Abstract
Daily torpor can provide significant energy and water savings in bats during cold ambient temperatures and food scarcity. However, it may reduce rates of foetal and juvenile development. Therefore, reproductive females should optimize development by minimizing times in torpor. To test this hypothesis, the use of torpor by female and male free-ranging Daubenton’s bats (Myotis daubentonii) during reproduction (gestation, lactation, and post-lactation period) was investigated in 1998 and 1999. Temperature-sensitive radio transmitters were attached to the bats to measure skin temperature. Simultaneously, ambient temperature was recorded. While both sexes became torpid during daytime, male bats used daily torpor (>6°C below individual active temperature) significantly more often during reproductive period (mean: 78.4 % of day time in May and 43 % in June) than females. Female bats went into daily torpor, particularly in late summer when juveniles were weaned (mean: 66.6 % of daytime). Lowest skin temperatures occurred in a female bat with 21.0°C during post-lactation. Skin temperatures of male bats fluctuated from 16.8°C in torpor to 37.2°C during times of activity. There was a significant effect of reproductive period on skin temperature in females whereas mean ambient temperature had no significant effect. However, mean ambient temperature affected mean skin temperatures in males. Our findings indicate that female Daubenton’s bats adopt their thermoregulatory behaviour in particular to optimize the juvenile development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bats occurring in temperate zones are subject to a strong reproductive periodicity, reflecting seasonal variations in food supply. They hibernate in deep torpor and give birth in summer when insect availability is highest (e.g. Hickey and Fenton 1996; Racey and Swift 1985; Rydell 1992). In most temperate zones, vespertilionid bats mate in late summer and early autumn when sperm production reaches a peak and females are in oestrus (Encarnação et al. 2004; Racey and Tam 1974). After mating, sperm is stored in the oviduct throughout the winter (Racey 1973). Pregnancy starts immediately after fertilisation following hibernation. During pregnancy and lactation, energy demand of females grows continuously (Kurta et al. 1989; McLean and Speakman 2000). This is reflected in higher food consumption by lactating females that increases, for example, by about 45 % in Myotis lucifugus (Anthony and Kunz 1977) and Myotis velifer (Kunz 1974) from pregnancy to lactation. Food availability dictates timing of parturition, as shown by Arlettaz et al. (2001) for the insectivorous mouse-eared bat Myotis blythii.
When food availability is low at low-ambient temperatures, bats in the temperate zone become torpid during daytime in summer to reduce daily energy expenditure (DEE) (Coburn and Geiser 1998; Hosken 1997; Kurta 1990). Such torpor patterns as a reaction of low-ambient temperatures and fluctuating food supply are also known for other small mammals like lemurs (Schmid et al. 2000; Schmid 2000) and hedgehogs (Fowler 1988), as well as for birds (e.g. Körtner et al. 2001; Prinzinger et al. 1981). However, breeding females of mammals face an obvious dilemma, because milk production is less during torpor. This may considerably slow down fetal development and postnatal growth (Eisentraut 1937; Racey and Swift 1981; Wilde et al. 1995, 1999). Because of the brief gestation and the particularly short lactation period at temperate latitudes, a delay of the development of juvenile bats reduces their time to prepare for hibernation. It is therefore crucial that yearlings deposit enough fat reserves in late summer to survive the winter (Ransome 1968; Thomas et al. 1990).
So, we assume that breeding female bats optimise progress of reproduction by minimizing use of torpor during pregnancy and lactation. However, current knowledge about thermoregulatory strategies of free-ranging bats during reproductive season is scarce and limited to a few Nearctic bats, particularly to Eptesicus fuscus (Audet and Fenton 1988; Grinevitch et al. 1995; Hamilton and Barclay 1994; Lausen and Barclay 2003), Lasiurus cinereus (Hickey and Fenton 1996), and Myotis evotis (Chruszcz and Barclay 2002). Furthermore, skin temperature as a measure of torpor was mainly measured while the bats were in the roost but not during foraging. The purpose of this study was to investigate thermoregulatory behaviour of female and male Daubenton’s bats (Myotis daubentonii) during pregnancy, lactation and post-lactation period.
We hypothesized that in order to optimise foetal development and milk production, females should maintain a constantly high-body temperature during pregnancy and lactation, as long as possible, while deep daily torpor would be used predominantly in the post-lactation period. We expected that ambient temperatures would affect thermoregulation of reproductive active females less than actual reproductive condition. In contrast, adult male M. daubentonii that are not reproductive active during early summer might be expected to react more strongly to ambient temperatures by reducing body temperature more often, especially at low-ambient temperatures during pregnancy and parturition, because they are not involved in the development and rearing of the young. However, as sperm production starts in late summer, thermoregulatory behaviour of the males should change accordingly.
Methods
Study area and animals
We radio-tracked 6 adult females and 5 adult males of M. daubentonii during 1998 and 1999 (Table 1). The females belonged to a maternity colony in the “Philosopher-Forest,” a small deciduous forest in the city of Gießen in central Germany. Males and females live in the same place and both roost in tree holes of Fagus sylvatica, Quercus robur and Fraxinus excelsior. The bats flew to their feeding grounds along a traditional flight-path. The feeding grounds consisted of two ponds at a distance of a few hundred meters to the roost, and the river Lahn at a distance of 2.5 km (Dietz and Fitzenräuter 1996). Population structure and feeding ecology of the Daubenton’s bats in the “Philosopher-Forest” have been investigated since 1992; therefore, seasonality and habitat use are well known. We caught bats with mist nets set in their flight-path or at their roosts to attach radio-transmitters. The age of the bats was determined by evaluating the closure of the epiphysis (Anthony 1988) and by subsequent banding. The reproductive status was classified as pregnant, non-pregnant, lactating and post-lactating according to the methods described by Racey (1988). Pregnancy was assessed by palpation of the abdomen directly after the emergence of the bats. A bare patch around the nipples and milk expression were used as evidence for lactation. The onset and progression of spermatogenesis in males was assessed by the size of the testes and the distension of the epididymis.
Telemetry
We used LB-2T temperature-sensitive radio-transmitters (Holohil Systems, Canada) with a weight of 0.5 g for tagging bats. The bats weighed between 7.1 and 10.7 g so that the transmitter corresponded to 4.6–6.8% of their body weight, which was slightly below and in a few cases slightly above the 5% that is suggested for radio-tracking studies (see Aldridge and Brigham 1988). All transmitters were attached between the shoulder blades of the bats with skin-bond to study skin temperature and torpor behaviour (Barclay et al. 1996). The transmitters fell off after 3–8 days. During this time we monitored the bats continuously by “homing-in-on-the-animal” (White and Garrott 1990) with the help of 2-element Yagi antennae and Yaesu receivers (FT-290RII) modified by Wagener, Cologne (Germany). Pulse repetition rate of the transmitters was measured 1 to 4 times per hour, as long as the transmitter stayed on the bat to determine skin temperature. The pulses for three 1-minute sets were counted, and then the average of the values was determined and finally compared with a calibration curve provided by the manufacturer. Usually, we tagged 2–3 bats simultaneously and monitored them with 2 or 3 observer-groups. For this study, our total sample was 1,114 recorded hours distributed over 45.5 bat-days and 43 bat-nights. Daily ambient temperature (mean, min and max; resolution 1/10°C) was taken from records of a station of the German Weather Service near the “Philosopher-Forest.”
The usefulness of temperature-sensitive transmitters for measuring skin temperatures of bats in the field was first demonstrated by Audet and Thomas (1996). They found that externally attached transmitters accurately reflected core temperature. The difference between measured skin and body temperature was linearly correlated with ambient temperature (T a), but they also found differences as high as 6°C at relatively high T a (>21°C). Barclay et al. (1996) confirmed that the readings were only slightly affected by T a. Their study revealed that skin temperature was within 2.0°C of rectal temperature in 35 out of 44 measurements with a maximum deviation of only 3.3°C. Willis and Brigham (2003) also showed that core body temperature is slightly higher than T s because external transmitters were cooled by lower ambient temperatures. In our study, we sometimes measured a decline of 2–3°C in skin temperature for a short time when the bats emerged from their roost. This may be the influence of the cooler ambient temperature on the sensor when the bats started to fly. However, overall differences between the last T s value measured in the roost before departure and the first value after leaving the roost were not significant (Wilcoxon Signed Rank Test, P=0.88).
Definitions
“Active temperature” refers to the average skin temperature of a bat when it leaves the roost. We based our values of active temperature on temperature measurements taken about 15 min before emergence. Definition of torpor is controversial and many definitions have been proposed in different studies (Barclay et al. 2001; Geiser and Ruf 1995). We refer to Hamilton and Barclay (1994) and Grinevitch et al. (1995) for definition of daily torpor to permit direct comparison of our data with other studies on bats. As recently reviewed by Willis and Brigham (2003), differences as high as 6°C have been observed between skin temperature and core body temperature influenced by ambient temperatures. So, we defined that torpor occurs when skin temperature drops more than 6°C below active temperature.
We further defined bat day and bat night to compare differences in thermoregulation. A “bat day” started at 6:00 am and ended at 9:00 pm in early summer during pregnancy and lactation and at 8:00 pm in late summer with shorter day length during post-lactation. A “bat night” is defined as the hours between daytimes and corresponded to the time between astronomical sunset and sunrise. With reference to the long-term studies in the research area since 1992, we divided the investigation periods (Table 1) into pregnancy, lactation and post-lactation periods. Female Daubenton’s bats give birth in the first week of June and the young become fledged at the beginning of July.
Statistical analyses
All results are given as mean ± SE. Individual skin temperatures (T s) of females and males were calculated as mean of all average values of T s of the recorded bat days (Table 1). Individual skin temperatures were averaged to calculate the mean skin temperature of the respective reproductive period (pregnancy, lactation, post-lactation). Influence of gender, different reproductive periods of the year and variant ambient temperatures of individual skin temperatures were tested using General Linear Model analysis (GLM). Backward stepwise selection was applied in order to exclude non-significant independent variables from the model. Active temperature is given as mean T s for each individual bat prior to leaving the roost. Using these data, we computed the individual percentage of daytime in torpor as well as the mean time for all female and male bats. Kruskal–Wallis non-parametric one-way ANOVA and Mann–Whitney U-test were applied to test for significant differences in mean temperature data. The Spearman rank correlations were calculated to test for significant correlations between skin and ambient temperatures. All statistical analyses were performed using statistical software (Statistica 6.0, SigmaStat, San Rsfael, CA, USA).
Results
Thermoregulation
For Daubenton’s bats GLM analysis (Table 2) revealed a significant effect of gender, reproductive period and the interaction of the two variables gender × period and period × ambient temperature (T a) on skin temperatures (T s). If we compare the individual temperature behaviour of the radio tracked bats, it is obvious that the progress in daily Ts does not follow a mechanism influenced by exogenous factors only. The comparison between a pregnant female and an adult male during the period of pregnancy shows that the male was heterotherm while the female remained homeotherm with constant T s’s (Fig. 1 and 2a). Those bats were radio-tracked simultaneously under similar ambient temperatures. The second example shows a second pregnant female in comparison to a post-lactating female in late summer. In both radio-tracking periods, mean ambient temperature varied identically between 14 and 18°C. During daytime, the post-lactating female was regularly in torpor in contrast to the normothermic pregnant bat (Fig. 2b).
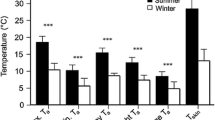
Mean daily skin temperature (T s) of all 11 radio-tracked female (F, n=6) and male (M, n=5) M. daubentonii during different reproductive periods (preg pregnancy, lac lactation, post-lac post-lactation period). Every dot represents one bat-day. Values were calculated as mean of all hourly recordings of T s during the bat days
Hourly absolute skin temperatures (°C) of a pregnant (preg) female (F) and a male (M) M. daubentonii during pregnancy period (a) with comparison of a second pregnant female and a post-lactating (post-lac) female (b). Dark bars represent bat nights between dusk and dawn. Filled squares indicate the time of a bat in the day roost and open squares the time it was in flight
Time of arousal from daily torpor varied, but both male and female raised their temperature from low values to active temperature during the last hour before leaving the roost (Fig. 2a,b). Sometimes, spontaneous arousals during the second and third daytime periods occurred (male bat in Fig. 2a).
It seemed obvious to us that the reduction of the body temperature is an individual behaviour, which initiated anew each day and so the daily T s’s were independent of the T s’s of the day before. Therefore, we defined the results of individual bat days as independent data. Figure 3 shows the mean T s of all bat days of one reproductive period for both sexes, which was recorded daily in the same time schedule. As hypothesized thermoregulatory behaviour of the radio tracked Daubenton’s bats was different during the reproductive periods in both sexes (Fig. 3, Kruskal–Wallis ANOVA: H=363.79, df=5, P<0.001). During pregnancy and lactation, reproductive females remained active in the day-roost and maintained their temperature at a high level (pregnancy: mean T s of 34.8°C±0.4 with a range from 30.7 to 39.5°C; lactation: 34.3°C ± 0.6 with a range from 27.9°C up to a maximum of and 36.3°C). Later in the year, when the young were weaned, body temperature of females decreased significantly after they had entered the roost (mean T s 26.2°C ± 0.9; Mann–Whitney U-test, P<0.001) with a minimum T s of 21.1°C.
Mean hourly skin temperature (°C) of female (F) and male (M) M. daubentonii during different reproductive periods. Temperature values are given as mean ± standard error (SE) representing the variance between the recorded bat-days. Data represent 14 bat days for 3 females and 8 bat days for 2 males during pregnancy period, 4 and 5 bat days for 1 female and 1 male during lactation period and 7 bat days each for 2 females and 2 males during post-lactation period
In comparison, T s of adult males varied considerably between night and day during pregnancy and lactation period. During this time, temperature dropped immediately after the males had returned to their day roost (pregnancy period: mean T s 22.6°C ± 1.3; lactation period: mean T s 27.1°C ± 2.1). The T s fluctuated to a much larger extent in males than in females, for example, during pregnancy period between 16.8°C in deep torpor and 37.2°C when the males were active. However, thermoregulatory behaviour of males changed later in the year when they maintained high temperatures during the first hours after they had entered the roost. Temperatures decreased only in the afternoon but they did not reach the low levels that we had measured in males during pregnancy and lactation period. During post-lactation period, mean T s of males differed significantly from T s of females and from T s of males during pregnancy and lactation period (Mann–Whitney U-test, P<0.001).
Use of torpor
There was a significant difference in the use of daily torpor by females and males between reproductive and post-lactation period (Kruskal–Wallis ANOVA H=36.15, df=5, P<0.001 and pairwise multiple comparison). Tagged pregnant and lactating females did not reduce their skin temperature more than 6°C below active temperature, whereas in the post-lactating period, females were on average torpid for more than half of daytime (60.0 and 73.3%, Table 3). Males were torpid during pregnancy period for about two thirds of daytime (88.0 and 68.9%). Later in the year, males remained mostly normothermic (Table 3).
Influence of ambient temperature
The relationship between skin temperature and ambient temperature (T a) was sex specifically assessed by calculating the Spearman rank correlations between the two variables. We found no significant influence of mean daily T a on mean T s of females (r s=−0.08, P=0.7, n=26) as opposed to male Daubenton’s bats where mean T s was positively correlated with mean T a (r s=0.67, P<0.01, n=20).
We further assessed the difference of mean T s—mean T a and minimum T a to assess the stability of T s in tagged female Daubenton’s bats in comparison to ambient temperature by calculating the Spearman rank correlations between the two variables. The two variables were negatively correlated (r s=−0.73, P<0.001, n=26). In contrast, we found a positive correlation between the two variables (r s=0.64, P<0.01, n=20) in male Daubenton’s bats. This indicates that body temperature of roosting female Daubenton’s bats was to a large extent, constant and therefore, independent of variations in ambient temperature as opposed to roosting male Daubenton’s bats where body temperature fluctuates to a large degree with ambient temperatures.
Discussion
The purpose of this study was to compare thermoregulation behaviour of female and male Daubenton’s bats during different reproductive periods. It is the first study where body temperature of both sexes of a free-ranging and tree-roosting insectivorous bat in the Palaearctic region has been examined with temperature-sensitive transmitters throughout roosting and foraging. Only Audet (1992) investigated skin temperatures of mouse-eared bats, Myotis myotis (Vespertilionidae) in the field, but measurements were limited to nursery colonies in attics. With our definition of torpor we take into consideration that ambient temperature slightly influences temperature measurements of external transmitters (see Willis and Brigham 2003). Compared with the possible influence of handling in captivity (e.g. physiological stress, disturbance), radio transmitters are less invasive for free-ranging bats and therefore, provide a better estimate of the importance of torpor in the daily thermoregulatory behaviour of small bats (Audet and Fenton 1988; Hamilton and Barclay 1994).
The results of our study support the hypothesis that there are significant differences between the thermoregulatory behaviour of female and male Daubenton’s bats in relation to reproductive status. Female Daubenton’s bats avoided deep daily torpor during pregnancy and lactation, whereas males became regularly torpid for several hours per day with lowest skin temperature of 16.8°C. As a consequence, lower energy expenditure achieved by the use of torpor may result in reduced foraging activities. This has been confirmed for Daubenton’s bats, as foraging times of male bats were significantly shorter during pregnancy period than that of female bats (Dietz et al. submitted). As we did not find a strict correlation between torpor and ambient temperature throughout the breeding season, it must be under active control of the bats in relation to their reproductive condition.
Torpor in mammals and birds is characterised by lowering the set point for body temperature regulation to achieve a hypo-metabolic state in order to conserve energy and water (e.g. Wang and Wolowyk 1988). Recent studies have demonstrated that the onset of non-seasonal daily torpor by small mammals and birds (e.g. Schmid et al. 2000; Körtner et al. 2001) and especially by bats can be initiated by food deprivation (Audet and Thomas 1997; Hickey and Fenton 1996; Racey and Swift 1981) and by low-ambient temperatures (Grinevitch et al. 1995). However, reproductive females have to balance energy savings through torpor with costs of decreased juvenile development. Juvenile development is depressed linearly, as the body temperature decreases (McNab 1982).
Assuming that the variance in T s with drops of 4–5°C below active temperature is not affected by T a (pregnant females in Fig. 2), reproductive female Daubenton’s bats used shallow daily torpor for short periods of time. Even such slight reduction in body temperature can already result in substantial energy savings by bats (Studier 1981; Webb et al. 1993). The same effect is also discussed for larger mammals like ungulates (Arnold et al. 2003).
Differences in the use of daily torpor by female and male bats were also described for Nearctic E. fuscus where free-living bats of both sexes had been tagged with temperature-sensitive radio-transmitters, as in our study. Both sexes went regularly into torpor but males used deeper torpor and were significantly more often torpid than reproductive females (Grinevitch et al. 1995; Hamilton and Barclay 1994). Lactating females were torpid significantly less often than pregnant and non-pregnant females (Audet and Fenton 1988; Chruszcz and Barclay 2002). Comparable results are described for pregnant and lactating females of European hedgehogs (Fowler 1988). It is reasonable that lactating females reduce time of torpor as much as possible because synthesis and secretion of milk is reduced with decreased body temperatures (Wilde et al. 1999). In contrast, assimilated energy requirement for lactation is much higher than for pregnancy (Kurta and Kunz 1988; Racey and Speakman 1987).
Besides shallow torpor, another strategy to compensate for energy demands during reproductive period is to cluster in nursery colonies (Kurta et al. 1987; Neuweiler 1993). Clustered females of M. lucifugus, for example, consumed only 19.9 ml oxygen per hour and per individual with a high-body temperature of 36.9°C. In contrast, solitary females needed 32.5 ml oxygen per hour with low-body temperature of only 32.2°C (Kurta 1986). In our study, we conclude that thermoregulation through sociality is an important factor that contributes to stable and warmer temperatures in the roosts for Daubenton’s bats. We found colonies of 20–40 Daubenton´s females during pregnancy and lactation, whereas the males mostly lived solitarily or in very small groups.
Selecting roosts with different qualities during reproductive season can also support the thermoregulatory behaviour of female bats. For instance, reproductive females of M. evotis and E. fuscus roosting in rock crevices chose roosts that differed in structure and thermal characteristics (Chruszcz and Barclay 2002; Lausen and Barclay 2003). Roosts used during lactation were more thermally stable and remained warmer at night compared to the shallow roosts used by pregnant and post-lactating females. Similar results were found in Myotis bechsteinii (Kerth et al. 2001). Females preferred tree roosts on colder nights especially before parturition and after weaning, whereas they favoured warm roosts in bat-boxes during the post-partum period. It is likely that the development of the young benefits from the warmer roosts. Female Daubenton’s bats in the “Philosopher-Forest” also switched between different roosts during the breeding season. However, we did not find obvious differences in the thermal properties of the tree holes used by Daubenton’s bats in our research area (unpublished data).
Thermoregulatory behaviour of female and male Daubenton’s bats changed significantly during post-lactation period after the juveniles had been weaned. During this time, females in our study used daily torpor more often and for longer time periods than males. This change suggests that males prepare for mating and therefore reduce periods of torpor to avoid the resulting negative impact on spermatogenesis (Enwistle et al. 1998; Jolly and Blackshaw 1987; Kurta and Kunz 1988). In our study area, sperm production started at the end of the lactation period. Epididymal filling continuously increased in July and August and reached its peak in the second half of September prior to the migration to the hibernacula (Encarnação et al. 2004).
We suppose that this period corresponds to the main mating season of Daubenton’s bats. Although copulations in Daubenton’s bats have only been observed in winter roosts (Grimmberger et al. 1987; Roer and Egsbaek 1969), we believe that it is likely that most copulation takes place already in late summer when the epididymes of males are fully developed and the animals are active. We found males with filled epididymes in tree roosts together with adult females at the end of July. Becoming torpid during this time would reduce copulation frequencies because adult females that have gained enough weight for hibernation already leave their breeding areas at the beginning of September to settle in the hibernation site (see Haarje 1994).
We conclude from our data on M. daubentonii that differences in reproductive status, in particular of females, explain the use of torpor better than exogenouos factors like ambient temperature or food deprivation alone. Our results on the female bats correspond well to those of other studies where the temperature behaviour of the nearctic E. fuscus was investigated (Audet and Fenton 1988; Grinevitch et al. 1995; Hamilton and Barclay 1994). As in female Daubenton’s bats, torpor was used to the greatest extent after weaning. But female E. fuscus also used daily torpor during pregnancy in response to ambient temperatures below 9°C.
We cannot exclude that this difference is partly due to low-sample size in comparison to studies on E. fuscus that have been examined in greater numbers. However, in our opinion, it is more probable that different results reflect the diverse feeding preferences and foraging strategies of the bat-species. For example, E. fuscus mainly feeds on Coleoptera, Hemiptera and Lepidoptera by aerial hawking in open spaces (e.g. Furlonger et al. 1987; Hamilton and Barclay 1998). For such prey, nightly availability has been shown to vary widely with ambient temperature (Kurtze 1974; Lewis and Taylor 1964). On colder nights below 10°C prey availability decreased and also the flight activity of bats foraging in open space (Hickey and Fenton 1996; Racey and Swift 1985). As a result, the energy intake on colder nights is lower than on warmer nights and so the use of torpor should be practised by individuals for whom the ratio of costs to benefits for thermoregulation was highest. In contrast to E. fuscus and other species, ambient temperature has no significant influence on flight duration and capture attempts of reproductive females of Daubenton’s bat (Dietz, in preparation). Moreover, tagged pregnant M. daubentonii hunted still at ambient temperatures below 5°C. Daubenton’s bats mainly feed on swarming chironomids (Beck 1995; Swift and Racey 1983), capturing them close to the water surface (Jones and Rayner 1988; Kalko and Schnitzler 1989). Apparently, insect density above water surfaces is less affected by short-term low-ambient temperatures because of the heat saving capacity of the water.
Assuming that M. daubentonii feeds more successfully during low-ambient temperatures compared to other species, this may compensate for the loss of energy to maintain high-body temperature. What is remarkable is that Reynolds and Kunz (2000) found changes in the gastrointestinal tract of lactating, ecologically similar females of the Neartic M. lucifugus, which suggest that increased food intake and assimilation are the primary factors with which bats compensate for the increased energy demand during pregnancy and lactation.
Overall, we conclude that bats in the temperate zone adapt their thermoregulatory behaviour primarily to their reproductive status and the influence of ambient temperature is specifically different for gender and species.
Abbreviations
- DEE:
-
Daily energy expenditure
- T a :
-
Ambient temperature
- T s :
-
Skin temperature
- Preg:
-
Pregnant
- Lac:
-
Lactating
- post-lac:
-
Post-lactating
References
Aldridge HDJN, Brigham RM (1988) Load carrying and maneuverability in an insectivorous bat: a test of the 5% “rule” of radiotelemetry. J Mammal 69:379–382
Anthony ELP (1988) Age determination in bats. In: Kunz TH (ed) Ecological and behavioural methods for the study of bats. London Smithsonian Institution Press, Washington DC, pp 1–28
Anthony ELP, Kunz TH (1977) Feeding strategies of the little brown bat, Myotis lucifugus, in southern New Hampshire. Ecology 58:775–786
Arlettaz R, Christe P, Lugon A, Perrin N, Vogel P (2001) Food availability dictates timing of parturition in insectivorous mouse-eared bats. Oikos 95:105–111
Arnold W, Ruf T, Reimoser S, Tataruch F, Onderschka K, Schober F (2003) Nocturnal hypometabolism as an overwintering strategy of red deer (Cervus elaphus). Am J Physiol Regul Integr Comp Physiol 286:174–181
Audet D, Fenton MB (1988) Heterothermy and the use of torpor by the bat Eptesicus fuscus (Chiroptera: Vespertilionidae): a field study. Physiol Zool 61:197–204
Audet D (1992) Roost quality, foraging and young production in the mouse-eared bat Myotis myotis: a test of the ESS model of group size selection. Phd. Dissertation, York University ON, Canada, pp 127
Audet D, Thomas DW (1996) Evaluation of the accuracy of body temperature measurement using external radio transmitters. Can J Zool 74:1778–1781
Audet D, Thomas DW (1997) Facultative hypothermia as a thermoregulatory strategy in the phyllostomid bats, Carollia perspicilliata and Sturnia lilium. J Comp Physiol B 167:146–152
Barclay RMR, Kalcounis MC, Crampton LH, Stefan C, Maarten JV, Wilkinson L, Brigham M (1996) Can external radiotransmitters be used to assess body temperature and torpor in bats? J Mammal 77:1102–1106
Barclay RMR, Lausen CL, Hollis L (2001) What’s hot and what’s not: defining torpor in free-ranging birds and mammals. Can J Zool 79:1885–1890
Beck A (1995) Fecal analyses of European bat species. Myotis 32/33:109–119
Coburn DK, Geiser F (1998) Seasonal changes in energetics and torpor patterns in the subtropical blossom-bat Syconycteris australis (Megachiroptera). Oecology 113:467–473
Chruszcz BJ, Barclay RMR (2002) Thermoregulatory ecology of a solitary bat, Myotis evotis, roosting in rock crevices. Func Ecol 16:18–26
Dietz M, Fitzenräuter B (1996) Zur Flugroutennutzung einer Wasserfledermauspopulation (Myotis daubentoni Kuhl, 1819) im Stadtbereich von Gießen. Säugetierkd Inf 4(20):107–116
Eisentraut M (1937) Die Wirkung niedriger Temperaturen auf die Embryonalentwicklung bei Fledermäusen. Biologisches Zentralbl 57:59–74
Encarnação J, Dietz M, Kierdorf U (2004) Reproductive condition and activity pattern of male Daubenton’s bats (Myotis daubentonii) in the summer habitat. Mamm biol 69:163–172
Enwistle AC, Racey PA, Speakman JR (1998) The reproductive cycle and determination of sexual maturity in male brown long.eared bats, Plecotus auritus (Chiroptera: Vespertilionidae). J Zool 244:63–70
Fowler PA (1988) Thermoregulation in the female hedgehog, Erinaceus europaeus, during the breeding season. J Reprod Fert 82:285–292
Furlonger CL, Dewar HJ, Fenton MB (1987) Habitat use by foraging insectivorous bats. Can J Zool 65:284–288
Geiser F, Ruf T (1995) Hibernation versus daily torpor in mammals and birds: physiological variables and classification of torpor patterns. Physiol Zool 68:935–966
Grimmberger E, Hackethal H, Urbanczyk Z (1987) Beitrag zum Paarungsverhalten der Wasserfledermaus, Myotis daubentoni (Kuhl, 1819), im Winterquartier. Zeitschr Säugetierkde 52:133–140
Grinevitch L, Holroyd SL, Barclay RM (1995) Sex differences in the use of daily torpor and foraging time by big brown bats (Eptesicus fuscus) during the reproductive season. J Zool 235:301–309
Hamilton IM, Barclay RMR (1994) Patterns of daily torpor and day-roost selection by male and female big brown bats (Eptesicus fuscus). Can J Zool 72:744–749
Hamilton IM, Barclay RMR (1998) Diets of juvenile, yearling, and adult big brown bats (Eptesicus fuscus) in southeastern Alberta. J Mammal 79:764–771
Haarje C (1994) Etho-ökologische Untersuchung der ganzjährigen Aktivität von Wasserfledermäusen (Myotis daubentoni Kuhl, 1819) am Winterquartier. Mitt Natf Ges Schaffhausen 39:15–52
Hickey MBC, Fenton MB (1996) Behavioural and thermoregulatory responses of female hoary bats, Lasiurus cinereus (Chiroptera: Vespertilionidae), to variations in prey availability. Ecoscience 3:414–422
Hosken DJ (1997) Thermal biology and metabolism of the greater long-eared bat, Nyctophilus major (Chiroptera: Vespertilionidae). Aust J Zool 45:145–156
Jolly SE, Blackshaw AW (1987) Prolonged epididymal sperm storage, and the temporal dissociation of testicular and accessory gland activity in the common sheat-tail bat. J Reprod Fert 81:205–211
Jones G, Rayner JMV (1988) Flight performance, foraging tactics and echolocation in free-living Daubenton’s bats Myotis daubentonii (Chiroptera: Vespertilionidae). J Zool 215:113–132
Kalko EKV, Schnitzler HU (1989) The echolocation and hunting behaviour of Daubenton’s bat, Myotis daubentoni. Behav Ecol Sociobiol 24:225–238
Kerth G, Weissmann K, König B (2001) Day roost selection in female Bechstein`s bats (Myotis bechsteinii): a field experiment to determine the influence of roost temperature. Oecology 126:1–9
Körtner G, Brigham RM, Geiser F (2001) Torpor in free-ranging tawny frogmouth (Podargus strigoides). Physiol Biochem Zool 74:789–797
Kunz TH (1974) Feeding ecology of a temperate insectivorous bat (Myotis velifer). Ecology 55:693–711
Kurta A (1986) Factors affecting the resting and postflight body temperature of little brown bats, Myotis lucifugus. Physiol Zool 59:429–438
Kurta A, Johnson KA, Kunz TH (1987) Oxygen consumption and body temperature of female little brown bats (Myotis lucifugus) under simulated roost conditions. Physiol Zool 60:386–397
Kurta A, Kunz TH (1988) Roosting metabolic rate and body temperature of male little brown bats (Myotis lucifugus) in summer. J Mammal 69:645–651
Kurta A, Bell GP, Nagy KA, Kunz TH (1989) Energetics of pregnancy and lactation in free-ranging Little Brown Bats (Myotis lucifugus). Physiol Zool 62:804–818
Kurta A (1990) Torpor patterns in food-deprived Myotis lucifugus (Chiroptera: Vespertilionidae) under simulated roost conditions. Can J Zool 69:255–257
Kurtze W (1974) Synökologische und experimentelle Untersuchungen zur Nachtaktivität von Insekten. Zool Jb Syst 101:297–344
Lausen CL, Barclay RMR (2003) Thermoregulation and roost selection by reproductive female big brown bats (Eptesicus fuscus) roosting in rock crevices. J Zool 260:235–244
Lewis T, Taylor LR (1964) Diurnal periodicity of flight insects. Proc Trans R Ent Soc Lond 116:393–476
McLean JA, Speakman JR (2000) Effects of body mass and reproduction on the basal metabolic rate of brown-long-eared bats (Plecotus auritus). Physiol Biochem Zool 73:12–21
McNab BK (1982) Evolutionary alternatives in the physiological ecology of bats. In: Kunz TH (ed) Ecology of bats. Plenum Publishing, London New York, pp 151–197
Neuweiler G (1993) Biologie der Fledermäuse. Thieme, Stuttgart New York
Prinzinger R, Göppel R, Lorenz A, Kulzer E (1981) Body temperature and metabolism in the Red-backed Mousebird (Colius castanotus) during fasting and torpor. Comp Biochem Physiol 69A:689–692
Racey PA (1973) The viability of spermatozoa after prolonged storage by male and female European bats. Period Biol 75:201–205
Racey PA, Swift SM (1981) Variations in gestation length in a colony of pipistrelle bats (Pipistrellus pipistrellus) from year to year. J Reprod Fert 61:123–129
Racey PA (1988) Reproductive assessement in bats. In: Kunz TH (eds) Ecological and behavioural methods for the study of bats. Smithsonian Institute Press, Washington DC, pp 31–46
Racey PA, Tam WH (1974) Reproduction in male Pipistrellus pipistrellus (Mammalia: Chiroptera). J Zool 172:101–122
Racey PA, Swift SM (1985) Feeding ecology of Pipistrellus pipistrellus (Chiroptera: Vespertilionidae) during pregnancy and lactation. I. Foraging behaviour. J Anim Ecol 54:205–215
Racey PA, Speakman JR (1987) The energy costs of pregnancy and lactation in heterothermic bats. Symp Zool Soc Lond 57:107–125
Ransome R (1968) The distribution of the greater horseshoe bat, Rhinolophus ferrumequinum, during hibernation, in relation to environmental factors. J Zool 154:77–112
Reynolds DS, Kunz TH (2000) Changes in body composition during reproduction and postnatal growth in the Little Brown bat, Myotis lucifugus (Chiroptera: Vespertilionidae). Ecoscience 7(1):10–17
Roer H, Egsbaek W (1969) Über die Balz der Wasserfledermaus (Myotis daubentoni) (Chiroptera) im Winterquartier. Lynx 10:85–91
Rydell J (1992) Occurrence of bats in northernmost Sweden (65°N) and their feeding ecology in summer. J Zool 227:517–529
Schmid J (2000) Torpor in the tropics: the case of the gray mouse lemur (Microcebus murinus). Basic Appl Ecol 1:133–139
Schmid J, Ruf T, Heldmaier G (2000) Metabolism and temperature regulation during daily torpor in the smallest primate, the pygmy mouse lemur (Microcebus myoxinus) in Madagascar. J Comp Physiol 170:59–68
Studier EH (1981) Energetic advantages of slight drops in body temperature in little brown bat, Myotis lucifugus. Comp Biochem Physiol A 70:537–540
Swift SM, Racey P (1983) Ressource partitioning in two species of vespertilionid bats (Chiroptera: Vespertilionidae) occupying the same roost. J Zool 200:249–259
Thomas DW, Dorais M, Bergeron JM (1990) Winter energy budgets and cost of arousals for hibernating Little Brown bats, Myotis lucifugus. J Mammal 71:475–479
Wang LCH, Wolowyk MW (1988) Torpor in mammals and birds. Can J Zool 66:133–137
Webb PI, Speakman JR, Racey PA (1993) The implication of small reductions in body temperature for radiant and convective heat loss in resting endothermic brown long-eared bats (Plecotus auritus). J Therm Biol 18:131–135
White GC, Garrott RA (1990) Analysis of wildlife radio-tracking data. Academic Press, San Diego
Wilde CJ, Kerr MA, Knight CH, Racey PA (1995) Lactation in vespertilionid bats. Symp Zool Soc Lond 67:139–149
Wilde CJ, Knight CH, Racey PA (1999) Influence of torpor on milk protein composition and secretion in lactating bats. J Exp Zool 284:35–41
Willis CKR, Brigham RM (2003) Defining torpor in free-ranging bats: experimental evaluation of external temperature-sensitive radiotransmitters and the concept of active temperature. J Comp Physiol B 172:379–389
Acknowledgements
The authors gratefully acknowledge the help of all the people who supported the time intensive field-work. We are particularly grateful to Dr. Jutta Schmid, Dr. Jorge Encarnação, Sabine Springer and two anonymous referees, who greatly improved the manuscript by valuable comments. Handling of Daubenton’s bats was done under licence from the nature conservancy department of the Regierungspräsidium Gießen.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier
Rights and permissions
About this article
Cite this article
Dietz, M., Kalko, E.K. Seasonal changes in daily torpor patterns of free-ranging female and male Daubenton’s bats (Myotis daubentonii). J Comp Physiol B 176, 223–231 (2006). https://doi.org/10.1007/s00360-005-0043-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-005-0043-x