Abstract
The present study aimed to establish zebrafish as an experimental model for investigations into obesity and physical exercise, as well as to assess the effects of these factors on metabolism. The experiment spanned twelve weeks, comprising a feeding trial during which the last four weeks incorporated a physical exercise protocol. This protocol involved placing fifteen animals in a five-liter aquarium, where they were subjected to swimming at an approximate speed of 0.08 m/s for 30 min daily. Throughout the experiment, histological analyses of visceral, subcutaneous, and hepatic adipose tissues were conducted, along with biochemical analyses of total cholesterol and its fractions, triglycerides, glucose, lactate, and alanine aminotransferase (ALT) levels. Additionally, oxidative stress markers, such as reactive oxygen species (ROS) levels, superoxide dismutase (SOD) activity, and catalase activity and the formation of thiobarbituric acid-reactive substances, were investigated. The results revealed that the group fed a high-fat diet exhibited an increase in ROS production and SOD activity. In contrast, the group administered the high-fat diet and subjected to physical exercise demonstrated a notable reduction in visceral adipocyte area, hepatic steatosis levels, ALT levels, and SOD activity. These findings indicate that physical exercise has a positive effect on obesity and oxidative stress in zebrafish, providing promising evidence for future investigations in this field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is a condition characterized by the excessive accumulation of both visceral and subcutaneous adipose tissue (Montalbano et al. 2021). This condition has serious health implications, such as the development of diseases such as atherosclerosis, type 2 diabetes mellitus, and cardiovascular diseases (Nakayama et al. 2020; WHO 2021). The etiology of obesity is multifactorial and results from complex interactions between genetic and environmental factors. Among the environmental factors involved, the consumption of an unbalanced diet and a lack of physical exercise can play significant roles (Le Lay et al. 2014; Li et al. 2019). Consequently, a positive energy imbalance occurs in which caloric intake exceeds energy expenditure, directly contributing to the development of obesity (Montalbano et al. 2021).
Obesity is associated with severe cellular damage that is mainly attributed to an exacerbated inflammatory state and high production of free radicals, such as hydroxyl and superoxide radicals (França et al. 2013). Under normal homeostatic conditions, these free radicals are effectively neutralized by antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT) and glutathione S-transferase (GST) (Hayes et al. 2005; Barbosa et al. 2010; Brieger et al. 2012; Mazari et al. 2023; Jena et al. 2023). However, in obese organisms, the capacity of antioxidant enzymes to counteract the high percentage of free radicals is insufficient, resulting in a redox imbalance that promotes lipid peroxidation, also known as oxidations (DNA, protein) (França et al. 2013).
Mitigating the damage caused by obesity can be achieved through two main strategies: dietary modifications and adopting a lifestyle that includes regular physical exercise (Ming Fock and Khoo 2013). However, sporadic physical exercise to exhaustion can lead to muscle cell damage, as evidenced by an increase in the plasma levels of cytosolic enzymes (Armstrong et al. 1983; Inglés et al. 2016). This phenomenon is a result of high free radical production, culminating in oxidative stress (Gomez-Cabrera et al. 2008). Conversely, when physical exercise is regularly practiced at moderate intensity, the generation of free radicals, such as reactive oxygen species (ROS), is counterbalanced by the simultaneous increase in antioxidant enzymes, such as SOD, CAT, and GPX (Viña et al. 2000; Simioni et al. 2018; Powers et al. 2023). Importantly, the production of ROS during moderate physical exercise is beneficial because these reactive species are essential for inducing the adaptive effects of physical exercise (Powers et al. 2020).
Zebrafish (Danio rerio) have emerged as a model organism in research addressing various metabolic alterations, including diabetes, obesity, and their resulting oxidative stress effects (Dandin et al. 2022; Chen and Liu 2022). Their advantages, such as low maintenance costs; high reproductive rate; short generation time (approximately 3 months); large number of eggs per spawn; and, most importantly, high genetic, anatomical, and physiological homology with mammals, make them a highly attractive model (Goldsmith and Jobin 2012; Ribas and Piferrer 2014). These characteristics have established zebrafish as a potential model for exercise-related studies, as demonstrated in previous studies (Heinkele et al. 2021; Wang et al. 2022; Martins et al. 2023). However, despite the growing interest in using zebrafish in exercise-related research, the effects of exercise-induced oxidative stress in zebrafish fed a high-fat diet have not been adequately elucidated. Therefore, this study aimed to establish zebrafish as an experimental model for investigating obesity and physical exercise, as well as to assess the effects of these factors on metabolism.
Materials and methods
Ethical aspects
All the experimental procedures were conducted at the Central Animal Facility of the Federal University of Lavras (Lavras, Minas Gerais, Brazil) and were approved by the Institutional Animal Ethics Committee of the Federal University of Lavras (protocol 042/2019).
Fish
Zebrafish (D. rerio) males at five months postfertilization were obtained from a local supplier and acclimated for a period of 10 days in a 500 L tank before obesity induction. Throughout the acclimation period, the animals were fed commercial flaked feed containing 43% crude protein and 6.5% crude fat (Ovo•Vit®, Chorzów, Poland). The photoperiod was maintained at 14:10 h, and water quality parameters such as temperature and pH were monitored and maintained within the ideal range for the species (Lawrence 2007).
Diet
Two semipurified diets with different levels of fat were formulated (Table 1) using SuperCrac 6.1 diet formulation software (TD Software, Viçosa, MG, Brazil). The ingredients were carefully mixed and moistened with approximately 40% water. The mixture was then forced through a meat grinder to produce pellets, which were subsequently dried in a forced-air oven at 55 °C for 24 h. Following drying, the pellets were crushed and standardized to an average particle size of approximately 0.5 mm.
The approximate composition of the diets (Table 1) was analyzed according to AOAC methodologies (2005) for crude protein (Method 984.13), ether extract (Method No. 920.39), moisture (Method 930.15), and ash (Method 942.05). Gross energy was determined using a calorimeter (Model-IKA C5000).
Experimental design
Throughout the experimental period, the animals were housed in polycarbonate aquaria (11.5 cm × 34.5 cm × 15.5 cm) connected to a water recirculation rack (Hydrus ZEB-40, Alesco, SP, BRA) with automated control of temperature, pH, and conductivity. A photoperiod of 14:10 h was established throughout the experimental period. Water quality parameters, including temperature, pH, and conductivity, were measured daily, with average values of 28.07 ± 0.94 °C, 8.03 ± 0.11, and 0.522 ± 1.120 mS, respectively. Total ammonia (0.03 ± 0.02 mg/L) was measured weekly using a digital photometer (AT 100 PB, Alfakit, SC, Brazil).
The experiment was conducted in two distinct phases. In the first phase, the obesity induction protocol was performed, during which the animals (weight 0.381 ± 0.01 g) were transferred to individual 3.0 L aquaria (i.e., the experimental unit). The animals were randomly divided into two groups (n = 15 animals per aquarium, for a total of 90 animals per treatment): the control group and the high-fat diet (HFD) group, each with six replicate tanks (n = 12 experimental units). The animals were fed their respective diets at a rate of 4% of their body weight for 8 weeks, which were divided into four daily meals (7 a.m., 10 a.m., 1 p.m., and 4 p.m.). The animals were weighed weekly to adjust the amount of feed provided based on their weight.
In the second phase of the experiment, animals from each group were captured with the aid of a net, dried on paper towels, weighed (Shimadzu analytical balance, 220 g, 0.0001 g), and redistributed, creating four experimental groups: the control, control + exercise (Control-EX), HFD, and HFD + exercise (HFD-EX) groups. Each group was replicated across three aquaria, with 15 animals in each. The same diet from the first phase was maintained, and a four-week exercise protocol was introduced for the Control-EX and HFD-EX groups (Fig. 1).
Physical exercise protocol
The exercise protocol was adapted from Boskovic et al. (2018) and Blazina et al. (2013). In this protocol, 15 fish per aquarium were placed in a 5 L glass beaker (with an external diameter of 170 mm) containing a 60 × 10 mm magnetic stirring bar. The beaker was filled with 4 L of water from the recirculating system and placed on a magnetic stirrer (Fisatom, model 754 A, BR). The fish were exposed to 30 min of exercise per day for 30 days. The magnetic stirrer was programmed to create a flow of ~ 0.08 m/s. If any fish were unable to sustain swimming and were drawn into the vortex, they were removed from the experiment.
To quantify the hydrodynamic velocity experienced by the zebrafish, a plastic fragment was anchored to allow free rotation on the water surface in the beaker. The rotation frequency (rpm) of this fragment was then recorded, serving as a direct indicator of water velocity. This value was subsequently converted to meters per second (m/s), thus enabling a more precise quantification of the swimming conditions faced by the fish.
The sedentary group of zebrafish underwent the same procedure as the exercise group, except that the magnetic stirrer was kept off, generating no flow in the beaker.
Biometric parameters
At the eighth week and at the end of the experiment, the fish were captured using a net, gently dried with paper towels, weighed using a Shimadzu analytical balance, and immediately returned to the water. The standard length was measured using a caliper from the tip of the snout to the end of the caudal peduncle. The body mass index (BMI) was determined for each fish using the following formula:
Histological analyses
For histological analysis, three whole zebrafish per aquarium (n = 9/group) were fixed in 10% buffered formalin, embedded in paraffin, and sectioned using a microtome. Subcutaneous and visceral region sections were stained with hematoxylin-eosin. Microscopy images (Motic, USA; Moticam 3+, USA) were obtained, and the cell area of adipocytes from each animal was manually measured using ImageJ software (National Institutes of Health) (Landgraf et al. 2017).
For cryostat sectioning, three animals per aquarium were dissected (for a total of 9 animals per treatment), and the livers were collected. Subsequently, the tissues were immediately fixed in 10% buffered formalin at -4 °C for 24 h. Subsequently, the livers were cryoprotected in solutions with increasing concentrations of sucrose (10, 20, and 30%) at -4 °C. The tissues were then embedded in Tissue-Plus O.C.T. (Tissue-Plus™, Thermo Fisher, Houston, TX-USA), frozen by immersion in P.A. N-hexane, and immersed in liquid nitrogen. Section (6 μm thick) were obtained using a cryostat (CM 1850-3-1; Leica Microsystems). The sections were then submerged in P.A. propylene glycol for 2 min and stained with Oil red O. The qualitative analyses of hepatic steatosis were evaluated by a veterinary pathologist.
Biochemical parameters
At the end of the experiment, the animals were anesthetized via benzocaine immersion (250 mg/L) and euthanized (Ross et al. 2009). Blood from three animals per aquarium was collected following a previously used protocol (Flávia et al. 2018), and glucose levels were immediately measured using a portable glucometer (Accu-Check, Roche Diagnostics, Rotkreuz, Switzerland).
For the remaining biochemical analyses, homogenates were prepared with a pool of 3 animals per repetition according to the protocol described by Sancho et al. (2009). Five volumes of 0.1 M phosphate buffer (pH 7.2 at 0 °C) were added to each sample. Subsequently, the homogenates were centrifuged at 5500 rpm (4 °C) for 30 min, and the resulting supernatants were diluted five times in buffer and frozen at -80 °C for subsequent analyses.
Biochemical determinations of total cholesterol (TCHO) (Bioclin, Ref. K083), low-density lipoprotein (LDL) (Bioclin, Ref. K015-1), high-density lipoprotein (HDL) (Bioclin, Ref. K015-1), triglycerides (TG) (Bioclin, Ref. K117-2), lactate (BioTécnica, Ref. 10.018.00), and alanine aminotransferase (ALT) (Kovalent, Ref. MS 80115310051) were performed following the manufacturer’s instructions.
Oxidative stress evaluation
A pool of three animals per repetition was homogenized in phosphate buffer (0.1 M, pH 7) at a ratio of 1:5 (weight: volume) and centrifuged at 5500 rpm for 30 min at 4 °C. The supernatant was used to assess the formation of reactive oxygen species (ROS), the activity of the enzymes superoxide dismutase (SOD) and catalase (CAT), and the formation of thiobarbituric acid reactive substances (TBARS).
The ROS content was measured using the fluorescence probe 2,7-dichlorofluorescein diacetate (DCFH-DA; Sigma Aldrich, St. Louis, USA). A DCFH-DA solution (10 µM) was prepared by diluting DCFH-DA in 50 mM phosphate buffer (pH 7.2). The quantification of ROS was determined by the conversion of DCFH-DA to dichlorofluorescein (DCF) at a fluorescence wavelength of 485/530 nm (excitation/emission) using a microplate reader spectrophotometer (Infinite 200 PRO, Tecan, Männedorf, Switzerland) (Driver et al. 2000).
SOD activity was measured based on the auto-oxidation of pyrogallol, with an absorbance of 560 nm (Madesh and Balasubramanian 1997). The CAT activity was measured at 240 nm (AEBI, 1984), as a decrease in absorbance at 240 nm indicates the degradation of hydrogen peroxide into oxygen and water. The absorbance was measured every 15 s for 3 min. Lipid peroxidation was determined by measuring the formation of total bilirubin substances (TBARS) as described by Buege and Aust (1978). Malondialdehyde (MDA), formed from the degradation of polyunsaturated fatty acids, was used as an index to determine the extent of lipid peroxidation, considering its reaction with thiobarbituric acid, which results in the formation of a reddish solution with an absorbance of 535 nm.
All analyses were performed in triplicate, and the results were normalized to total protein values determined using the Bradford method (Bradford 1976). SOD, CAT, and TBARS levels were measured using a microplate reader spectrophotometer (Multiskan Go, Thermo Scientific, Waltham, Massachusetts, USA).
Statistical analysis
The data are presented as descriptive statistics (mean ± standard deviation). Normality and homogeneity of variance were assessed using the Shapiro‒Wilk and Levene tests, respectively. Statistical analyses were performed using Student’s t-test and one-way ANOVA (with post hoc Tukey analysis). A p-value < 0.05 was considered to indicate statistical significance (Prism 9.02, GraphPad Software, La Jolla, CA, USA).
Results
Biometric parameters
At the end of the eighth week of the feeding period, a significant increase in body weight was observed in the HFD group compared to the control group (Fig. 1A). After the exercise protocol was implemented, the weights of the fish in the HFD-EX group significantly increased compared to those of the Control and Control-EX groups. Furthermore, the HFD group also exhibited significantly greater weight than the Control-EX group (Fig. 1B).
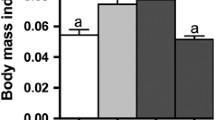
When comparing weight gain between the eighth and twelfth weeks, a reduction was observed in the Control-EX group compared to the Control, HFD, and HFD-EX groups (Fig. 1C). Concerning BMI, the HFD group had significantly greater BMI values than did the Control-EX group (Fig. 1D). Additionally, the standard length was significantly greater in the HFD-EX group than in the Control and Control-EX groups (Fig. 1E).
Biometric parameters after HFD-induced obesity and the effects of exercise in zebrafish were evaluated as follows: (A) Animal weights after 8 weeks of feeding, (B) Effects of 12 weeks of feeding and physical exercise on body weights, (C) Weight gain after 4 weeks of exercise, (D) Body mass indexes (BMI) after 12 weeks of feeding and physical exercise, and (E) Standard lengths after 12 weeks of feeding and physical exercise. The data are presented as the mean ± SD. Significant differences between groups are denoted as * p < 0.05, ** p < 0.01, and ***p < 0.001 (t-test and one-way ANOVA followed by Tukey’s post hoc test)
Body fat and hepatic lipid accumulation
The areas of adipocytes located in the visceral compartment were significantly greater in the HFD group than in the HFD-EX, Control, and Control-EX groups (Fig. 2A and C). A significant difference in the areas of adipocytes located in the subcutaneous compartment was observed between the HFD group and the Control and Control-EX groups (Fig. 2B and C). Figure 2D qualitatively illustrates the markedly increased hepatic fat accumulation in the HFD group compared to that of the other groups.
Effects of physical exercise on HFD-induced body fat accumulation in zebrafish. (A) Effects of diet and physical exercise on the visceral adipocyte area. (B) Effects of diet and physical exercise on the subcutaneous adipocyte area. (C) Representative images of visceral and subcutaneous fat distribution. (D) Liver fat distribution. Distribution of liver fat. The arrows indicate the fat staining by Oil Red O, while the triangles indicate the nuclei of the hepatocytes. Data are presented as mean ± SD. Significant differences between groups are indicated as * p < 0.05, ** p < 0.01, and ***p < 0.001 (ANOVA followed by Tukey’s post hoc test)
Biochemical parameters
ALT activity was significantly greater in the HFD group than in the HFD-EX, Control, and Control-EX groups (Table 2). No significant differences were observed in the TCHO, HDL, LDL, TG, lactate, or glucose parameters between any of the groups (Table 2).
Oxidative stress
A significantly greater production of ROS was observed in the HFD group than in the other groups (Fig. 3A). Additionally, the production of ROS in the HFD-EX group was significantly lower than that in the Control-EX group (Fig. 3A). However, the HFD-EX group exhibited significantly greater ROS production than the Control group (Fig. 3A). There was a significant reduction in ROS production in the Control group compared to that in the Control-EX group (Fig. 3A).
An increase in SOD activity was found in the HFD group compared to the other groups (Fig. 3B). A significant reduction in SOD activity was observed in the HFD-EX group compared to the other groups (Fig. 3B). The CAT activity and TBARS levels did not significantly differ among the experimental groups (Fig. 3C and D).
Effects of physical exercise and diet on the production of (A) reactive oxygen species (ROS), (B) superoxide dismutase (SOD) activity, (C) catalase (CAT) activity, and (D) thiobarbituric acid reactive substances (TBARS). The data are presented as the mean ± SD. Significant differences between groups are denoted as * p < 0.05, ** p < 0.01, ***p < 0.001, and ****p < 0.0001 (ANOVA followed by Tukey’s post hoc test)
Discussion
Obesity is characterized by a positive energy balance, leading to excessive accumulation of body fat in the subcutaneous and visceral regions, consequently causing weight gain (Montalbano et al. 2021). The results of the present study demonstrated a significant increase in body weight after 8 weeks of high-fat diet consumption. These findings align with the results of Landgraf et al. (2017) and Picolo et al. (2021), both of which revealed that a high-fat diet leads to weight gain in zebrafish, inducing an obese phenotype.
Physical exercise represents a nonpharmacological approach in the treatment of obesity, as it increases energy expenditure, generating a caloric deficit and thus contributing to a reduction in body weight (Swift et al. 2018). Although physical exercise at a flow rate of ~ 0.08 m/s for 30 min daily has been shown to increase the metabolic rate of zebrafish by three to four times (Seebacher and James 2019), the implementation of this exercise program for four weeks did not result in a decrease in body weight, weight gain, or body mass index (BMI) in the HFD-EX group compared to the HFD group. These results suggest that while physical exercise plays a relevant role in reducing body weight, its effectiveness may be limited in the absence of a balanced diet (Strasser and Fuchs 2016).
However, physical exercise had an effect on the Control-EX group, in which there was a significant decrease in body weight, weight gain, and BMI. The HFD-EX group, conversely, exhibited an increase in standard length compared to that of the Control group, supporting the finding that teleosts subjected to physical exercise tend to develop greater muscle mass and, consequently, greater growth (Palstra and Planas 2011). This factor, combined with the high energy availability from the 20% fat diet administered to the HFD-EX group, resulted in a greater standard length in this group.
Several studies addressing HFD-induced obesity in zebrafish have provided evidence that the alterations in metabolic and histological variables with obesity are comparable to those of mammals (Oka et al. 2010; Landgraf et al. 2017). Adipocyte hypertrophy and the presence of hepatic steatosis are conditions present in both obese mammals and zebrafish (Landgraf et al. 2017; Lee et al. 2020). We demonstrated that the physical exercise protocol used in obese zebrafish was sufficient to decrease the visceral adipocyte area and the degree of hepatic steatosis, which can be attributed to increased lipolysis during exercise due to increased energy demand (Prescott et al. 2023).
Factors that could contribute to the reduction in hepatic steatosis include the upregulation of lipogenesis genes (acaca, fasn, srebf1, and pparg) and the expression of β-oxidation genes (pgc1α, pparab, acox1, and cpt1a), as demonstrated in a previous study by Zou et al. (2021). Conversely, exercise was not sufficient to generate a decrease in subcutaneous adipose tissue, which may have been due to the intensity of the exercise not being sufficient to lead to a reduction in subcutaneous tissue. Similar results were reported by dos Santos et al. (2017), who reported that low-intensity swimming activity (approximately 1.35 body length/second) during physical exercise in pacu (Piaractus mesopotamicus) increased the density of ventral subcutaneous adipocytes by 20%.
An increase in TCHO, HDL, LDL, TG, and glucose is frequently associated with obesity (Sikaris 2004), as obesity and hyperlipidemia are major risk factors for cardiovascular diseases (Emami et al. 2016) and the development of type II diabetes mellitus (Sikaris 2004). However, no significant differences were observed in the aforementioned biochemical variables, which could be explained by the possibility that zebrafish present a metabolically healthy obesity phenotype, as observed in the study by Landgraf et al. (2017). The evaluation of ALT levels is recommended as an initial screening tool for hepatic steatosis in obese individuals (Chalasani et al. 2018), while LDH levels are used to indicate liver damage (Jafari et al. 2012). The increase in plasma ALT levels is mainly due to insulin resistance in adipose tissue and hepatic triglyceride content (Maximos et al. 2015), as well as the fact that approximately 50–70% of ingested glucose is converted to lactate in adipose tissue (Lin et al. 2022). We assessed the levels of ALT and lactate in the animals and found no differences in the lactate levels. However, we detected a significant decrease in ALT levels in the HFD-EX group. This decrease could be attributed to the reduction in lipid content in the liver and the prevention of hepatic damage due to the antioxidant potential of physical exercise (Delemasure et al. 2012). A similar result was found in a study by Thompson et al. (2010), in which they reported that 6 months of aerobic exercise significantly reduced serum ALT level in men.
High-fat deposition in obesity pathology is closely related to redox imbalance, which leads to oxidative stress (OS) (Savini et al. 2013). In other words, obese individuals present with elevated levels of reactive oxygen species (ROS) and decreased levels of antioxidant defenses, such as those related to the enzymes SOD and CAT (Olusi 2002; Keaney et al. 2003; Huang et al. 2015). This OS is attributed to excess adipose tissue, where adipocytes and preadipocytes act as sources of inflammatory cytokines such as TNF-α, IL-1, and IL-6, which stimulate the production of ROS and RNS by macrophages and monocytes (Fernández-Sánchez et al. 2011). Physical exercise is beneficial for health independent of changes related to body weight (Strasser 2013). In mammals, exercise reduces ROS production and inflammation (Feairheller et al. 2009) by increasing the levels of anti-inflammatory cytokines such as IL-1 and IL-10 while reducing the generation of the proinflammatory cytokine TNF-α (Hopps et al. 2011; Teixeira De Lemos et al. 2012). Therefore, physical exercise appears to be a promising antioxidant and anti-inflammatory strategy to prevent complications arising from obesity (Savini et al. 2013).
In the present study, we observed that regular physical exercise reduced ROS levels in animals fed a HFD. Conversely, compared with those in the control group, the animals in the Control-EX group had increased ROS levels. Physical exercise is an important factor in reducing ROS production in obese individuals (Gómez-Barroso et al. 2020). However, in nonobese individuals, strenuous physical exercise may lead to an increase in ROS production, especially in the muscle. Nevertheless, this exercise-induced increase in ROS is considered beneficial and can contribute to the adaptive effects of exercise (Powers et al. 2020).
The antioxidant enzymes SOD and CAT play crucial roles in cellular defenses by removing superoxide anions (O2•−) and hydrogen peroxide (H2O2). A decrease in the activity of these enzymes can lead to an excess of O2•− and H2O2, which subsequently generate hydroxyl radicals (HO•), resulting in lipid peroxidation and modification of membrane proteins (Halliwell and Gutteridge 2015; Sassetti et al. 2021). The increase in SOD activity is proportional to the increase in ROS production (De Farias et al. 2013). In our study, we demonstrated that SOD activity decreased in the exercise groups, as moderate exercise maintains a balance between pro-oxidative and antioxidant factors (Powers et al. 2023). Although no significant differences were observed in CAT activity, there was a trend toward a decrease in CAT levels in the HFD groups. The reduction in CAT activity may be attributed to its rapid consumption in the absorption of free radicals during obesity (Noeman et al. 2011).
Lipid peroxidation (LP) is a common phenomenon in obese individuals. MDA is one of the end products of LP and can be used as a marker of tissue oxidative stress (Huang et al. 2015). We did not find significant differences in TBARS levels. However, we observed a trend toward a decrease in the HFD + EX group, although this trend did not reach statistical significance. Similar results were observed in studies by Phillips et al. (2012) and Shin et al. (2008), where a decrease in TBARS, total lipid hydroperoxide (PEROX), and MDA was noted after exercise intervention. This decreasing trend may have occurred due to increases in maximal oxygen consumption and lean body mass and/or reductions in total body fat (Vincent et al. 2006; Huang et al. 2015).
Conclusion
In this study, we showed that the zebrafish is a potential model for physical exercise studies. Physical exercise is an important nonpharmacological treatment for obesity, as demonstrated in model organisms, particularly for reducing oxidative stress levels and hepatic steatosis. Notably, additional studies are needed to examine the molecular-level metabolic effects of physical exercise in zebrafish fed a high-fat diet.
Data availability
In reference WHO, DOI number was substituted by following link document https://www.who.int/publications/m/item/who-discussion-paper-draft-recommendations-for-the-prevention-andmanagement-of-obesity-over-the-life-course-including-potential-targets.
References
WHO (2021) Draft recommendations for the prevention and management of obesity over the life course, including potential targets. https://www.who.int/publications/m/item/who-discussion-paper-draft-recommendations-for-the-prevention-and-management-of-obesity-over-the-life-course-including-potential-targets
Armstrong RB, Ogilvie RW, Schwane JA (1983) Eccentric exercise-induced injury to rat skeletal muscle. J Appl Physiol 54:80–93. https://doi.org/10.1152/JAPPL.1983.54.1.80
Barbosa K, Costa N, Alfenas R et al (2010) Estresse oxidativo: conceito, implicações e fatores modulatórios. Rev Nutr 23:629–643. https://doi.org/10.1590/S1415-52732010000400013
Blazina AR, Vianna MR, Lara DR (2013) The spinning task: a new protocol to easily assess motor coordination and resistance in zebrafish. Zebrafish 10:480–485. https://doi.org/10.1089/zeb.2012.0860
Boskovic S, Marín-Juez R, Jasnic J et al (2018) Characterization of zebrafish (Danio rerio) muscle ankyrin repeat proteins reveals their conserved response to endurance exercise. PLoS ONE 13:1–21. https://doi.org/10.1371/journal.pone.0204312
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Brieger K, Schiavone S, Miller FJ, Krause KH (2012) Reactive oxygen species: from health to disease. Swiss Med Wkly 142. https://doi.org/10.4414/SMW.2012.13659
Buege JA, Aust SD (1978) [30] microsomal lipid peroxidation. Methods Enzymol 52:302–310
Chalasani N, Younossi Z, Lavine JE et al (2018) The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the study of Liver diseases. Hepatology 67:328–357. https://doi.org/10.1002/hep.29367
Chen C, Liu D (2022) Establishment of zebrafish models for diabetes Mellitus and its microvascular complications. J Vasc Res 59:251–260. https://doi.org/10.1159/000522471
Dandin E, Üstündağ ÜV, Ünal İ et al (2022) Stevioside ameliorates hyperglycemia and glucose intolerance, in a diet-induced obese zebrafish model, through epigenetic, oxidative stress and inflammatory regulation. Obes Res Clin Pract 16:23–29. https://doi.org/10.1016/J.ORCP.2022.01.002
De Farias JM, Bom KF, Tromm CB et al (2013) Effect of physical training on the adipose tissue of diet-induced obesity mice: Interaction between reactive oxygen species and lipolysis. Horm Metab Res 45:190–196. https://doi.org/10.1055/s-0032-1323740
De Teixeira E, Oliveira J, Páscoa J, Reis F (2012) Regular physical Exercise as a strategy to improve antioxidant and anti-inflammatory status: benefits in type 2 diabetes Mellitus. Oxid Med Cell Longev 2012:15. https://doi.org/10.1155/2012/741545
Delemasure S, Richard C, Gambert S et al (2012) Impact of high-fat diet on antioxidant status, vascular wall thickening and cardiac function in adult female LDLR-/-mice. World J Cardiovasc Dis 2:184–192. https://doi.org/10.4236/wjcd.2012.23031
dos Santos VB, de Oliveira MWM, Salomão RAS et al (2017) Influence of temperature and exercise on growth performance, muscle, and adipose tissue in pacus (Piaractus mesopotamicus). J Therm Biol 69:221–227. https://doi.org/10.1016/J.JTHERBIO.2017.08.004
Driver AS, Kodavanti PRS, Mundy WR (2000) Age-related changes in reactive oxygen species production in rat brain homogenates. Neurotoxicol Teratol 22:175–181. https://doi.org/10.1016/S0892-0362(99)00069-0
Emami SR, Jafari M, Haghshenas R, Ravasi A (2016) Impact of eight weeks endurance training on biochemical parameters and obesity-induced oxidative stress in high fat diet-fed rats. J Exerc Nutr Biochem 20:29. https://doi.org/10.20463/JENB.2016.03.20.1.5
Feairheller DL, Brown MD, Park JY et al (2009) Exercise training, NADPH oxidase p22phox gene polymorphisms, and hypertension. Med Sci Sports Exerc 41:1421–1428. https://doi.org/10.1249/MSS.0b013e318199cee8
Fernández-Sánchez A, Madrigal-Santillán E, Bautista M et al (2011) Inflammation, Oxidative Stress, and Obesity. Int J Mol Sci 2011, Vol 12, Pages 3117–3132 12:3117–3132. https://doi.org/10.3390/IJMS12053117
Flávia T, Castro D, Gilmar J et al (2018) Anxiety-associated behavior and genotoxicity found in adult Danio rerio exposed to tebuconazole-based commercial product. https://doi.org/10.1016/j.etap.2018.06.011
França BK, Melo Alves MR, Silveira Souto FM et al (2013) Peroxidação lipídica E obesidade: Métodos para aferição do estresse oxidativo em obesos. GE J Port Gastrenterologia 20:199–206. https://doi.org/10.1016/J.JPG.2013.04.002
Goldsmith JR, Jobin C (2012) Think small: zebrafish as a model system of human pathology. J Biomed Biotechnol 817341. https://doi.org/10.1155/2012/817341
Gómez-Barroso M, Moreno-Calderón KM, Sánchez-Duarte E et al (2020) Diazoxide and Exercise Enhance Muscle Contraction during Obesity by Decreasing ROS Levels, Lipid Peroxidation, and Improving Glutathione Redox Status. Antioxidants 2020, Vol 9, Page 1232 9:1232. https://doi.org/10.3390/ANTIOX9121232
Gomez-Cabrera MC, Domenech E, Viña J (2008) Moderate exercise is an antioxidant: Upregulation of antioxidant genes by training. Free Radic Biol Med 44:126–131. https://doi.org/10.1016/j.freeradbiomed.2007.02.001
Halliwell B, Gutteridge J (2015) Free radicals in biology and medicine. Oxford Univ Press USA
Hayes JD, Flanagan JU, Jowsey IR (2005) Glutathione transferases. Annu Rev Pharmacol Toxicol 45:51–88
Heinkele FJ, Lou B, Erben V et al (2021) Metabolic and transcriptional adaptations improve physical performance of zebrafish. Antioxidants 10:1581. https://doi.org/10.3390/ANTIOX10101581/S1
Hopps E, Canino B, Caimi G (2011) Effects of exercise on inflammation markers in type 2 diabetic subjects. Acta Diabetol 48:183–189. https://doi.org/10.1007/s00592-011-0278-9
Huang CJ, McAllister MJ, Slusher AL et al (2015) Obesity-Related Oxidative Stress: the Impact of Physical Activity and Diet Manipulation. Sport Med - Open 2015 11 1:1–12. https://doi.org/10.1186/S40798-015-0031-Y
Inglés M, Serra-Añó P, Gambini J et al (2016) Active paraplegics are protected against exercise-induced oxidative damage through the induction of antioxidant enzymes. Spinal Cord 2016 5410 54:830–837. https://doi.org/10.1038/sc.2016.5
Jafari M, Salehi M, Asgari A et al (2012) Effects of paraoxon on serum biochemical parameters and oxidative stress induction in various tissues of Wistar and Norway rats. Environ Toxicol Pharmacol 34:876–887. https://doi.org/10.1016/J.ETAP.2012.08.011
Jena AB, Samal RR, Bhol NK, Duttaroy AK (2023) Cellular Red-Ox system in health and disease: the latest update. Biomed Pharmacother 162:114606. https://doi.org/10.1016/J.BIOPHA.2023.114606
Keaney JF, Larson MG, Vasan RS et al (2003) Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol 23:434–439. https://doi.org/10.1161/01.ATV.0000058402.34138.11
Landgraf K, Schuster S, Meusel A et al (2017) Short-term overfeeding of zebrafish with normal or high-fat diet as a model for the development of metabolically healthy versus unhealthy obesity. BMC Physiol 17:1–10. https://doi.org/10.1186/S12899-017-0031-X
Lawrence C (2007) The husbandry of zebrafish (Danio rerio): a review. Aquaculture 269:1–20. https://doi.org/10.1016/J.AQUACULTURE.2007.04.077
Le Lay S, Simard G, Martinez MC, Andriantsitohaina R (2014) Oxidative stress and metabolic pathologies: from an adipocentric point of view. Oxid Med Cell Longev 2014. https://doi.org/10.1155/2014/908539
Lee ES, Kwon MH, Kim HM et al (2020) Curcumin analog CUR5–8 ameliorates nonalcoholic fatty liver disease in mice with high-fat diet-induced obesity. Metabolism 103:154015. https://doi.org/10.1016/J.METABOL.2019.154015
Li X, Xue Y, Pang L et al (2019) Agaricus Bisporus-derived β-glucan prevents obesity through PPAR γ downregulation and autophagy induction in zebrafish fed by chicken egg yolk. Int J Biol Macromol 125:820–828. https://doi.org/10.1016/J.IJBIOMAC.2018.12.122
Lin Y, Bai M, Wang S et al (2022) Lactate is a key mediator that links obesity to Insulin Resistance via modulating cytokine production from adipose tissue. Diabetes 71:637–652. https://doi.org/10.2337/DB21-0535
Madesh M, Balasubramanian KA (1997) A microtiter plate assay for superoxide using MTT reduction method. Indian J Biochem Biophys 34:535–539
Martins MSA, Carneiro WF, Rodrigues Da Cunha Barreto, Vianna A et al (2023) The zebrafish (Danio rerio) as a model for studying Voluntary Physical Exercise and its effects on Behavior and Metabolism. Brazilian Arch Biol Technol 66:e23220279. https://doi.org/10.1590/1678-4324-2023220279
Maximos M, Bril F, Portillo Sanchez P et al (2015) The role of liver fat and insulin resistance as determinants of plasma aminotransferase elevation in nonalcoholic fatty liver disease. Hepatology 61:153–160. https://doi.org/10.1002/hep.27395
Mazari AMA, Zhang L, Ye ZW et al (2023) The Multifaceted Role of Glutathione S-Transferases in Health and Disease. Biomol 2023, Vol 13, Page 688 13:688. https://doi.org/10.3390/BIOM13040688
Ming Fock K, Khoo J (2013) Diet and exercise in management of obesity and overweight. J Gastroenterol Hepatol 28:59–63. https://doi.org/10.1111/JGH.12407
Montalbano G, Mhalhel K, Briglia M et al (2021) Zebrafish and Flavonoids: Adjuvants against Obesity. Mol 2021, Vol 26, Page 3014 26:3014. https://doi.org/10.3390/MOLECULES26103014
Nakayama H, Hata K, Matsuoka I et al (2020) Anti-Obesity Natural Products Tested in Juvenile Zebrafish Obesogenic Tests and Mouse 3T3-L1 Adipogenesis Assays. Mol 2020, Vol 25, Page 5840 25:5840. https://doi.org/10.3390/MOLECULES25245840
Noeman SA, Hamooda HE, Baalash AA (2011) Biochemical study of oxidative stress markers in the liver, kidney and heart of high fat diet induced obesity in rats. Diabetol Metab Syndr 3:17. https://doi.org/10.1186/1758-5996-3-17
Oka T, Nishimura Y, Zang L et al (2010) Diet-induced obesity in zebrafish shares common pathophysiological pathways with mammalian obesity. BMC Physiol 10:21. https://doi.org/10.1186/1472-6793-10-21
Olusi SO (2002) Obesity is an independent risk factor for plasma lipid peroxidation and depletion of erythrocyte cytoprotectic enzymes in humans. Int J Obes Relat Metab Disord 26:1159–1164. https://doi.org/10.1038/SJ.IJO.0802066
Palstra AP, Planas JV (2011) Fish under exercise. Fish Physiol Biochem 37:259. https://doi.org/10.1007/S10695-011-9505-0
Phillips MD, Patrizi RM, Cheek DJ et al (2012) Resistance training reduces subclinical inflammation in obese, postmenopausal women. Med Sci Sports Exerc 44:2099–2110. https://doi.org/10.1249/MSS.0b013e3182644984
Picolo VL, Quadros VA, Canzian J et al (2021) Short-term high-fat diet induces cognitive decline, aggression, and anxiety-like behavior in adult zebrafish. Prog Neuro-Psychopharmacology Biol Psychiatry 110:110288. https://doi.org/10.1016/J.PNPBP.2021.110288
Powers SK, Deminice R, Ozdemir M et al (2020) Exercise-induced oxidative stress: friend or foe? J Sport Heal Sci 9:415–425. https://doi.org/10.1016/J.JSHS.2020.04.001
Powers SK, Goldstein E, Schrager M, Ji LL (2023) Exercise training and skeletal muscle antioxidant enzymes: an update. https://doi.org/10.3390/ANTIOX12010039. Antioxidants 12:
Prescott LA, Symonds JE, Walker SP et al (2023) Long-term sustained swimming improves swimming performance in Chinook salmon, Oncorhynchus tshawytscha, with and without spinal scoliosis. Aquaculture 574:739629. https://doi.org/10.1016/J.AQUACULTURE.2023.739629
Ribas L, Piferrer F (2014) The zebrafish (Danio rerio) as a model organism, with emphasis on applications for finfish aquaculture research. Rev Aquac 6:209–240. https://doi.org/10.1111/RAQ.12041
Ross LG, Ross B, Ross B (2009) Anaesthetic and sedative techniques for aquatic animals: Third Edition. Blackwell Publishing Ltd., Oxford, UK
Sancho E, Fernández-Vega C, Villarroel MJ et al (2009) Physiological effects of tricyclazole on zebrafish (Danio rerio) and post-exposure recovery. Comp Biochem Physiol - C Toxicol Pharmacol 150:25–32. https://doi.org/10.1016/j.cbpc.2009.02.004
Sassetti E, Clausen MH, Laraia L (2021) Small-molecule inhibitors of reactive oxygen species production. J Med Chem 64:5252–5275. https://pubs.acs.org/doi/10.1021/acs.jmedchem.0c01914
Savini I, Catani MV, Evangelista D et al (2013) Obesity-Associated Oxidative Stress: Strategies Finalized to Improve Redox State. Int J Mol Sci 2013, Vol 14, Pages 10497–10538 14:10497–10538. https://doi.org/10.3390/IJMS140510497
Seebacher F, James RS (2019) Increased physical activity does not improve obesity-induced decreases in muscle quality in zebrafish (Danio rerio). J Appl Physiol 127:1802–1808. https://doi.org/10.1152/japplphysiol.00433.2019
Shin YA, Lee JH, Song W, Jun TW (2008) Exercise training improves the antioxidant enzyme activity with no changes of telomere length. Mech Ageing Dev 129:254–260. https://doi.org/10.1016/j.mad.2008.01.001
Sikaris KA (2004) The clinical biochemistry of obesity. Clin Biochem Rev 25:165
Simioni C, Zauli G, Martelli AM et al (2018) Oxidative stress: role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget 9:17181. https://doi.org/10.18632/ONCOTARGET.24729
Strasser B (2013) Physical activity in obesity and metabolic syndrome. Ann N Y Acad Sci 1281:141–159. https://doi.org/10.1111/J.1749-6632.2012.06785.X
Strasser B, Fuchs D (2016) Diet Versus Exercise in Weight loss and maintenance: focus on Tryptophan. Int J Tryptophan Res 9:9. https://doi.org/10.4137/IJTR.S33385
Swift DL, McGee JE, Earnest CP et al (2018) The effects of Exercise and physical activity on weight loss and maintenance. Prog Cardiovasc Dis 61:206–213. https://doi.org/10.1016/J.PCAD.2018.07.014
Thompson D, Markovitch D, Betts JA et al (2010) Time course of changes in inflammatory markers during a 6-mo exercise intervention in sedentary middle-aged men: a randomized-controlled trial. J Appl Physiol 108:769–779. https://doi.org/10.1152/japplphysiol.00822.2009
Viña J, Gomez-Cabrera MC, Lloret A et al (2000) Free radicals in exhaustive physical exercise: mechanism of production, and protection by antioxidants. IUBMB Life 50:271–277. https://doi.org/10.1080/15216540051080994
Vincent HK, Bourguignon C, Vincent KR (2006) Resistance training lowers exercise-induced oxidative stress and homocysteine levels in overweight and obese older adults. Obes (Silver Spring) 14:1921–1930. https://doi.org/10.1038/OBY.2006.224
Wang L, Ma J, Wu W et al (2022) Effect of aerobic exercise as a treatment on type 2 diabetes mellitus with depression-like behavior zebrafish. Life Sci 300:120578. https://doi.org/10.1016/J.LFS.2022.120578
Zou Y, Chen Z, Sun C et al (2021) Exercise intervention mitigates pathological liver changes in NAFLD zebrafish by activating SIRT1/AMPK/NRF2 signaling. Int J Mol Sci 22:10940. https://doi.org/10.3390/IJMS222010940/S1
Acknowledgements
We thank Bioclin-Quibasa Química Básica Ltda. and Biotécnica Indústria e Comércio Ltda. for providing the biochemical kits, as well as the Central Bioterium of the Universidade Federal de Lavras and the Rede Mineira de Bioterismo-FAPEMIG.
Funding
This study was supported by the Conselho Nacional de Desenvolvimento Científico Tecnológico, grant number 308359/2019–4; the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, grant number 88881.117641/2016–01; Fundação de Amparo à Pesquisa do Estado de Minas Gerais, grant number 11542; Rede ZebraMinas, grant number: FAPEMIG 00083-23, Projeto Multiusuário Biotério UFLA, grant number FAPEMIG 02386/22 and Rede Mineira de Bioterismo-FAPEMIG.
Author information
Authors and Affiliations
Contributions
Martins, M.S.A. Conceptualization; Formal analysis; Investigation; Methodology; Resources; Validation; Visualization; Roles/Writing - original draft; Writing, review and editing. Carneiro, W.F. Investigation; Methodology; Formal analysis & Writing original draft, Writing review & editing. Monteiro, K.S. Investigation; Methodology; Formal analysis; Visualization. Souza, S.P. Investigation; Methodology; Formal analysis; Visualization. Vianna, A.R.C.B. Resources; Validation; Visualization; Roles/Writing - original draft; Writing, review and editing. Murgas, L.D.S. Conceptualization; Resources; Project administration; Supervision; Writing original draft; Writing review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflicts of interest.
Additional information
Communicated by Martin Klingenspor
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Martins, M.S., Carneiro, W.F., Monteiro, K.S. et al. Metabolic effects of physical exercise on zebrafish (Danio rerio) fed a high-fat diet. J Comp Physiol B (2024). https://doi.org/10.1007/s00360-024-01577-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00360-024-01577-x