Abstract
Background/Objectives:
Obesity can affect muscle phenotypes, and may thereby constrain movement and energy expenditure. Weight loss is a common and intuitive intervention for obesity, but it is not known whether the effects of obesity on muscle function are reversible by weight loss. Here we tested whether obesity-induced changes in muscle metabolic and contractile phenotypes are reversible by weight loss.
Subjects/Methods:
We used zebrafish (Danio rerio) in a factorial design to compare energy metabolism, locomotor capacity, muscle isometric force and work-loop power output, and myosin heavy chain (MHC) composition between lean fish, diet-induced obese fish, and fish that were obese and then returned to lean body mass following diet restriction.
Results:
Obesity increased resting metabolic rates (P<0.001) and decreased maximal metabolic rates (P=0.030), but these changes were reversible by weight loss, and were not associated with changes in muscle citrate synthase activity. In contrast, obesity-induced decreases in locomotor performance (P=0.0034), and isolated muscle isometric stress (P=0.01), work-loop power output (P<0.001) and relaxation rates (P=0.012) were not reversed by weight loss. Similarly, obesity-induced decreases in concentrations of fast and slow MHCs, and a shift toward fast MHCs were not reversed by weight loss.
Conclusion:
Obesity-induced changes in locomotor performance and muscle contractile function were not reversible by weight loss. These results show that weight loss alone may not be a sufficient intervention.
Similar content being viewed by others
Introduction
Skeletal muscle is essential for locomotion and posture,1 as well as for whole-body energy homoeostasis and metabolism.2, 3 Hence, any impairment of muscle function will impact on the health and fitness of the whole organism. Obesity has detrimental effects on metabolic phenotypes and muscle function.4 The consequences of these effects will be compounded if they persist in individuals that have undergone successful weight-loss therapy. It is therefore essential to determine the reversibility of obesity-induced physiological changes to predict the potential consequences of obesity and the efficacy of treatments. Our aim was to determine whether diet-induced obesity impairs locomotor capacity and muscle function, and whether any effects are reversible with weight loss. Skeletal muscle function is dependent on the contractile and calcium signalling proteins that mediate contraction and relaxation, and on energy metabolism to supply the necessary ATP.5 We therefore investigated metabolism in parallel with muscle contractile function.
Many signalling pathways associated with energy homoeostasis are conserved among vertebrates and are similar in a number of model species, including humans, rodents and zebrafish.6, 7 One of the principal mediators of obesity-induced metabolic dysfunction is a reduction in concentration and activity of the sirtuin SIRT1.4, 8 SIRT1 is a NAD+-dependent histone deacetylase that promotes expression of a range of metabolic regulators such as PGC-1alpha,9 and thereby regulates mitochondrial function.10 Mice lacking SIRT1 had reduced activity and reduced rates of oxygen consumption, leading to an overall decrease in energy expenditure.11 Conversely, increased expression of SIRT1 led to increased energy expenditure.12 Obesity-induced mitochondrial dysfunction in skeletal muscle manifests as decreased tricarboxylic acid cycle (citrate synthase; CS) activity and electron transport chain flux.13 It would be expected therefore that this reduction in maximal metabolic capacities would lead to a reduction in metabolic scope, which represents the energy available for activity and locomotion.
Locomotor performance is determined by dynamic muscular contractility rather than by isometric force production, and the capacity of muscles to produce work during the shortening and lengthening cycle, and the passive resistance to stretch determine muscle power output (work-loop performance).14, 15 When normalised to muscle mass, work-loop power output of isolated skeletal muscle was reduced in faster muscle fibre types of obese mice.16 A possible cause for changes in muscle power output are obesity-induced shifts in the expression of slow (oxidative) type I myosin heavy chains (MHCs),17, 18, 19 although fibre type shifts can differ between males and females.19 Obesity also altered locomotor capacity20 and the metabolic cost of locomotion,21 which could be associated with changes in muscle contractile properties. Although obesity is well known to constrain physical performance,22, 23 beyond a single mouse study24 the effect of obesity on muscle power output are unknown, and it remains to be shown whether there is a link between muscle power output and locomotion, and whether any obesity-induced changes are reversible with weight loss.
Weight loss reversed obesity-induced increases in pro-inflammatory proteins,25 reductions in adiponectin levels,26 impaired lymphatic function,27 metabolic dysfunction28, 29 and reduction in slow type I MHCs.17 However, at least with respect to kidney function it is not clear whether the effects of obesity are reversible by weight loss.30 Overall, it may be expected that obesity-induced declines in physiological function are reversed by weight loss. However, the physiological effects of obesity are so complex that it is difficult to extrapolate between physiological systems. Hence, we tested whether obesity-induced impacts on muscle and locomotor function are reversible by weight loss.
We used zebrafish to test the hypotheses that (a) obesity reduces metabolic scope and muscle CS activity because of mitochondrial dysfunction; (b) obesity reduces isolated muscle power output; we predicted that these changes in contractile function are associated with decreases in whole-animal locomotor performance, and changes in MHC composition; (c) obesity-induced changes in metabolism, skeletal muscle and locomotor phenotypes are reversible by weight loss.
Materials and Methods
Study animals and treatments
All procedures were performed with the approval of the University of Sydney Animal Ethics Committee (approval #723). Adult zebrafish (Danio rerio) were obtained from a commercial supplier (Livefish, Bundaberg, Australia) and maintained in plastic tanks (600 × 450 × 250 mm; 1–2 fish per l) with dechlorinated water at 25 °C, and a 12 h dark:12 h light photoperiod for 2 weeks before experimentation, and fed with commercial fish flakes (Wardley's, The Hartz Mountain Company, Secaucus, USA; 46% protein, 6% fat). After 2 weeks, fish were randomly allocated to one of three groups: (1) control fish were fed once a day to satiety for 9–10 weeks; (2) obese fish fed three times per day to satiety for 9–10 weeks; (3) obese–lean fish were fed three times per day for 4–5 weeks, then once per day for 4–5 weeks. We took photos of each fish (with an Exilim camera, Casio, Tokyo, Japan) to determine standard length (in ImageJ software, NIH, USA), and we weighed fish before treatments, again at the time when obese–lean fish were switched to the lean diet, and at the end of the treatments immediately before measurements were taken.
Metabolism and swimming performance
Metabolic scope, that is the difference between resting and maximal metabolic rates, represents the energy (ATP) available for activity.31 Resting metabolic rate represents the energetic costs to maintain membrane potential, protein synthesis and other processes occurring while the animal is at rest. Maximal metabolic rate reflects the maximal mitochondrial and cardiovascular capacities.31 We measured (n=12 lean fish, 14 obese and 9 obese–lean fish for all measures of oxygen consumption) resting and maximal oxygen consumption rates according to our previously published protocols32, 33 at 25 °C.
CS is a rate-limiting enzyme in the tricarboxylic acid cycle, and its activity reflects mitochondrial densities in tissue samples.34 Fish (n=8 fish per treatment group) were anaesthetised in buffered ethyl 3-aminobenzoate methanesulfonate (MS222; 0.3 g l−1; Sigma-Aldrich, Castle Hill, NSW, Australia) and euthanized by decapitation. Dorsal (back) and caudal (tail) skeletal muscle was extracted and immediately transferred to liquid nitrogen and stored at −80 °C. Muscle samples were homogenised (in a TissueLyser LT; Qiagen, Venlo, Netherlands) in nine volumes Radioimmunoprecipitation assay buffer (20 mM TrisCl pH 7.5, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid, 1 mM EGTA, 1% NP40, 1% sodium deoxycholate) and protease inhibitor cocktail (complete, ethylenediaminetetraacetic acid-free; Roche Life Sciences, Mannheim, Germany) solution. Homogenate was further diluted by a factor of 10 to a final 1:100 dilution. Following published protocols,35 enzyme activities were determined using a UV/visible spectrophotometer (Ultrospec 2100 Pro; Biochrom, Cambridge, UK) with a temperature controlled cuvette holder. Assays were performed in duplicate at 25 °C.
Sustained swimming performance was measured (in n=12 lean, 14 obese and 9 obese–lean fish) as critical sustained swimming speed (Ucrit)36 in a Blazka-type swimming flume according to published protocols.32 The Ucrit protocol uses an incremental increase in speed (Ui) for predetermined time intervals (Ti) until fish are fatigued as a measure of maximum locomotor capacity.36
Muscle biomechanics
Fish (n=10 per treatment group) were euthanized via a blow to the head, and transection of the spinal cord. The skin was removed and a section of rostral (anterior dorsal) muscle fibres of five to seven myotomes in length was dissected from one side of the fish in cooled (<5 °C) aerated fish Ringer’s solution (composition in mmol l−1: NaCl 115.7; sodium pyruvate 8.4; KCl 2.7; MgCl2 1.2; NaHCO3 5.6; NaH2PO4 0.64; 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid sodium salt 3.2; HEPES 0.97; CaCl2 2.1; pH 7.4 at 20 °C).37 The spine was removed from most of the muscle preparation leaving one myotome attached to the residual amount of spine at either end.
We conducted isometric studies to determine the twitch and tetanus kinetics of the isolated muscle according to published protocols.38 We calculated rates of force production as peak tetanic stress (force per cross-sectional area) divided by 2 × time to half peak tetanus, and muscle relaxation as peak tetanic stress divided by 2 × time from last stimulus to half relaxation as measures of the contractile performance of muscle.
We used the work-loop technique to determine the power output (average of each work-loop cycle) of muscles during cyclical length changes.15 Unlike fixed-length isometric studies and fixed load isotonic studies of muscle performance, the work-loop technique allows measures of muscle power output under length and activation changes that are generally more indicative of in vivo contractile performance.39 In the absence of in vivo strain (length change) data for rostral muscle in zebrafish, each muscle preparation was subjected to a set of four sinusoidal length changes symmetrical around the length found to generate maximal twitch force. In vivo rostral muscle length changes have been found to approximate a sinusoidal length change waveform in fish undergoing steady swimming, with the primary function of such muscle to produce power.40 The muscle was stimulated using the stimulation amplitude and stimulation frequency found to yield maximal isometric force. Electrical stimulation and length changes were controlled via a data acquisition board (KUSB3116, Keithley Instruments, Ohio, USA) and a custom-designed programme developed via TestPoint software (CEC Testpoint version 7, Measurement Computing, Norton, MA, USA). Muscle force was plotted against muscle length for each cycle to generate a work loop, the area of which equated to the net work produced by the muscle during the cycle of length change.41 Instantaneous power output was calculated for every data point in each work loop (2000 data points per work loop) by multiplying instantaneous velocity by instantaneous force. These instantaneous power output values were then averaged to generate an average net power output for each work-loop cycle. Every 5 min, the muscle was subjected to a further set of four work-loop cycles with length change cycle frequency (between 3 and 22 Hz), strain, stimulation duration and stimulation phase parameters being altered in between each set until maximum net work was achieved at each cycle frequency and maximal power output had been determined.
Every fourth or fifth set of work-loop cycles was used as a control run whereby a fixed set of strain and stimulation parameters were repeated regularly throughout the experiment to monitor underlying changes in the performance of the muscle over time. On average the net mean muscle power output per cycle, produced in control runs, decreased by 8.7% over the time course of each experiment. Therefore, the power produced by each preparation was corrected to the control run that yielded the highest power output (average power per cycle), assuming that alterations in power generating ability were linear over time between control runs.
After a further 5 min rest, fatigue resistance was determined by subjecting the muscle preparation to a series of tetani, each of 150 ms stimulation duration, at a rate of one tetanus per second for 25 s. For each muscle, fatigue resistance was calculated as the maximal force produced in the 25th tetanus as a percentage of the maximal force produced in the 1st tetanus for the same muscle. Ten minutes after the fatigue run each preparation was stimulated to produce a further tetanus to determine recovery from the fatigue run. The mean recovery of all 30 muscle preparations was 81.1%, indicating that reversible fatigue had been induced.
At the end of the muscle mechanics experiments, bone and connective tissue were removed and each muscle preparation was blotted on absorbent paper to remove excess Ringer’s solution. Wet muscle mass was determined to the ~0.1 mg using an electronic balance (Sartorius, Dandenong South, VIC, Australia). Mean muscle cross-sectional area was calculated from muscle length and mass assuming a density of 1060 kg m−3.42 The overall mean cross-sectional area±s.e. of all 30 muscle preparations was 2.65±0.17 mm2. Maximum isometric muscle stress (kN m−2) was then calculated for each tetanic response as the maximum tetanic force within that response divided by mean cross-sectional area. Normalised muscle power output (W kg−1) was calculated as average power output per length change cycle divided by wet muscle mass.
MHC concentrations
We prepared tissue homogenates as described above for measures of CS activity. The identification and quantification of slow and fast MHC isoforms was performed by capillary electrophoresis in a ‘Wes’ Simple Western System (ProteinSimple, San Jose, CA, USA) following the manufacturer's instructions. The antibodies (all from Developmental Studies Hybridoma Bank, University of Iowa, USA) we used were: EB165 to determine fast MHC concentrations; BA-F8 to determine slow MHC concentrations; 12G10 (α-tubulin) as internal control. We expressed normalised MHC concentrations by dividing MHC peaks by α-tubulin peaks measured for the same sample on the same plate. The concentrations of protein extracts were determined using a bicinchoninic acid assay kit (Sigma-Aldrich) following the manufacturer’s instructions.
Statistical analyses
We analysed data with permutational tests for linear models in the package lmPerm43 in R.44 Permutational analyses do not make assumptions about underlying data distributions, but use the data per se to infer significant differences. This approach is preferable to parametric tests, especially for sample sizes that are small relative to the total population of all possible samples.45 We analysed all dependent variables (body mass index, Ucrit, metabolic rates, muscle mechanics, MHC concentrations and enzyme activities) with treatment (levels: lean, obese, obese–lean) as factor. In the analysis comparing power output at different cycle frequencies, we use treatment as fixed factor, and we used fish id as a random factor within which we nested the different cycle frequencies to account for repeated measures of the same muscle preparation at different cycle frequencies. In analyses of Ucrit (in m s−1) we used body length as covariate, but we show data in units of body lengths per second. In case of significant results, we used pair-wise permutational tests for post hoc comparisons, and we used P<0.05 to indicate significant differences between treatment groups. Sample sizes were based on the power we achieved using similar techniques on zebrafish in past experiments.38, 46
Results
Obese fish had greater body mass index
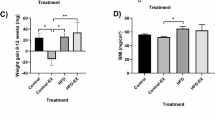
Body mass indices differed significantly between treatments (P<0.001; Figure 1), and lean and obese–lean fish had significantly lower body mass indices than obese fish (both P<0.001), but there was no difference in body mass index between lean and obese–lean fish (P=0.63). There was no difference in body mass index between the obese and the obese–lean groups (P=0.82) just before the start of diet restriction of the obese–lean group when the feeding regime was switched from feeding three time per day to once per day.
Body mass indices of the treatment groups. Lean (L) and obese–lean (OL; obese fish that underwent weight loss) fish had similar body mass indices (BMI), which were significantly lower that those of obese (O) fish. The BMI of the obese group was not different from that of the obese–lean group just before diet restriction when the feeding regime was switched from three times to once per day (OL/O). Means±s.e. are shown, and letters above bars indicate significant differences.
Obesity caused reversible decreases in metabolic scope independently from CS activity
Resting metabolic rates differed significantly between treatments (P=0.0068; Figure 2a). Lean and obese–lean fish had similar resting metabolic rates (P=0.98), and the rates of both groups were lower than that of the obese fish (P<0.001 and P=0.040, respectively, for lean and obese–lean). Maximal metabolic rates also differed between treatments (P=0.035; Figure 2b), and obese fish had significantly lower maximal metabolic rates than lean fish (P=0.030), but there were no differences between obese–lean and obese (P=0.72) or lean (P=0.13) fish. These responses of resting and maximal metabolic rates led to differences in metabolic scope between treatments (P=0.0026; Figure 2c), and lean fish had higher metabolic scope than obese fish (P=0.0060), but obese–lean fish did not differ from either of the other groups (P=0.13 and P=0.75, respectively).
Metabolic responses of zebrafish to obesity and weight loss. Obese (O) fish had significantly greater resting metabolic rates than lean (L) or obese–lean (OL) fish (a), but maximal metabolic rates of obese fish were lower than in lean fish (b). Maximal metabolic rates of obese–lean fish were not different from lean or obese fish. Metabolic scope was reduced in obese fish (c), but at least partly restored after weight loss in obese–lean fish. Citrate synthase activity, an indicator of mitochondrial density, did not differ significantly between treatment groups (d). Means±s.e. are shown, and letters above bars indicate significant differences. n=12 lean, 14 obese and 9 lean-obese fish for all metabolic rate measures, and n=8 fish per treatment group for citrate synthase activity.
CS activity, an indicator of mitochondrial density and metabolic capacity, did not differ between the treatment groups (P=0.43; Figure 2d).
Obesity caused irreversible changes in locomotor performance and muscle contractile properties
There were significant differences in Ucrit between treatments (P=0.0034; Figure 3A). Compared with lean fish, swimming performance was significantly lower in obese (P=0.044) and in obese–lean (P=0.0066) fish, but there was no difference between the latter two groups (P=0.27).
Muscle mechanics and locomotor performance in response to obesity and weight loss. Sustained swimming performance (Ucrit; A), isometric stress (force per unit area, F), dynamic muscle power output determined by the work-loop technique (C), and muscle relaxation rates (H) were lower in obese (O) individuals compared with lean (L) fish, and stayed at a reduced level even after weight loss (obese–lean, OL). An example of a typical work-loop shape (D) demonstrates that muscle of lean fish (broken line) produced greater stress and maintained stress to a greater extent during shortening (decreasing strain) compared with obese–lean fish (solid line). Differences in muscle power output between treatments were apparent at high cycle frequencies (E; significant differences indicated by an asterisk). Activation rate (G) showed similar reductions in obese and obese–lean individuals, but the differences were significant at a one-tailed probability only. There was no effect of treatment on muscle fatigue (B). Means±s.e. are shown, and letters above bars indicate significant differences. A hash next to a letter (for example, a#) indicates differences with a one-tailed probability. For Ucrit, n=12 lean, 14 obese and 9 obese–lean fish, and n=10 fish per treatment group for measures of muscle mechanics.
There were significant effects of treatment on muscle isometric stress (force per unit area; P=0.023; Figure 3F) and muscle work-loop power output (power produced per muscle mass; P=0.016; Figure 3C), and both were lowest in obese–lean fish (lean vs obese–lean: stress P=0.0098, power P<0.001; obese vs obese–lean: stress P=0.029, power P=0.011). There was a reduction in power and stress in obese fish compared with lean fish, but this was significant at a one-tailed probability only (stress P=0.092, power P=0.086; Figures 3C and D). Work-loop shapes indicated that most of the difference in normalised power output between lean and obese–lean fish was due to lean fish generating a higher peak stress in the work loop and maintaining higher stress during shortening (Figure 3D). Differences in power output between treatments were apparent only at higher cycle frequencies (interaction between treatment and cycle frequency P<0.0001; Figure 3E). At cycle frequencies of 12 Hz and above, power output was significantly lower in obese–lean fish compared with lean fish (all P<0.05), and obese–lean fish produced less power than obese fish at cycle frequencies of 14 Hz and above (all P<0.05; Figure 3E). Power output of obese fish was variable and we detected no differences between lean and obese fish (all P>0.2; Figure 3E).
Muscle activation rates were significantly different at a one-tailed probability only (P=0.074; Figure 3G). Muscle relaxation rates differed significantly between treatments (P=0.02; Figure 3H), and relaxation rate was significantly faster in muscle of lean fish compared with obese–lean fish (P=0.012); obese fish differed from lean fish with a one-tailed probability (P=0.067), but there was no difference in relaxation rate between obese and obese–lean fish. There were no differences between treatments in muscle fatigue (P=0.98; Figure 3B).
MHCs changed irreversibly with obesity
Obesity treatment had a significant effect on (normalised) slow MHC concentrations (P<0.001; Figure 4a). Lean fish had significantly greater concentrations than obese (P=0.012), and obese–lean (P=0.0062) fish, but there was no difference between the latter two groups (P=0.75). Similarly, concentrations of fast MHCs changed with treatment (P=0.044; Figure 4b), and compared with lean fish obese–lean fish had significantly lower concentrations of fast MHCs (P=0.025), but the decrease in obese fish was significant at a one-tailed probability only (P=0.078). Muscle composition changed with treatment (treatment effect P=0.0096, Figure 4c), and the ratio between slow:fast MHCs was significantly lower in obese (P=0.029) and obese–lean fish (P=0.010) than in lean fish, but obese fish were not different from obese–lean fish (P=0.96).
Myosin heavy chain concentrations in response to obesity and weight loss. Slow (a) and fast (b) myosin heavy chain concentrations (MHC; normalised to α-tubulin) were significantly lower in obese (O) and obese–lean (OL) individuals compared with lean controls (L). The slow:fast MHC ratio was lower in obese and obese–lean individuals, indicating a shift in muscle composition (c). Means±s.e. are shown, and letters above bars indicate significant differences. A hash next to a letter (for example, a#) indicates differences with a one-tailed probability. N=6 individuals for each treatment group.
Discussion
We have shown that declines in metabolic scope in obese individuals are reversed by weight loss, but declines in muscle contractile function and locomotion are not. These results indicate that weight gain and loss influence metabolic responses directly, but that the effect of obesity on muscle phenotypes is not mediated directly by changes in body mass index. The implication of our finding is that weight loss alone may be an insufficient treatment for obese pathologies.
Zebrafish are well established now in the literature as a model for obesity,6, 7, 47 metabolic disease48 and exercise.49, 50, 51 Overfeeding in zebrafish led to rapid weight gain and a significant increase in body mass index (1.1–1.3 fold) compared with control fish after 1–2 weeks.7 Similar to the effects of weight gain and obesity in humans, the increase in body mass resulted in pathophysiological conditions such as hypertriglyceridemia and hepatosteatosis.7, 52 Hence, that level of weight gain (>1.1–1.3 increase from lean body mass index) may be defined functionally as obese.53, 54 As in mice and humans, disruption of the adipostat system caused obesity in zebrafish,6 and leptin receptor deficiency in zebrafish disrupted glucose homoeostasis, but it did not cause hyperphagia.55 Exercise training in zebrafish increased muscle mass,51 myogenin levels and shifted skeletal muscle to a slower and more aerobic fibre type.56 These responses are broadly similar to those of other vertebrates,2, 57 which makes zebrafish a good exercise model for biomedical research.50
The (patho)physiological similarities between zebrafish and humans, combined with the lower cost and increased tractability of conducting experimental and screening studies on zebrafish compared with rodents or humans47, 48, 58, 59 mean that zebrafish have increasing translational impact59, 60 Zebrafish are particularly suitable for studies on muscle function and exercise because the methodologies to determine muscle and locomotor performance are well estabished in fish.61, 62, 63 We recently optimised isometric techniques to measure muscle performance in zebrafish,38 which we extended here to include the work-loo technique. These techniques are particularly powerful in a zebrafish model, because here it utilises most of the locomotory muscle assembly to provide a functional measure of muscle performance, which is more realistic than approaches that use only single fibres from biopsies as is the case for human studies. Hence, for our study, as well as for many others,47 zebrafish were a superior model than humans, in terms of quality of data, sample sizes, and practicality in terms of manipulating weight gain and weight loss under controlled experimental conditions.
Chronic feeding on high-energy diets and a sedentary lifestyle lead to an imbalance in glucose metabolism and insulin signalling, which can lead to obesity and metabolic diseases.4 A mechanism by which these effects can be mediated is the action of the SIRT1, which in association with AMPK activity regulates fatty-acid oxidation and energy homoeostasis.10 SIRT1 levels are increased by caloric restriction and are decreased by overfeeding.4 Adiponectin, the levels of which decrease with obesity but are restored by weight loss,26 stimulates the SIRT1/AMKP axis.64 Together, the actions of these molecules provide a mechanistic link between excessive feeding and obesity on the one hand, and metabolic dysfunction on the other,65 and may explain why metabolic dysfunction is reversible by decreased feeding and weight loss. Our finding that obesity-induced decreases in metabolic scope are reversible by reduced feeding and weight loss are similar to responses from mammals. The decrease in (mass specific) maximal metabolic rates was expected from obesity-induced metabolic dysfunction, and from the increase in adiposity in obese individuals. However, the decreases in maximal metabolic rates were not associated with decreased CS activities, which indicates that mitochondrial densities in muscle were not altered by obesity.34 The observed increases in resting metabolic rate may be due to increased inflammation and its attendant increase in resting metabolic demand,66 but this suggestion should be verified in a zebrafish model.
Surprisingly, the obesity-induced decreases in muscle contractile function and locomotor capacity were not reversible by weight loss in our zebrafish. High-fat diet caused a shift in MHCs toward faster isoforms in rhesus monkeys, and that shift was partly reversed with resveratrol, a drug that stimulates the SIRT1 pathway and promotes mitochondrial proliferation.67 Decreases in adiponectin and its receptor AdipoR1 can also decrease oxidative type I myofibres.64 However, if adiponectin and SIRT1 levels are restored by weight loss, as suggested in the literature, some other mechanisms must regulate muscle function and MHC expression in our zebrafish. Our data indicate that muscle of obese and obese–lean fish had low myofibrillar density (low MHC concentrations), and this decrease can explain the decreases in muscle stress and power output. The increasing difference in power output between treatments with increasing cycle frequency confirm this suggestion, because the effect of low myofibrillar density would be particularly pronounced as muscle works harder and at higher cycle frequencies. The cycle frequencies of the work-loop assays are proportional to tail beat frequencies in swimming fish,15 and tail beat frequencies are proportional to swimming speed.68 Hence, the reduction in MHC concentrations and power output at high cycle frequencies can explain the decreases in Ucrit we observed in obese and obese–lean individuals. The reduction in MHC concentration, and the shift from slow to fast MHC could be owing to impaired signalling pathways that mediate expression of muscle proteins. For example, expression of calcium handling and contractile proteins in skeletal muscle is regulated by the interaction between myocyte enhancer factor 2 and histone deacetylases.2 Obesity can lead to a disruption of the transcriptional regulation of muscle phenotypes, thereby leading to decreased muscle mass and strength,69 which provides a explanatory model for the changes in MHC levels we observed that can be tested in zebrafish and other obesity models.
Tail beat frequency, and hence swimming performance, is sensitive to calcium release and re-sequestration into the sarcoplasmic reticulum.68 Obesity can alter calcium (Ca2+) concentrations70 and reduce SERCA (sarco-endoplasmic reticulum Ca2+-ATPase) activity,71 which provides a second avenue by which obesity can constrain locomotion at higher speeds (that is, cycle frequencies—tail beat frequencies). Muscle contraction is mediated by the release of Ca2+ from the sarcoplasmic reticulum following neural stimulation of dihydropyridine receptors and their interaction with ryanodine receptors.72 Free Ca2+ mediates muscle contraction by binding to troponin in a concentration-dependent manner. Muscle relaxation is mediated by re-sequestration of Ca2+ into the sarcoplasmic reticulum via SERCA.57 Disruption of Ca2+ dynamics will attenuate muscle contractile properties57 and decrease locomotor performance.68 The reduction in relaxation rate indicates that obesity reduced SERCA activity and thereby slowed re-sequestration of Ca2+ into the sarcoplasmic reticulum and muscle relaxation. Similarly, the (one-tailed) decrease in activation rate suggests that the rate of Ca2+ release from the sarcoplasmic reticulum is reduced following stimulation. Depletion of Ca2+ stores in the sarcoplasmic reticulum can also reduce stress and power output.57 However, store depletion is unlikely, because fatigue resistance, which is at least partly determined by store depletion,73 was not affected by obesity.
Zebrafish are an excellent model to test obesity-induced changes on skeletal muscle, because it is easier to isolate the effects of obesity on muscle per se. In terrestrial animals, including humans, obesity increases the in vivo strength of postural ‘antigravity’ muscles as a result of a training effect from the increased load during standing and locomotion,74 which can obscure the effects of obesity on locomotor muscle. Our data are important because we show that the effects of obesity persist beyond weight loss. Weight loss is an essential intervention for obesity, but our data indicate that it is not sufficient to restore healthy, pre-obese phenotypes. The average lifespan of zebrafish is ~5% that of humans.75 Hence, the period of diet restriction (4–5 weeks) in our experiments represents a reasonably long time in human terms. An important outstanding question now is whether the observed changes, such as MHC concentrations and composition, can revert back to pre-obesity levels. Even though there can be a training effect of postural muscle as a result of supporting larger mass,74 obesity leads to reductions in motor control76 and it is often associated with sedentary lifestyles77 so that the mass-induced training effect would be minimised.78 Exercise intervention could be effective in restoring muscle function as well as weight loss.79 An interesting future direction will be to determine the link between transcriptional regulation of muscle phenotypes and changes in the contractile apparatus of skeletal muscle during obesity and following weight loss in both the zebrafish model and in humans directly. Understanding the role of exercise in influencing these pathways during or following weight loss could lead to developing effective programmes to reverse the negative effects of obesity on muscle function and locomotor capacity.
Data accessibility
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.5k303.
References
Dickinson M, Farley C, Koehl M, Kram R . How animals move: an integrative view. Science 2000; 288: 100–106.
Bassel-Duby R, Olson EN . Signaling pathways in skeletal muscle remodeling. Annu Rev Biochem 2006; 75: 19–37.
O’Neill HM, Holloway GP, Steinberg GR . AMPK regulation of fatty acid metabolism and mitochondrial biogenesis: Implications for obesity. Mol Cell Endocrinol 2013; 366: 135–151.
Pérez LM, Pareja-Galeano H, Sanchis-Gomar F, Emanuele E, Lucia A, Gálvez BG . ‘Adipaging’: ageing and obesity share biological hallmarks related to a dysfunctional adipose tissue. J Physiol (Lond) 2016; 594: 3187–3207.
Gordon AM, Homsher E, Regnier M . Regulation of contraction in striated muscle. Physiol Rev 2000; 80: 853–924.
Song Y, Cone RD . Creation of a genetic model of obesity in a teleost. FASEB J 2007; 21: 2042–2049.
Oka T, Nishimura Y, Zang L, Hirano M, Shimada Y, Wang Z et al. Diet-induced obesity in zebrafish shares common pathophysiological pathways with mammalian obesity. BMC Physiol 2010; 10: 21.
Rodgers J, Lerin C, Gerharthines Z, Puigserver P . Metabolic adaptations through the PGC-1α and SIRT1 pathways. FEBS Lett 2008; 582: 46–53.
Canto C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M et al. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab 2010; 11: 213–219.
Price NL, Gomes AP, Ling AJY, Duarte FV, Martin-Montalvo A, North BJ et al. SIRT1 Is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab 2012; 15: 675–690.
Ramadori G, Fujikawa T, Anderson J, Berglund ED, Frazao R, Michán S et al. SIRT1 deacetylase in SF1 neurons protects against metabolic imbalance. Cell Metab 2011; 14: 301–312.
Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschöp MH . Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci USA 2008; 105: 9793–9798.
Jheng H-F, Huang S-H, Kuo H-M, Hughes MW, Tsai Y-S . Molecular insight and pharmacological approaches targeting mitochondrial dynamics in skeletal muscle during obesity. Ann NY Acad Sci 2015; 1350: 82–94.
James RS, Altringham JD, Goldspink DF . The mechanical-properties of fast and slow skeletal-muscles of the mouse in relation to their locomotory function. J Exp Biol 1995; 198: 491–502.
Syme DA. Functional Properties of Skeletal Muscle In Fish Physiology Volume 23 Academic Press, 2005. pp 179–240.
Tallis J, Hill C, James RS, Cox VM, Seebacher F . The effect of obesity on the contractile performance of isolated mouse soleus, EDL and diaphragm muscles. J Appl Physiol 2017; 122: 170–181.
Tanner CJ, Barakat HA, Dohm GL, Pories WJ, MacDonald KG, Cunningham PRG et al. Muscle fiber type is associated with obesity and weight loss. Am J Physiol Endocrinol Metab 2002; 282: E1191–E1196.
Stuart CA, McCurry MP, Marino A, South MA, Howell MEA, Layne AS et al. Slow-twitch fiber proportion in skeletal muscle correlates with insulin responsiveness. J Clin Endocrinol Metab 2013; 98: 2027–2036.
DeNies MS, Johnson J, Maliphol AB, Bruno M, Kim A, Rizvi A et al. Diet-induced obesity alters skeletal muscle fiber types of male but not female mice. Physiol Rep 2014; 2: e00204.
Garland T, Schutz H, Chappell MA, Keeney BK, Meek TH, Copes LE et al. The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: human and rodent perspectives. J Exp Biol 2011; 214: 206–229.
Browning RC, Baker EA, Herron JA, Kram R . Effects of obesity and sex on the energetic cost and preferred speed of walking. J Appl Physiol 2006; 100: 390–398.
Larsson UE, Mattsson E . Functional limitations linked to high body mass index, age and current pain in obese women. Int J Obes 2001; 25: 893–899.
Maffiuletti NA, Ratel S, Sartorio A, Martin V . The impact of obesity on in vivo human skeletal muscle function. Curr Obes Rep 2013; 2: 251–260.
Tallis J, James RS, Little AG, Cox VM, Duncan MJ, Seebacher F . Early effects of ageing on the mechanical performance of isolated locomotory (EDL) and respiratory (diaphragm) skeletal muscle using the work-loop technique. Am J Physiol Regul Integr Comp Physiol 2014; 307: R670–R684.
Yang WS, Lee WJ, Funahashi T, Tanaka S, Matsuzawa Y, Chao CL et al. Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. J Clin Endocrinol Metab 2001; 86: 3815–3819.
Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA 2003; 289: 1799–1804.
Nitti MD, Hespe GE, Kataru RP, García Nores GD, Savetsky IL, Torrisi JS et al. Obesity-induced lymphatic dysfunction is reversible with weight loss. J Physiol (Lond) 2016; 594: 1–15.
Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI . Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 2005; 54: 603–608.
Jiang S, Wang Q, Huang Z, Song A, Peng Y, Hou S et al. Gastric bypass surgery reverses diabetic phenotypes in Bdnf-deficient mice. Am J Pathol 2016; 186: 2117–2128.
Li K, Zou J, Ye Z, Di J, Han X, Zhang H et al. Effects of bariatric surgery on renal function in obese patients: a systematic review and meta analysis. PLoS One 2016; 11: e0163907–e0163918.
Clark TD, Sandblom E, Jutfelt F . Aerobic scope measurements of fishes in an era of climate change: respirometry, relevance and recommendations. J Exp Biol 2013; 216: 2771–2782.
Seebacher F, Borg J, Schlotfeldt K, Yan Z . Energetic cost determines voluntary movement speed only in familiar environments. J Exp Biol 2016; 219: 1625–1631.
Seebacher F, Ward AJW, Wilson RS . Increased aggression during pregnancy comes at a higher metabolic cost. J Exp Biol 2013; 216: 771–776.
Larsen S, Nielsen J, Hansen CN, Nielsen LB, Wibrand F, Stride N et al. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J Physiol (Lond) 2012; 590: 3349–3360.
Garenc C, Silversides F, Guderley H . Burst swimming and its enzymatic correlates in the threespine stickleback (Gasterosteus aculeatus: full-sib heritabilities. Can J Zool 1998; 76: 680–688.
Hammer C . Fatigue and exercise tests with fish. Comp Biochem Physiol A 1995; 112: 1–20.
Wakeling JM, Cole NJ, Kemp KM, Johnston IA . The biomechanics and evolutionary significance of thermal acclimation in the common carp Cyprinus carpio. Am J Physiol Regul Integr Comp Physiol 2000; 279: R657–R665.
Seebacher F, Little AG, James RS . Skeletal muscle contractile function predicts activity and behaviour in zebrafish. J Exp Biol 2015; 218: 3878–3884.
James RS, Young IS, Cox VM, Goldspink DF, Altringham JD . Isometric and isotonic muscle properties as determinants of work loop power output. Pflüg Arch 1996; 432: 767–774.
Altringahm JD, Wardle CS, Smith CI . Myotomal muscle function at different locations in the body of a swimming fish. J Exp Biol 1993; 182: 191–206.
Josephson R . Contraction dynamics and power output of skeletal muscle. Annu Rev Physiol 1993; 55: 527–546.
Mendez J, Keys A . Density and composition of mammalian muscle. Metabolism 1960; 9: 184–188.
Wheeler RE, Torchiano M Permutation tests for linear models in R. R Package Version. 2016.
R Development Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria, 2013.
Drummond GB, Vowler SL . Different tests for a difference: how do we do research? J Physiol (Lond) 2012; 590: 235–238.
Little AG, Kunisue T, Kannan K, Seebacher F . Thyroid hormone actions are temperature-specific and regulate thermal acclimation in zebrafish (Danio rerio. BMC Biol 2013; 11: 26.
Lieschke GJ, Currie PD . Animal models of human disease: zebrafish swim into view. Nat Rev Gen 2007; 8: 353–367.
Schlegel A, Gut P . Metabolic insights from zebrafish genetics, physiology, and chemical biology. Cell Mol Life Sci 2015; 72: 2249–2260.
McClelland GB, Craig PM, Dhekney K, Dipardo S . Temperature- and exercise-induced gene expression and metabolic enzyme changes in skeletal muscle of adult zebrafish (Danio rerio. J Physiol (Lond) 2006; 577: 739–751.
Palstra AP, Tudorache C, Rovira M, Brittijn SA, Burgerhout E, van der Thillart GEEJM et al. Establishing zebrafish as a novel exercise model: swimming economy, swimming-enhanced growth and muscle growth marker gene expression. PLoS One 2010; 5: e14483–e14489.
Hasumura T, Meguro S . Exercise quantity-dependent muscle hypertrophy in adult zebrafish (Danio rerio. J Comp Physiol B 2016; 186: 603–614.
Shimada Y, Kuninaga S, Ariyoshi M, Zhang B, Shiina Y, Takahashi Y et al. E2F8 promotes hepatic steatosis through FABP3 expression in diet-induced obesity in zebrafish. Nutr Metab (Lond) 2015; 12: 17.
Nguyen M, Yang E, Neelkantan N, Mikhaylova A, Arnold R, Poudel MK et al. Developing ‘integrative’ zebrafish models of behavioral and metabolic disorders. Behav Brain Res 2013; 256: 172–187.
Montalbano G, Mania M, Guerrera MC, Abbate F, Laurà R, Navarra M et al. Morphological differences in adipose tissue and changes in BDNF/Trkb expression in brain and gut of a diet induced obese zebrafish model. Annals Anat 2016; 204: 36–44.
Michel M, Page-McCaw PS, Chen W, Cone RD . Leptin signaling regulates glucose homeostasis, but not adipostasis, in the zebrafish. Proc Natl Acad Sci USA 2016; 113: 3084–3089.
van der Meulen T, Schipper H, van den Boogaart JGM, Huising MO, Kranenbarg S, van Leeuwen JL . Endurance exercise differentially stimulates heart and axial muscle development in zebrafish (Danio rerio. Am J Physiol Regul Integr Comp Physiol 2006; 291: R1040–R1048.
Gundersen K . Excitation-transcription coupling in skeletal muscle: the molecular pathways of exercise. Biol Rev 2011; 86: 564–600.
Newman T, Jhinku N, Meier M, Horsfield J . Dietary intake influences adult fertility and offspring fitness in zebrafish. PLoS One 2016; 11: e0166394–21.
Bournele D, Beis D . Zebrafish models of cardiovascular disease. Heart Fail Rev 2016; 21: 803–813.
Patton EE, Dhillon P, Amatruda JF, Ramakrishnan L . Spotlight on zebrafish: translational impact. Dis Model Mech 2014; 7: 731–733.
Altringham J, Ellerby DJ . Fish swimming: patterns in muscle function. J Exp Biol 1999; 202: 3397–340.
Swank D, Rome LC . The influence of thermal acclimation on power production during swimming. II. Mechanics of scup red muscle under in vivo conditions. J Exp Biol 2001; 204: 419–430.
Anttila K . Effects of different training protocols on Ca2+ handling and oxidative capacity in skeletal muscle of Atlantic salmon (Salmo salar L.). J Exp Biol 2006; 209: 2971–2978.
Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M et al. Adiponectin and AdipoR1 regulate PGC-1α and mitochondria by Ca2+ and AMPK/SIRT1. Nature 2010; 464: 1313–1319.
Guarente L . Calorie restriction and sirtuins revisited. Genes Dev 2013; 27: 2072–2085.
Utaka S, Avesani CM, Draibe SA, Kamimura MA, Andreoni S, Cuppari L . Inflammation is associated with increased energy expenditure in patients with chronic kidney disease. Am J Clin Nutr 2005; 82: 801–805.
Hyatt J-PK, Nguyen L, Hall AE, Huber AM, Kocan JC, Mattison JA et al. Muscle-specific myosin heavy chain shifts in response to a long-term high fat/high sugar diet and resveratrol treatment in nonhuman primates. Front Physiol 2016; 7: 77.
Seebacher F, Pollard SR, James RS . How well do muscle biomechanics predict whole-animal locomotor performance? The role of Ca2+ handling. J Exp Biol 2012; 215: 1847–1853.
Watts R, McAinch AJ, Dixon JB, O'Brien PE, Cameron-Smith D . Increased Smad signaling and reduced MRF expression in skeletal muscle from obese subjects. Obesity 2013; 21: 525–528.
Bruton JD, Aydin J, Yamada T, Shabalina IG, Ivarsson N, Zhang SJ et al. Increased fatigue resistance linked to Ca2+-stimulated mitochondrial biogenesis in muscle fibres of cold-acclimated mice. J Physiol (Lond) 2010; 588: 4275–4288.
Funai K, Song H, Yin L, Lodhi IJ, Wei X, Yoshino J et al. Muscle lipogenesis balances insulin sensitivity and strength through calcium signaling. J Clin Invest 2013; 123: 1229–1240.
Bannister RA . Bridging the myoplasmic gap II: more recent advances in skeletal muscle excitation-contraction coupling. J Exp Biol 2016; 219: 175–182.
Allen DG, Lamb GD, Westerblad H . Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 2008; 88: 287–332.
Garcia-Vicencio S, Coudeyre E, Kluka V, Cardenoux C, Jegu A-G, Fourot A-V et al. The bigger, the stronger? Insights from muscle architecture and nervous characteristics in obese adolescent girls. Int J Obes 2016; 40: 245–251.
Arslan-Ergul A, Adams MM . Gene expression changes in aging zebrafish (Danio rerio brains are sexually dimorphic. BMC Neurosci 2014; 15: 29.
Teasdale N, Simoneau M, Corbeil P, Handrigan G, Tremblay A, Hue O . Obesity alters balance and movement control. Curr Obes Rep 2013; 2: 235–240.
Vickers MH, Breier BH, McCarthy D, Gluckman PD . Sedentary behavior during postnatal life is determined by the prenatal environment and exacerbated by postnatal hypercaloric nutrition. Am J Physiol Regul Integr Comp Physiol 2003; 285: R271–R273.
Stodden DF, Goodway JD . The dynamic association between motor skill development and physical activity. J Phys Ed Recr Dance 2007; 78: 33–49.
Booth FW, Roberts CD, Laye MJ . Lack of exercise is a major cause of chronic diseases. Compr Physiol 2012; 2: 1143–1211.
Acknowledgements
We would like to thank Alec Simmons and Isabella Loughland for help with laboratory work. The research was supported by the University of Sydney and Coventry University, and Australian Research Council Discovery Grant DP160102260 to FS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Seebacher, F., Tallis, J., McShea, K. et al. Obesity-induced decreases in muscle performance are not reversed by weight loss. Int J Obes 41, 1271–1278 (2017). https://doi.org/10.1038/ijo.2017.81
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2017.81
- Springer Nature Limited
This article is cited by
-
The potential effect mechanism of high-fat and high-carbohydrate diet-induced obesity on anxiety and offspring of zebrafish
Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity (2022)
-
Investigating a dose–response relationship between high-fat diet consumption and the contractile performance of isolated mouse soleus, EDL and diaphragm muscles
European Journal of Applied Physiology (2019)