow filtrate colorimetrically after color development of the soil suspension with 1 mL 0.5 mol L−1 CaCl2 and 4 mL of tris (hydroxymethyl) aminomethane buffer (pH = 12). Three replicates and two controls were set up during the assay with the same procedure, except that the substrate (PNG) was added to controls after the incubation described above, immediately prior to the end of the reaction. The mean control value was subtracted from the corresponding sample values. The intensity of the color of the filtrate that developed was determined at 400 nm, and the amount of p-nitrophenyl released was calculated using a calibration curve established with standards containing 0, 1, 2, 3, 4, and 5 μg mL−1of p-nitrophenyl. The β-glucosidase activity ω (PN) was expressed as milligrams of p-nitrophenyl released per kilogram dry soil per hour.
where A = the amount of p-nitrophenyl in a soil sample, in milligrams; m = the amount of soil used, in kilograms; and b = 1 − soil moisture content, in percent.
The method used to determine dehydrogenase activity was based on extraction with methanol and colorimetric determination of the triphenyl formazan (TPF) produced from the reduction of 2,3,5-triphenyltetrazolium chloride (TTC) in soils (Tabatabai 1994). Six grams of soil was mixed with 1 mL 3 % TTC, 0.07 g CaCO3, and 2.5 mL distilled water and then incubated at 37 °C for 24 h. The TPF in the filtrate produced from TTC was extracted with four successive portions of methanol totaling 100 mL. The control was processed using the same procedure except that the substrate (TTC) was added to the control after the incubation. The intensity of the reddish color developed was measured at a wavelength of 485 nm. The amount of TPF produced was calculated using a calibration curve prepared from TPF standards containing 0, 5, 10, 15, and 20 μg mL−1 of TPF. Dehydrogenase activity ω (TPF) was expressed as milligrams of TPF produced per kilogram of dry soil per hour.
where m 1 = TPF produced by soil samples, in milligrams; m 2 = the amount of soil used for the assay, in grams; and t = incubation time, in hours. The color of the filtrate and solution described above was measured with a Spectronic 10 Genesys spectrophotometer (Thermo Electron Scientific Instruments Corp., Madison, WI, USA).
Analysis of soil, straw, and biochar properties
Soil texture was determined using the hydrometer method (Gee and Or 2002). The pH of the soil and biochar was measured with a pH meter in 0.01 mol L−1 CaCl2 (pHCaCl2) using 10 g of soil/biochar in 20 mL of solution. CEC was determined after extracting the sample with 1 mol L−1 NH4OAc at pH 7 following Carter and Gregorich (2006). The total C, N, H, O, and S concentrations of the soil, straw, or biochar samples were analyzed using a CHNS/O elemental analyzer. Soil total P and K concentrations were determined on an autoanalyzer (for P) or an atomic absorption spectrophotometer (K) after the samples were digested in a HNO3/HClO4 solution (Sparks et al. 1996). Soil available NO3 −N was measured on an autoanalyzer after extracting the soil sample with 2 mol L−1 KCl. Available soil PO4-P and K were determined following a modification of Kelowna extraction (Qian et al. 1994). The ash content was determined after burning off the organic matter in a sample in a muffle furnace at 500 °C. The volatile matter content was determined following American Society for Testing and Materials method E872, by placing 1 g sample in a lidded silica crucible in a 950 + 20 °C furnace for 7 min and determining the weight loss. The amount of fixed C in a sample of material was determined by subtracting water, ash, and volatile matter content from the total mass. The bulk density of the biochar was determined by placing and weighing the material in a container with a known volume. Aeration porosity and water holding capacity were measured following the methods in Ingram et al. (1990).
Statistical analysis
The rates of CO2, CH4, and N2O emissions were determined from the increase in gas concentrations in the headspace over the 1-h incubation period, and the values are expressed as arithmetic means (n = 5) and standard deviations (SD) of the replications. Variances were analyzed using the repeated measures analysis, the treatments consisted of a control (CK), two levels of biochar amendments (B1 and B2), and two levels of straw amendments (S1 and S2). One-way analysis of variance was applied to determine treatment efforts, and significance level was assumed at p = 0.05. Mean separations were conducted using the LSD multiple range test. All statistical analyses were performed using version 9.01 of SAS (SAS Institute, Inc., Cary, NC, USA).
Results
Soil trace gas emissions
Straw treatments had greater CO2 emission rates than the biochar treatments during the incubation, and the difference between the treatments gradually decreased over time in the first 30 days (Fig. 1a). The straw treatments had greater mean CO2 emission rates than the biochar treatments (which was not different from the CK) during the 100-day incubation period, with CO2 emission rates increased with increased rate of straw addition (Fig. 1b). Overall, CO2 emissions in the biochar treatments were reduced by 73 and 81 % for the low and high rates of biochar C addition, respectively, as compared with the corresponding straw treatments.
The effects of straw and its biochar application on a the dynamics of CO2 emission (mean ± SD) and b mean CO2 emission rates during a 100-day incubation experiment. The insert plots the data from the first 10 days of the incubation. Treatment codes are CK control, no biochar or straw application; B1 biochar application at a rate equivalent of 10 t ha−1; B2 biochar application at a rate equivalent of 25 t ha−1; S1 straw application at a rate equivalent of 15 t ha−1; and S2 straw application at a rate equivalent of 37.5 t ha−1
The rates of CH4 emissions were generally similar across the treatments over the incubation period (Fig. 2a). Rates of CH4 emissions fluctuated over the incubation period with minimal emission rates observed on day 5, and obvious absorption of CH4 was found in all treatments on day 68. Biochar addition increased mean CH4 emission rates in comparison with the CK (Fig. 2b). There were no significant differences in the mean CH4 emission rates among the B1, B2, and CK treatments. Rates of CH4 emissions in the straw treatments (S1 and S2) were lower than that in the biochar treatments. Compared to straw addition (S1 andS2), biochar application (B1 and B2) increased mean CH4 emission rates by 13 and 35 %, respectively.
The effects of straw and its biochar application on a the dynamics of CH4 emission (mean ± SD) and b mean CH4 emission rates during a 100-day incubation experiment. The insert plots the data from the first 10 days of the incubation. Treatment codes are CK control, no biochar or straw application; B1 biochar application at a rate equivalent of 10 t ha−1; B2 biochar application at a rate equivalent of 25 t ha−1; S1 straw application at a rate equivalent of 15 t ha−1; and S2 straw application at a rate equivalent of 37.5 t ha−1
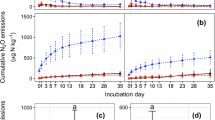
The highest N2O emission rates were observed between days 2 and 4, and then the rates decreased to their lowest level on day 13 in all treatments, thereafter N2O emission rates stabilized or increased with time (the CK in particular) in the incubation period (Fig. 3a). The N2O emission rates from treatment with the high rate of biochar addition (B2) were negligible during the entire incubation period. There was an increasing trend of N2O production after day 36 in all treatments, with N2O production from the CK greater than that from the other treatments. Both biochar and straw application suppressed the production of N2O based on the mean emission rates, with the suppressing effects greater in the treatments with greater biochar and straw application rates (Fig. 3b). However, at the equivalent C addition rate, biochar addition had a much greater effect on suppressing N2O emission rates than did the straw treatments, with the B1 and B2 treatments reducing the mean N2O emission rates by 55 and 96 % as compared with the respective straw treatment or by 66 and 99 % as compared with the CK.
The effects of straw and its biochar application on a the dynamics of N2O emission (mean ± SD) and b mean N2O emission rates during a 100-day incubation experiment. The insert plots the data from the first 10 days of the incubation. Treatment codes are CK control, no biochar or straw application; B1 biochar application at a rate equivalent of 10 t ha−1; B2 biochar application at a rate equivalent of 25 t ha−1; S1 straw application at a rate equivalent of 15 t ha−1; and S2 straw application at a rate equivalent of 37.5 t ha−1
Enzyme activities
Biochar applications had no effect on dehydrogenase activities as compared to the CK; however, straw additions significantly increased dehydrogenase activities relative to biochar additions and the CK (P < 0.01), and the greater the straw addition rate, the greater the dehydrogenase activity (Fig. 4a). Activities of β-glucosidase increased with the increasing rate of straw addition. Biochar (B1 and B2) treatments reduced β-glucosidase activities by 28 and 34 %, respectively, as compared with the straw treatments (S1 and S2) (Fig. 4b). Both biochar and straw treatments reduced urease activities as compared to the CK (Fig. 4c). Straw additions significantly decreased urease activities in comparison to biochar additions and CK. Urease activities decreased with the increasing rate of biochar and straw additions.
The effects of straw and its biochar application on a soil dehydrogenase (mean ± SD), b β-glucosidase, and c urease activities after an 100-day incubation. Treatment codes are CK control, no biochar or straw application; B1 biochar application at a rate equivalent of 10 t ha−1; B2 biochar application at a rate equivalent of 25 t ha−1; S1 straw application at a rate equivalent of 15 t ha−1; and S2 straw application at a rate equivalent of 37.5 t ha−1
Relationships among greenhouse gas emissions and enzyme activities
Mean CO2 production was found to be related to all three enzyme activities, among which positive correlations were observed with dehydrogenase (r s = 0.44, P < 0.05; data not shown, same below) and β-glucosidase activities (r s = 0.85, P < 0.01), while a negative relationship was found with urease activities (r s = −0.82, P < 0.01). There was a negative correlation between mean production of CH4 and β-glucosidase activities (r s = −0.45, P < 0.05). Mean production of N2O was positively correlated with urease activities (r s = 0.57, P < 0.05). A positive correlation was found between β-glucosidase and dehydrogenase activities (r s = 0.67, P < 0.01). No significant correlations were found among the rates of the three GHG emissions.
Discussion
Biochar addition did not affect soil CO2 emissions as compared to the CK (Fig. 1b), indicating that probably biochar had no effect on microbial activities and that little biochar decomposition occurred during the incubation period (Jones et al. 2011; Spokas et al. 2009). However, an increasing CO2 emission was observed in the straw treatments, and approximately 77 % of straw C was respired over the incubation period (data not shown), indicating increased microbial activities due to the relatively high amounts of easily decomposable organic matter in the straw (Singh et al. 2010). Those results are consistent with another study that used the same biochar and straw on a different soil and with a much shorter incubation period of 12 days (Cheng et al. 2012).
Biological oxidation of organic compounds is generally a dehydrogenation process (Tabatabai 1994), and thus, dehydrogenases are important enzymes during the biological oxidation of organic compounds. The products of hydrolysis involving the enzyme β-glucosidase are believed to be important energy sources for microorganisms in soils which make β-glucosidase an indicator of changes of soil organic matter (Bell et al. 2010; Garcia and Hernandez 1994). The fact that the activities of those two enzymes were highly correlated (P < 0.01) and that both enzyme activities were correlated with the production of CO2 (P < 0.05) in our study was in agreement with the role of these two enzymes in the soil (Gärdenäs et al. 2011). Our results also showed that straw additions significantly increased dehydrogenase and β-glucosidase activities as compared to biochar additions and the CK (Fig. 4a, b). These results indicate that when the form of C added was biochar (relative to straw), the C was more recalcitrant and did not stimulate soil microbial or enzyme (dehydrogenase and β-glucosidase) activities.
There have been reports of reduction of CH4 emissions from soils after biochar addition (Rondon et al. 2005, 2006; Spokas et al. 2009). However, CH4 production also occurs under anaerobic conditions (Fenchel et al. 1998). In our research, biochar addition did not affect the CH4 production rate as compared to the CK (Fig. 2b). The effects of straw application on CH4 emission have been studied mostly in rice paddy systems and showed that CH4 emissions increased due to straw application (Bossio et al. 1999; Ma et al. 2008). However, we found a significant reduction in CH4 production rate (Fig. 2) by straw addition, indicating that under aerobic conditions, which are similar to upland conditions, straw application increased the soil’s ability to take up CH4 (Tate et al. 2007).
Emissions of N2O from soils are primarily from microbially driven nitrification and denitrification processes. In the latter case, oxygen is limiting and anaerobic (mainly bacteria) reduction of nitrate occurs where nitrate becomes the electron acceptor. Results from our study (Fig. 3a) demonstrate that biochar additions were effective in reducing overall emissions of N2O as compared to straw additions and the CK. The effect on the reduction of N2O emission was better by the application of biochar than that of straw residual was similar to the finding of Van Zwieten et al. (2010b). This means that organic materials such as straw when converted into biochar will not only provide energy but will also reduce emissions of soil N2O, a potent greenhouse gas (Clough et al. 2010; Singh et al. 2010). The suppression of N2O emissions may be related to the alkali (liming effects resulting from the addition of ash that had a pH of 10) added to the soil that had the potential to increase the N2O-reducing activity of denitrifying communities which shifted the main microbial source of N2O from ammonia oxidation to denitrification (Baggs et al. 2010; Cavigelli and Robertson 2000, 2001; Clough et al. 2004; Van Zwieten et al. 2010a; Yanai et al. 2007).
The decomposition of straw and thus its ability to immobilize N may be the cause for the lower rates of N2O emissions from straw added soils (Cheng et al. 2012; Ryszkowski and Kędziora 2007). The process of straw decomposition is influenced by enzymes such as urease and the activity of urease in the soil seems to be mainly the result of its release from decaying microbial and plant cells which were primarily associated with soil organic matter and clay minerals (Geisseler et al. 2010; Kandeler et al. 1999; Reynolds et al. 1985). In our study, urease activities were reduced by both biochar and straw treatments as compared to the CK. Our results of reduced N2O emission and urease activity by the biochar and straw addition treatments are consistent with reports that soil urease activity is closely connected with N2O emissions (Bai et al. 2002, 2003). The abnormally low N2O emissions with straw addition were due to its high rate of addition without any additional inorganic N. The low and high rates of straw addition were equivalent to an addition of 15 and 37.5 Mg ha−1 of straw, compared to typical straw yields in Alberta of less than 5 Mg ha−1. The amount of C added at the high rate of straw (1,500 mg per jar) would immobilize about 100–200 mg of N, but initial soil NO3-N was only 2 mg N per jar and the added straw only contained about 38 mg N per jar. High N immobilization in straw-amended treatments thus explains the reduction of N2O emissions from straw addition. The implication is, however, the effectiveness of biochar addition for reducing N2O emissions may be greater if more available N were present in the soil.
Overall, straw applications to the soil increased CO2 production but reduced N2O production, while biochar amendments had no effect on CO2 emission and almost completely suppressed N2O emission when the high biochar application rate was used. The lack of biochar application effect on CH4 production was in contrast with the depressed CH4 emission after straw application. The global warming potential of CH4 and N2O are 23 and 296 times greater than that of CO2 in a 100-year period (Muñoz et al. 2010). Therefore, the benefit for reducing the greenhouse effect from the substantial reduction of N2O emissions after biochar application outweighs that of the reduction in CH4 emission after straw application. In addition, toxins produced when straw residue break down can inhibit plant seed germination and growth (Ma et al. 2008; Procházková et al. 2003; Thomsen and Christensen 1998). Straw incorporation can also increase disease transfer from previous crops (Janya et al. 2010). Pyrolysis of straw would resolve all these issues while contributing to C sequestration, soil amelioration, and reduction of GHG emissions. Further research is needed to gain more information about the environmental vs. economic benefits of biochar vs. straw application.
Although extrapolation of the findings from this short-term laboratory study to long-term field results should be conducted with caution, field applications of biochar could possibly be used to suppress GHG emissions from arable soils, depending on the moisture content and aeration status of the soil. Further research is needed to broaden our understanding of the effectiveness of biochar application on reducing GHG emissions from different soils in Alberta under field conditions.
References
Asmar F, Eiland F, Nielsen NE (1994) Effect of extracellular-enzyme activities on solubilization rate of soil organic nitrogen. Biol Fertil Soils 17:32–38
Baggs EM, Smales CL, Bateman EJ (2010) Changing pH shifts the microbial source as well as the magnitude of N2O emission from soil. Biol Fertil Soils 46:793–805
Bai H, Han J, Zhang Y (2002) Correlations between physical, chemical and biological properties and denitrifying enzymes activity and N2O flux in soil profiles. Agro- Environ Prot 21:193–196
Bai H, Zhang Y, Han J, Li C (2003) Nitrous oxide emission and urease activity in wheat. Environ Contam Toxicol 71:1282–1288
Bailey VL, Fansler SJ, Smith JL, Bolton H (2011) Reconciling apparent variability in effects of biochar amendment on soil enzyme activities by assay optimization. Soil Biol Biochem 43:296–301
Baum C, Leinweber P, Schlichting A (2003) Effects of chemical conditions in re-wetted peats on temporal variation in microbial biomass and acid phosphatase activity within the growing season. Appl Soil Ecol 22:167–174
Bell TH, Klironomos JN, Henry HAL (2010) Seasonal responses of extracellular enzyme activity and microbial biomass to warming and nitrogen addition. Soil Biol Biochem 74:820–828
Bossio DA, Horwath WR, Mutters RG, van Kessel C (1999) Methane pool and flux dynamics in a rice field following straw incorporation. Soil Biol Biochem 31:1313–1322
Brzezińska M, Wodarczyk T, Gliński J (2004) Effect of methane on soil dehydrogenase activity. Int Agrophys 18:213–216
Carter MR, Gregorich EG (2006) Soil sampling and methods of analysis, 2nd edn. Canadian Society of Soil Science, CRC Press, Taylor & Francis Group, Boca Raton
Cavigelli MA, Robertson GP (2000) The functional significance of denitrifier community composition in a terrestrial ecosystem. Ecology 81:1402–1414
Cavigelli MA, Robertson GP (2001) Role of denitrifier in rates of nitrous oxide consumption in a terrestrial ecosystem. Soil Biol Biochem 33:297–310
Cheng Y, Cai ZC, Chang SX, Wang J, Zhang JB (2012) Wheat straw and its biochar have contrasting effects on inorganic N retention and N2O production in a cultivated Black Chernozem. Biol Fertil Soils. doi:10.1007/s00374-012-0687-0
Clough TJ, Kelliher FM, Sherlock RR, Ford CD (2004) Lime and soil moisture effects on nitrous oxide emissions from a urine patch. Soil Sci Soc Am J 68:1600–1609
Clough TJ, Bertram JE, Ray JL, Condron LM, O’Callaghan M, Sherlock RR, Wells NS (2010) Unweathered wood biochar impact on nitrous oxide emissions from a bovine-urine-amended pasture soil. Soil Biol Biochem 74:852–860
Cole CV, Duxbury J, Freney J, Heinemeyer O, Minami K, Mosier A, Paustian K, Rosenberg N, Sampson N, Sauerbeck D, Zhao Q (1997) Global estimates of potential mitigation of greenhouse gas emissions by agriculture. Nutr Cycl Agroecosy 49:221-228
Day D, Evans RJ, Lee JW, Reicosky D (2005) Economical CO2, SOx, and NOx capture from fossil-fuel utilization with combined renewable hydrogen production and large-scale carbon sequestration. Energy 30:2558–2579
Fenchel T, King GM, Blackburn TH (1998) Bacterial biogeochemistry: the ecophysiology of mineral cycling. Academic, London, p 307
Gao XS, Zeng M, Deng LJ, Zhong CB (2010) Present situation and benefit analysis of the straw recycling in southwest China. Asian Agr Res 2:40–44
Garcia C, Hernandez T (1994) Microbial activity in soils under Mediterranean environmental conditions. Soil Biol Biochem 26:1185–1191
Gärdenäs AI, Ågren GI, Bird JA, Clarholm M, Hallin S, Ineson P, Kätterer T, Knicker H, Ingvar Nilsson S, Näsholm S, Ogle S, Paustian K, Persson T, Stendahl J (2011) Knowledge gaps in soil carbon and nitrogen interactions—from molecular to global scale. Soil Biol Biochem 43:702–717
Gee GW, Or D (2002) Particle size analysis. In: Dane JH, Topp C (eds) Methods of soil analysis. Part 4, physical methods. ASA, Wisconsin, pp 255–293
Geisseler D, Horwath WR, Joergensen RG, Ludwig B (2010) Pathways of nitrogen utilization by soil microorganisms—a review. Soil Biol Biochem 42:2058–2067
Glaser B, Lehmann J, Zech W (2002) Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—a review. Biol Fertil Soils 35:219–230
Graham JP, Ellis FB, Christian DG, Cannell RQ (1986) Effects of straw residues on the establishment, growth and yield of autumn-sown cereals. J Agric Eng Res 33:39–49
Han RJ, Yin DQ, Zhao YZ (2003) The present situation of the researching of straw turnover. Agric Mech Asia 2003:39–40
Hao X, Chang C, Carefoot JM, Janzen HH, Ellert BH (2001) Nitrous oxide emissions from an irrigated soil as affected by fertilizer and straw management. Nutr Cycl Agroecosy 60:1–8
Ingram DL, Henley RW, Yeager TH (1990) Diagnostic and monitoring procedures for nursery crops. University of Florida, Florida Cooperative Extension Service. Circular 556. November 1990. 11 p.
IPCC (2007) Climate change 2007: the physical science basis. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) Summary for policymakers. Contribution of working group I to the fourth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, pp 1–18
Janya SA, Eiji Y, Jureerat B (2010) Promoting plant residue utilization for food security and climate change mitigation in Thailand. In: Fukushi K, Hassan KM, Honda R, Sumi A (eds) Sustainability in food and water: an Asian perspective. Springer, Dordrecht, pp 343–352
Janzen HH (2006) The soil carbon dilemma: shall we hoard it or use it? Soil Biol Biochem 38:419–424
Jones DL, Murphy DV, Khalid M, Ahmad W, Edwards-Jones G, DeLuca TH (2011) Short-term biochar-induced increase in soil CO2 release is both biotically and abiotically mediated. Soil Biol Biochem 43:1723–1731
Kandeler E, Stemmer M, Klimanek EM (1999) Response of soil microbial biomass, urease and xylanase within particle size fractions to long-term soil management. Soil Biol Biochem 31:261–273
Katyal S, Thambimuthu K, Valix M (2003) Carbonisation of bagasse in a fixed bed reactor: influence of process variables on char yield and characteristics. Renew Energy 28:713–725
Kumar K, Goh KM (2000) Crop residues and management practices: effects on soil quality, soil nitrogen dynamics, crop yields, and nitrogen recovery. Adv Agron 68:197–319
Lammirato C, Miltner A, Kaestner M (2011) Effects of wood char and activated carbon on the hydrolysis of cellobiose by β-glucosidase from Aspergillus niger. Soil Biol Biochem 43:1936–1942
Lehmann J, Rondon M (2005) Bio-char soil management on highly-weathered soils in the humid tropics. In: Uphoff N (ed) Biological approaches to sustainable soil systems. CRC, Boca Raton, pp 517–530
Lehmann J, Da Silva JPJ, Rondon M, Da Silva CM, Greenwood J, Nehls T, Steiner C, Glaser B (2002) Slash-and-char: a feasible alternative for soil fertility management in the central Amazon? Presentation poster of the 17th World Congress of Soil Science, Bangkok Thailand, Symposium 13, pp 1–12
Lehmann J, Da Silva JPJ, Steiner C, Nehls T, Zech W, Glaser B (2003) Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: fertilizer, manure and charcoal amendments. Plant Soil 249:343–357
Lehmann J, Gaunt J, Rondon M (2006) Bio-char sequestration in terrestrial ecosystems—a review. Mitig Adapt Strat Glob Change 11:403–427
Ma J, Xu H, Yagi K, Cai Z (2008) Methane emission from paddy soils as affected by wheat straw returning mode. Plant Soil 313:167–174
Major J, Lehmann J, Rondon M, Goodale C (2010) Fate of soil-applied black carbon: downward migration, leaching and soil respiration. Glob Change Biol 16:1366–1379
Muñoz C, Paulino L, Monreal C, Zagal E (2010) Greenhouse gas (CO2 and N2O) emission from soils: a review. Chil J Agr Res 70:485–497
Nannipieri P, Ascher J, Ceccherini MT, Landi L, Pietranellera G, Renella G (2003) Microbial diversity and soil functions. Eur J Soil Sci 54:655–670
Paz-Ferreiro J, Gascó G, Gutiérrez B, Méndez A (2011) Soil biochemical activities and the geometric mean of enzyme activities after application of sewage sludge and sewage sludge biochar to soil. Biol Fertil Soils 48:511–517
Procházková B, Hrubý J, Dovrtěl J, Dostál O (2003) Effects of different organic amendment on winter wheat yields under long-term continuous cropping. Plant Soil Environ 49:433–438
Qian P, Schoenaru JJ, Karamanos RE (1994) Simultaneous extraction of available phosphorus and potassium with a new soil test: a modification of Kelowna extraction. Commun Soil Sci Plant Anal 25:627–635
Reynolds CM, Wolf DC, Armbruster JA (1985) Factors related to urea hydrolysis in soils. Soil Sci Soc Am J 49:104–108
Roldán A, Salinas-García JR, Alguacil MM, Díaz E, Caravaca F (2005) Soil enzyme activities suggest advantages of conservation tillage practices in sorghum cultivation under subtropical conditions. Geoderma 129:178–185
Rondon MA, Ramirez JA, Lehmann J (2005) Greenhouse gas emissions decrease with charcoal additions to tropical soils. http://soilcarboncenter.kstate.edu/conference/USDA%20Abstracts%20html/Abstract%20Rondon.htm
Rondon MA, Molina D, Hurtado M, Ramirez J, Lehmann J, Major J, Amezquita E (2006) Enhancing the productivity of crops and grasses while reducing greenhouse gas emissions through biochar amendments to unfertile tropical soils. In: Proceedings of the 18th World Congress of Soil Science, Philadelphia, PA, pp 138–168
Ryszkowski L, Kędziora A (2007) Modification of water flows and nitrogen fluxes by shelterbelts. Ecol Eng 29:388–400
Shevtsova L, Romanenkov V, Sirotenko O, Smith P, Smith JU, Leech P, Kanzyvaa S, Rodionova V (2003) Effect of natural and agricultural factors on long-term soil organic matter dynamics in arable soddy-Podzolic soils—modeling and observation. Geoderma 116:165–189
Singh BP, Hatton BJ, Singh B, Cowie AL, Kathuria A (2010) Influence of biochars on nitrous oxide emission and nitrogen leaching from two contrasting soils. J Environ Qual 39:1224–1235
Snyder CS, Bruulsema TW, Jensen TL, Fixen PE (2009) Review of greenhouse gas emissions from crop production systems and fertilizer management effects. Agr Ecosyst Environ 133:247–266
Sohi S, Loez-Capel E, Krull E, Bol R (2009) Biochar’s roles in soil and climate change: a review of research needs. CSIRO Land and Water Science Report 05/09, p 64
Sombroek W, Nachtergaele FO, Hebel A (1993) Amounts, dynamics and sequestering of carbon in tropical and subtropical soils. Ambio 22:417–426
Sparks DL, Page AL, Helmke PA, Loeppert RH, Soltanpour PN, Tabatai MA, Johnson CT, Sumner ME (1996) Methods of soil analysis: part 3—chemical methods. ASA-CSSA-SSSA, Madison
Sparling GP (1997) Soil microbial biomass, activity and nutrient cycling as indicators of soil health. In: Pankhurst C, Doube BM, Gupta VVSR (eds) Biological indicators of soil health. CAB International, Wallingford, pp 97–119
Spokas KA, Koskinen WC, Baker JM, Reicosky DC (2009) Impacts of woodchip biochar additions on greenhouse gas production and sorption/degradation of two herbicides in a Minnesota soil. Chemosphere 77:574–581
Tabatabai MA (1994) Soil enzymes. In: Weaver RW, Angle S, Bottomley P, Bezdicek D, Smith S, Tabatabai A, Wollum A (eds) Method of soil analysis part 2. Microbiological and biochemical properties. SSSA, Madison, pp 775–833
Tate KR, Ross DJ, Saggar S, Hedley CB, Dando J, Singh BK, Lambie SM (2007) Methane uptake in soils from Pinus radiata plantations, a reverting shrubland and adjacent pastures: effects of land-use change, and soil texture, water and mineral nitrogen. Soil Biol Biochem 39:1437–1449
Thomsen IK, Christensen BT (1998) Cropping system and residue management effects on nitrate leaching and crop yields. Agr Ecosyst Environ 68:73–84
Tiedje JM (1998) Ecology of denitrification and dissimilatory nitrate reduction to ammonium. In: Zehnder AJB (ed) Biology of anaerobic microorganisms. Wiley, New York, pp 179–244
van Zwieten L, Kimber S, Morris S, Chan KY, Downie A, Rust J, Joseph S, Cowie A (2010a) Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 327:235–246
van Zwieten L, Kimber S, Morris S, Downie A, Berger E, Rust J, Scheer C (2010b) Influence of biochars on flux of N2O and CO2 from Ferrosol. Aust J Soil Res 48:555–568
Wang J, Zhang M, Xiong Z, Liu P, Pan G (2011) Effects of biochar addition on N2O and CO2 emissions from two paddy soils. Biol Fertil Soils 47:887–896
Wick B, Kühne RF, Vlek PLG (1998) Soil microbiological parameters as indicators of soil quality under improved fallow management systems in south-western Nigeria. Plant Soil 202:97–107
Wick B, Veldkamp E, De Mello WZ, Keller M, Crill P (2005) Nitrous oxide fluxes and nitrogen cycling along a pasture chronosequence in Central Amazonia, Brazil. Biogeosci Discuss 2:499–535
Włodarczyk T, Stępniewski W, Brzezińska M (2002) Dehydrogenase activity, redox potential, and emissions of carbon dioxide and nitrous oxide from Cambisols under flooding conditions. Biol Fertil Soils 36:200–206
Woolf D, Amonette JE, Street-Perrott FA, Lehmann J, Joseph S (2010) Sustainable biochar to mitigate global climate change. Nat commun 1(5):56
Yanai Y, Toyota K, Okazaki M (2007) Effects of charcoal addition on N2O emissions from soil resulting from rewetting air-dried soil in short-term laboratory experiments. Soil Sci Plant Nutr 53:181–188
Zhang A, Cui L, Pan G, Li L, Hussain Q, Zhang X, Zheng J, Crowley D (2010) Effect of biochar amendment on yield and methane and nitrous oxide emissions from a rice paddy from Tai Lake plain, China. Agr Ecosyst Environ 139:469–475
Acknowledgments
The authors thank Donna Friesen and Pak Chow (Department of Renewable Resources, University of Alberta) for providing excellent technical support with the laboratory work. Our thanks are also due to Tim Anderson, Jin Tak, and Rob Hughes (Alberta Innovates—Technology Futures) for providing and testing of soil and biochar used in this experiment. We would also like to thank Sawyer Desaulniers, Beibei Zhang, Lin Yang, Zheng Shi, and Jenna Zee for their help during the experiment. We thank the China Scholarship Council, Alberta Innovates—Technology Futures, and the Natural Sciences and Engineering Research Council of Canada for their financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, F., Jia, Z., Wang, S. et al. Contrasting effects of wheat straw and its biochar on greenhouse gas emissions and enzyme activities in a Chernozemic soil. Biol Fertil Soils 49, 555–565 (2013). https://doi.org/10.1007/s00374-012-0745-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-012-0745-7