Abstract
A variety of definitions involving body temperature (T b), metabolic rate and behavior have been used to define torpor in mammals and birds. This problem is confounded in some studies of free-ranging animals that employ only skin temperature (T sk), a measure that approximates but may not precisely reflect T b. We assess the accuracy of T sk in the context of a recent definition for torpor called active temperature. We compared the active temperatures of individual big brown bats (Eptesicus fuscus), which aggregate in cavities, with solitary, foliage-roosting hoary bats (Lasiurus cinereus). In captive big brown bats, we compared T sk and core T b at a range of ambient temperatures for clustered and solitary roosting animals, compared T sk and T b during arousal from torpor, and quantified the effect of flight on warming from torpor. Hoary bats had significantly lower active temperatures than big brown bats despite having the same normothermic T sk. T sk was significantly lower than T b during normothermia but often greater than T b during torpor. Flight increased the rate of warming from torpor. This effect was more pronounced for T sk than T b. This suggests that bats could rely on heat generated by flight muscles to complete the final stages of arousal. Using active temperature to define torpor may underestimate torpor due to ambient cooling of external transmitters or animals leaving roosts while still torpid. Conversely, active temperature may also overestimate shallow torpor use if it is recorded during active arousal when shivering and non-shivering thermogenesis warm external transmitters. Our findings illuminate the need for laboratory studies that quantify the relationship between metabolic rate and T sk over a range of ambient temperatures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many mammals and some birds lower their body temperature (T b) setpoint and metabolic rate (MR) to offset thermoregulatory costs during periods of cold ambient temperature (T a) and or food shortage (Wang and Wolowyk 1988; Wang 1989). This heterothermy or torpor can save animals up to 99% of their daily energy requirements and, thus, is of tremendous importance for short- and long-term energy budgets (Wang 1989). Heterothermy is traditionally divided into hibernation (multi-day bouts) and daily torpor (bouts restricted to a single circadian cycle) (Geiser and Ruf 1995). In both situations, laboratory studies have revealed much about torpor patterns and associated energy savings by direct measurement of oxygen consumption or MR and T b based on rectal temperature or using surgically implanted temperature dataloggers or temperature-sensitive radiotransmitters (e.g., Geiser et al. 1996; Geiser and Brigham 2000; Ortmann et al. 1996). However, torpor patterns may differ markedly between free-ranging and captive (Geiser et al. 2000) or captive-bred individuals (Geiser and Ferguson 2001) and metabolic rates are logistically difficult to measure in the field (although see Schmid 1996). To address questions regarding the physiological ecology of torpor, including its proximate energetic benefits and ultimate selective implications, field studies that quantify energy expenditure in free-ranging heterotherms are required. For example, how much time do animals spend torpid under different natural conditions? What is the level of energy saving associated with an individual's use of torpor in the wild? How do inter- and intra-specific differences in biology and life history influence torpor use? These questions require an accurate measure of T b and, more importantly, inference about MR in free-ranging animals.
For relatively large animals, dataloggers or radiotransmitters can be surgically implanted to measure T b in the field (e.g., Barnes 1989). Body size and reception range limitations of implanted transmitters mean that studies of small heterotherms typically rely on a measure of skin temperature (T sk). In free-ranging bats and birds T sk is measured using external temperature-sensitive radiotransmitters affixed dorsally between the scapulae (e.g., Brigham 1992; Hamilton and Barclay 1994; Hickey and Fenton 1996; Brigham et al. 2000; Chruszcz and Barclay 2002). Few studies have rigorously evaluated T sk as a measure of T b. Audet and Thomas (1996) found that T sk and T b (measured rectally) were similar, but that T a influenced the relationship between T b and T sk with differences as high as 6 °C even at relatively high T a (i.e., >21 °C). Likewise, Barclay et al. (1996) demonstrated a correlation between T sk and T b but also found significant differences between T b and T sk particularly at low T a, presumably due to ambient cooling of external transmitters. Brigham et al. (2000) reported a strong correlation between T b and T sk in Australian Owlet Nightjars (Aegotheles cristatus) but noted instances where T b and T sk differed by as much as 6 °C (see Fig. 1 in Brigham et al. 2000). In an analysis based on over 11,000 measurements (from one individual), T b explained only 85% of the variation in T sk.
To date, defining a reliable boundary between torpor and normothermy (i.e., periods when an endotherm's core temperature is within ±1 SD of the range associated with the species' post-absorptive, resting thermoneutral zone; IUPS Thermal Commission 2001) has proven challenging in both the laboratory and field (Barclay et al. 2001). Traditionally an arbitrary temperature or behavioral boundary is used to delineate when animals are "in" and "out" of torpor. The accuracy of such a boundary is important because initial reductions in T b (e.g., from 35 °C to 30 °C) save animals more energy than reductions of the same increment at lower T b (e.g., from 25 °C to 20 °C; Studier 1981). Shallow torpor is likely of greatest energetic and ecological significance but is easy to overlook. In the laboratory, MR can be measured directly and the point at which metabolism declines can be identified. However, even in laboratory studies many different definitions of torpor have been used. Some studies employ specific T bs (e.g., 30 °C), while others rely on behavior to discriminate between torpor and normothermia (Barclay et al. 2001).
In a review of recent studies of topor in mammals and birds, Barclay et al. (2001) propose "active temperature" (T act) as a standard means of defining torpor. This method generates an individual-specific definition of torpor based on T b or T sk recorded in the field. T b or T sk is recorded each day at a time when individuals are assumed to be active (e.g., within 10 min of dusk emergence). To ensure a conservative estimate of torpor use, the lowest T sk at dusk for all the days a bat carries its transmitter is termed T act. This temperature is then considered the threshold below which an individual is assumed to be torpid. This method helps to control for variation resulting from differences in contact between a transmitter's temperature sensor and an animal's skin, slight differences in transmitter calibration, and individual and population variation in the relationship between T b and T sk (Barclay et al. 2001).
While the method of Barclay et al. (2001) is a relatively rigorous means to define torpor, it depends on a number of untested assumptions. The use of T act assumes that the relationship between T sk and T b is constant; this may not be true for a number of reasons. First, decreasing air temperatures at dusk could increase ambient cooling of transmitters and thus reduce T act and, therefore, the chance of detecting shallow torpor bouts. Second, transmitters must be attached to bats dorsally between the scapulae, the site of brown adipose tissue (BAT) storage (Eckert et al. 1988) and large flight muscles. During periods of active rewarming at dusk, T sk could overestimate T b because heat generated by BAT metabolism or flight muscle shivering warms the transmitter. Third, differences in roost structure or roosting behavior could influence the relationship between T b and T sk, and alter the measure of T act. Many mammals roost communally to reduce heat loss (e.g., Kunz 1982); thus T sk recorded for an individual may be affected by its proximity to roost mates. Fourth, and most fundamentally, T act assumes that animals are normothermic prior to becoming active. Some flying animals are capable of powered flight at T bs below normothermia, as defined above (Austin and Bradley 1969; Bradley and O'Farrell 1969; this study), and motor systems of bats remain active over a wide range of T b (Choi et al. 1998). Conceivably, bats and birds could leave roosts prior to complete rewarming and rely on heat generated by flight muscle activity to complete the final stages of warming. If this were the case, T act and the use of shallow torpor bouts would be underestimated.
Our objectives were to test the above assumptions and compare T sk to T b over a range of conditions. We used captive and free-ranging big brown bats (Eptesicus fuscus), free-ranging hoary bats (Lasiurus cinereus) and temperature telemetry in the field and laboratory to address four specific questions:
-
1.
Do active temperatures of free-ranging, solitary, foliage-roosting bats approximate those recorded for colonial, cavity-roosting bats?
-
2.
Does T sk approximate T b over a range of T a, during torpor, active warming from torpor, and normothermia?
-
3.
Does clustering behavior influence the relationship between T b and T sk?
-
4.
Are bats capable of flight at T bs below normothermia and, if so, does flight increase the rate of rewarming?
Materials and methods
Study animals
All methods were in accordance with the Canadian Council for animal care and were approved by the University of Regina President's Committee on Animal Care. For free-ranging bats, we compared T act of big brown bats to those for hoary bats. These species are well suited to this comparison because of known differences in roosting behavior. In our study area, big brown bats form maternity colonies in cavities of trembling aspen (Populus tremuloides; Kalcounis and Brigham 1998; Willis et al. 2003) while hoary bats roost solitarily, exposed in the open foliage of white spruce (Picea glauca; Willis 2003).
Active temperature
Fieldwork took place during the summers of 2000 and 2001 in the West Block of Cypress Hills Provincial Park, Saskatchewan, Canada (49°34′N, 109°53′W; see Sauchyn 1993 for description). The region is well suited to this study because it is characterized by dramatic diurnal fluctuations in T a.
We captured bats in mist nets set at foraging areas or at roosts. Fur was clipped between the shoulders and temperature-sensitive radio-transmitters (0.7 g BD-2AT for 19.6±2.6 g, range: 17.3–23.7 g big brown bats; 1.05 g BD-2T for 27.8±5.84 g, range: 20.1–34.5 g hoary bats, Holohil Systems Carp, ON Canada) were affixed using surgical cement (Skin-Bond, Smith and Nephew, Largo, Fla., USA). Transmitter mass represented less than 5% of each free-ranging bat's body mass (Aldridge and Brigham 1988). We released bats within several hours of capture and followed them to roost trees on as many days as possible using hand-held telemetry receivers (R-1000, Communication Specialists, Calif., USA) and 5-element yagi antennas (AF Antronics, Urbana Ill., USA). We used datalogging radiotelemetry receivers (SRX-400, Lotek Wireless, Newmarket, ON Canada) to record inter-pulse intervals of transmitter signals every 15 min. The receivers converted inter-pulse intervals into T sk values based on transmitter-specific calibration curves provided by the manufacturer, which were verified before transmitters were used.
We defined T act following Barclay et al. (2001). For each bat, on as many nights as possible, we identified the T sk recorded immediately prior to dusk departure and the lowest dusk departure temperature obtained during the life of a bat's transmitter was defined as T act. We identified dusk departure based on the variance in signal strength recorded by the datalogging receiver. We compared T act between hoary bats and big brown bats. We also compared resting T sk between species based on the mean value of all T sk greater than 32 °C. We selected 32 °C, because it fell between the T act of the two species and because it is higher than the lower critical temperature reported in the literature for hoary bats (Genoud 1993). Hence, 32 °C likely represents a normothermic T sk for this species. We only included bats in this analysis for which we recorded a dusk departure T sk on at least three nights.
Effect of T a, torpor and clustering on T b vs. T sk
To compare T b to T sk in the laboratory, we captured ten big brown bats as they emerged from a tree cavity at dusk on 9 June 2001. The bats were kept in cloth-lined wire cages at the University of Regina Biology Station, given ad lib water and handfed mealworms coated with powdered canine vitamin/iron supplement with a high essential fatty acid content (Vi-sorbits, SmithKline Beecham Animal Health, Westchester, Pa., USA). We kept cages outdoors but protected them from the elements in large, plastic containers (Rubbermaid, Wooster, Ohio, USA) with the lids elevated 20 cm above the rim on wooden stakes to increase ventilation. We attached temperature-sensitive radiotransmitters as described above (0.75 g BD-2AT, Holohil Systems). A second temperature-sensitive transmitter was surgically implanted (0.75 g BD-2ATH, Holohil Systems) into the intraperitoneal cavity of each bat under inhalant anesthesia (Isoflurane USP, Abbot Lboratories, Montreal, QC Canada). The combined mass of both transmitters represented between 6.3% and 8.7% of body mass, which exceeds Aldridge and Brigham's (1988) 5% rule for telemetry studies of flying animals. However, bats were not required to fly while in captivity and both internal and external transmitters were removed before bats were released at the end of the experiment.
We did not begin data collection until 1 week after implantation to allow bats to recover from surgery. Temperature dataloggers (iButton, Dallas Semiconductor, Dallas, Tex., USA) were used to record T a in the cages. For the first 5 days of the experiment, all bats were housed in a single cage (clustered) and were then put in separate cages for an additional 5 days (solitary). During the clustered treatment, we did not observe bats continuously but all were found clustered with at least one other individual when they were removed for feeding each day. T sk and T b of all bats was calculated concurrently based on signals from the external and implanted transmitters, respectively, using the datalogging radiotelemetry receiver described above.
Ideally, repeated measures analyses are most appropriate for our data (Zar 1999). However, premature failure of a number of transmitters reduced the sample size and precluded this analysis. Instead, based on each bat's temperature time course, we identified bouts of steady-state normothermia and steady-state torpor and analyzed the difference between T b and T sk for each. We excluded periods of warming and cooling and defined a bout of steady-state normothermia as any period greater or equal to 30 min duration (two sampling periods) during which a bat's T sk remained above 33.6 °C (mean T act for free-ranging big brown bats found in this study), and bouts of steady-state torpor as any period >30 min duration when a bat's T sk was within ±3 °C of the minimum temperature for that bout. We chose this value based on qualitative inspection of temperature time courses, which revealed that following changes in T sk greater than 3 °C, bats tended to continue warming or cooling. We calculated mean T b, T sk, and T a values for each bout of torpor and normothermia for each bat. We removed inter-individual autocorrelation effects by calculating a group mean T b for each treatment group. In other words we calculated four T b values: one for all normothermic clustered bats, one for all normothermic solitary bats, one for all torpid clustered bats and one for all torpid solitary bats. An individual's T sk for each bout of normothermia or torpor was then subtracted from the treatment-group-specific T b value to obtain a measure of the difference between T sk and T b. We used one-sample Bonferroni-adjusted T-tests to determine if the difference between the treatment-group-specific T b and individual T sk differed from zero. To determine the influence of T a, we also used a 2-factor ANCOVA, with T a as a covariate, to compare the dependent variable (difference between treatment group T b and individual T sk) between the factors normothermia vs. torpor and clustered vs. solitary roosting.
Effect of flight on T b vs. T sk
During late August 2000, we captured four big brown bats emerging from an aspen cavity in the Cypress Hills and transported them to the laboratory at the University of Regina. In September and October, we obtained four additional big brown bats from buildings in Regina. All bats were housed for the winter in cloth-lined wire cages, fed as described above and maintained at 18 °C and 12L:12D photoperiod. Additionally they were exercised in a flight room for ca. 20 min, 3–4 times per week. Bats were always torpid when removed from cages prior to feeding and re-warmed prior to being fed. Flight experiments were not performed until all bats were able to fly continuously in the flight room.
We conducted flight experiments over a 2-week period in April 2001. Bats were placed in a refrigerator for several hours prior to flight trials to ensure they were torpid (T sk<18 °C). During the warm-up period we measured T sk and T b at 2-min intervals using a teflon coated J-type thermocouple probe and thermometer (Model 600–1040, Barnant Company, Barrington, Ill., USA). To maintain consistency with the T sk data recorded using temperature telemetry in the field, we recorded T sk by holding the thermocouple wire in contact with the skin between the scapulae. We used rectal temperature as our measure of T b, following Barclay et al. (1996), by inserting the thermocouple probe 6 mm into the rectum. Each bat underwent a control trial when they were not induced to fly between sampling periods, and a flight trial when bats were dropped from a height of 1.5 m above a padded floor between each sampling period. Both control and flight trials were performed in a 21°C flight room. For each bat, we chose trial order (i.e., flight trial vs. control first) randomly. The two trials for each individual were separated by at least 4 days.
All bats underwent 2–3 practice trials 3–4 weeks prior to data recording to familiarize them with the flight trial protocol. During practice trials we devised a flight scoring system based on the ability of bats to fly in the flight room (Table 1). During experimental trials we used this system to score each flight attempt and recorded T b and T sk after each attempt. This allowed us to compare temperatures recorded when we knew that bats were capable of flying with T act recorded using temperature telemetry in the field.
Statistical analysis
We report values as means ±SD unless otherwise specified. All analyses were performed using Systat Version 9 (SPSS 1998) with significance for all tests assessed at P<0.05. For repeated measures analyses, conservative Greenhouse-Geiser and Huynh-Feldt adjusted P-values were equal and these are reported. All repeated measures analyses met homogeneity of slopes assumptions (Zar 1999).
Results
Active temperature
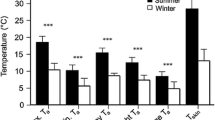
For free-ranging bats, the mean T act of eight colonial, cavity-roosting big brown bats was significantly higher than that of ten solitary, foliage-roosting hoary bats (Fig. 1A; T-test, T [8,10]=6.65, P<0.001). However, there was no difference between normothermic T sk (i.e., T sk>32 °C; Fig. 1B; T-test, T [8,10]=1.33, P=0.21).
A Box plots of hoary (n=10) and big brown bat (n=8) active temperatures recorded using temperature telemetry. Boxes represent quartile ranges, whiskers represent 5th and 95th percentiles and lines inside the boxes represent median values. B Box plots of resting normothermic T sk for the same individuals
Effect of T a, torpor, and clustering on T b vs. T sk
We found a significant difference between T b and T sk for normothermic bats that was significantly influenced by T a. We also found a significant difference between T b and T sk during active warming from torpor but found that clustering had no effect on the relationship between T b and T sk. Qualitatively, for big brown bats held in outdoor cages, T sk differed from T b, particularly at temperatures near the T act of free-ranging individuals (i.e., 30–35 °C), as well as during torpor. We plotted T sk vs. T b for each bat and these relationships approximated but did not match a 1:1 relationship (Fig. 2). We did not use linear regression to analyze relationships between T b and T sk for individual big brown bats because intra-individual autocorrelation would dramatically increase the probability of Type I error. Therefore, r 2 values are not reported. A typical time course of T b, T sk, and T a indicates the source of some of this variation (Fig. 3). T sk was typically lower than T b when bats were normothermic but usually exceeded T b at low T a when bats were torpid. T sk also typically exceeded T b early in periods of active warming from torpor; for example, at sunset (ca. 22:00) when bats re-warmed (Fig. 3).
Time course of T b (circles), T sk (triangles) and T a (solid line) obtained over 2 days from one individual big brown bat housed in an outdoor cage. Note that T sk typically exceeded T b during active warming bouts at dusk (ca. 22:00) and during torpor at very low T a, but that T b exceeded T sk during normothermia
In the T b vs. T sk experiment, we recorded eight bouts of spontaneous arousal from torpor by each of four individuals. All arousals were complete within three sampling periods (i.e., 45 min) or less. Overall, T sk warmed significantly more slowly than T b (Table 2; Paired T-test, T=−2.42, df=7, P=0.046). However, during the first 15 min of warming, T sk increased significantly more rapidly than T b (Table 2; Paired T-test, T=2.42, df=7, P=0.046).
We tested whether clustering and torpor affected the difference between treatment group-specific T b and individual T sk using Bonferroni-adjusted one sample T-tests. T b was significantly greater than T sk for clustered normothermic (Fig. 4; T=3.51, df=18, P=0.01) and solitary normothermic bats (T=6.94, df=7, P=0.001), but T b and T sk were not significantly different for clustered torpid (T=−2.17, df=10, P=0.22) or solitary torpid bats (T=−2.54, df=6, P=0.18). The difference between T b and T sk during torpor was highly variable, however (Fig. 4), and we recorded T sk values as much as 9.5 °C higher than T b during deep torpor at low T a.
Differences between treatment group T b and individual T sk for ten big brown bats. The data were divided into four groups: Clustered normothermic (ClustNorm, n=19 bouts), solitary normothermic (SolNorm, n=8 bouts), clustered torpid (ClustTorp, n=11 bouts), and solitary torpid (SolTorp, n=7 bouts). Values greater than zero (dashed line) indicate that T b exceeded T sk and values less than zero indicate that T sk exceeded T b
To test for the effect of clustering and T a on the difference between T b and T sk we used ANCOVA, with T a as the covariate, normothermia vs. torpor and roosting treatment (clustered vs. solitary) as factors, and the difference between treatment-group-specific T b and individual T sk as the dependent variable. The effect of T a was highly significant (F [1,40]=17.66, P<0.001) and the model was highly significant for effects of torpor vs. normothermia (Fig. 4; F [1,40]=62.12, n=27,18, P<0.001). There was no effect of clustered vs. solitary roosting (F [1,40]=0.10, P=0.75).
Effect of flight on T b vs. T sk
Captive big brown bats were capable of flight at low T b (29.2±1.1 °C) and flight significantly increased the rate of warming from torpor. To test for the influence of flight and T sk vs. T b on recorded temperature, we used a 3-factor repeated measures ANOVA with flight treatment, temperature treatment, and capture location as factors, and measured temperature as the dependent variable. The model was significant for between-subject effects of all three factors (flight treatment: F [1,18]=15.6, P<0.001; temperature treatment: F [1,18]=35.4, P<0.001; capture location: F [1,18]=14.4, P<0.001). There were significant within-subject/between-subject interactions between the repeated measure (i.e., time) and flight treatment (F [8,144]=7.46, P<0.001), temperature treatment (F [8,144]=10.43, P<0.001), and location (F [8,144]=4.92, P<0.001). This means that warming patterns of bats over the course of flight trials were influenced by all three factors (Fig. 5). We did not include body mass as a covariate in this analysis because mass was not significantly different between bats from the two study locations (Mann Whitney U-Test, U=9, n=4,4, P=0.56) and body mass did not influence arousal rates (see below).
Warm-up rates of T b and T sk for bats used in the flight experiments were greater than those recorded for bats housed in out-door cages (Table 3). We calculated the mean warm-up rate for each bout and also divided each bout in half (based on the duration of the bout) and calculated warm-up rates during the first- and second-halves of each arousal. To be conservative, we further divided these data by capture location, because of the significant difference between Cypress Hills and Regina bats. We compared mean, first-half, and second-half warm-up rates individually using two-factor blocked ANOVAs, treating each bat as a block and flight treatment (flight or control), and temperature treatment (T sk vs. T b) as factors. Flight significantly increased mean warm-up rate for bats from both locations but there were no significant differences between mean T b and T sk warm-up rates (Table 4). For the first half of rewarming bouts, flight significantly increased warm-up rate for bats from both capture locations. In contrast to mean warm-up rates, T sk warmed significantly more quickly than T b in the first half of warming bouts (Table 4). During the second half of warming, T b warmed significantly more quickly than T sk for bats from both locations (Table 4). Flight significantly increased warm-up rate for Regina bats but not for bats from the Cypress Hills during the second half of warming (Table 4). Overall, although absolute warming rates were faster for bats used in the flight experiment than for bats in the T b vs. T sk experiment, the pattern of more rapid initial warming rates for T sk relative to T b was consistent between experiments. When these analyses were repeated controlling for body mass by dividing each bat's warm-up rate by body mass, there was no change in the significance of any test. Therefore, we report analyses based on absolute and not mass-specific warm-up rates.
For flight trial data, we compared the dependent variable, measured temperature, at different flight scores using a 2-factor repeated measures ANCOVA with T sk vs. T b, and capture location (i.e., Regina vs. Cypress Hills) as factors, and flight (i.e., first and best; scores of 2 and 6 respectively) as the repeated measure. We included body mass as a covariate in this analysis because of the potential influence of mass on flight performance. There was a significant three-way interaction between the repeated measure (flight), capture location and T b vs. T sk (F [1,11]=7.11, P=0.022; Fig. 6). Several factors account for this interaction. First, T sk was consistently greater than T b but this effect was more pronounced at flight scores of 2 compared to scores of 6. Second, the T b–T sk differential was much greater for Regina bats than for Cypress Hills bats for flight scores of 2 but not 6. There was also a significant interaction between the repeated measure, flight, and the covariate body mass (F [1,11]=15.23, P=0.002) but when we subdivided the data by flight score and T b vs. T sk, the only significant relationship was a positive effect of body mass on the T b required for best flight (i.e., score=6; Fig. 7, Linear regression, F=26.19, r 2=0.81, n=8, P=0.02). There was no significant effect of body mass on T sk at best flight (Fig. 7, F=2.71, n=8, r 2=0.31, P=0.15), T b at first flight (F=0.12, n=8, r 2=0.02, P=0.74), or T sk at first flight (F=0.82, n=8, r 2=0.12, P=0.40).
We compared T act of free-ranging big brown bats to the skin temperatures associated with first and best flight for captive Cypress Hills big brown bats using ANCOVA with body mass as a covariate. T sk recorded when bats flew best (i.e., flight score=6, 41.3±2.1 °C) were significantly higher than T act recorded in the field (33.6±1.7 °C, F [1,9]=41.70, P<0.001). The T sk of bats first capable of flight (i.e., flight score=2, 33.6±2.0 °C) were not significantly different from T act (F [1,9]=0.001, P=0.98).
Discussion
The objectives of our study were to evaluate assumptions about using "T act" as the threshold between torpor and normothermia for free-ranging endotherms and to compare T sk with T b under a range of conditions. This is the first study to report concurrent measurements of T sk and T b in mammals, both recorded using temperature-sensitive radiotransmitters, and to report concurrent T sk and T b warm-up rates.
Active temperatures
We found that the T act of free-ranging hoary bats was significantly lower than that of big brown bats despite no difference between their resting normothermic T bs. The T act of hoary bats we recorded (28.6±1.5 °C) was also lower than the resting normothermic rectal temperature (34.8 °C) and lower critical temperature (31 °C) measured in laboratory trials (Genoud 1993). Low T a at dusk could mean greater ambient cooling of external transmitters on foliage roosting, solitary hoary bats relative to cavity-roosting, clustered big brown bats. Alternatively, if hoary bats are capable of powered flight at low Tb they could be more likely to leave their roosts while still rewarming, using heat produced as a by-product of flight muscle activity to complete the final stages of warm-up. In either case, the low T sk active temperatures of hoary bats clearly underestimate normothermic T b. This has dramatic consequences for estimates of the importance of shallow torpor to overall energy budgets. For example, in a 9 g little brown bat (Myotis lucifugus), a reduction in T b from 34.8 °C to 28.6 °C at T a=20 °C reduces energy expenditure by ca. 64% (ca. 0.80 kJ/h to 0.29 kJ/h; Studier 1981). Assuming similar rates for ca. 30 g hoary bats, in the absence of data from the literature, the active temperature we found could underestimate energy savings of shallow torpor by 1.70 kJ/h (2.65 kJ/h minus 0.95 kJ/h; Studier 1981). Acknowledging that hoary bat and little brown bat mass-specific metabolic rates will differ, T act will nonetheless compromise our ability to accurately assess torpor in the field.
Effect of Ta, torpor and clustering on T sk vs. T b
We found that the relationship between T b and T sk differed significantly, depending on T a and on whether or not bats were torpid. Consistent with previous research, we found that during normothermia T sk underestimated T b by up to 6 °C (Audet and Thomas 1996; Barclay et al. 1996). Our results are not surprising given that T a was typically cooler than T b during normothermic bouts, which likely cooled external transmitters. Such large differences present a problem for studies that employ T sk to infer metabolic savings. Again using equations derived by Studier (1981) for little brown bats, underestimating T b by 6 °C, from 30 °C to 24 °C at a T a of 20 °C, would lead to underestimating energy expenditure by ca. 79% (0.84 vs 0.18 kJ/h) for a 20-g bat. The ambient effect is also problematic since it likely occurs at sunset when T act is recorded. Underestimating T b at sunset likely overestimates energy expenditure because it will reduce T act and therefore conceal the importance of shallow torpor to the overall energy budget. T act is sensitive to ambient effects because the lowest dusk departure T sk or T b is adopted as the torpor threshold. For example, using T act may lead to the conclusion that, when T a at sunset is relatively low (e.g., early in the season), bats use less shallow torpor than during the warmest months of the year, simply because of differences in ambient cooling effects.
Consistent with Barclay et al. (1996), we found that T b and T sk were not significantly different during torpor. However, for bats in deep torpor at low T a, T sk exceeded T b by as much as 9.5 °C (Fig. 3). This could reflect two effects of metabolic heat production to defend a reduced T b setpoint while torpid. First, brown fat metabolism could warm external transmitters. Brown fat is stored dorsally, between the shoulders in many mammals (Eckert et al. 1988), the best site for transmitter attachment. Second, also due to the position of transmitters, shivering thermogenesis of the large dorsal flight muscles could further elevate T sk. This is most likely at torpid T bs of <20–25 °C when skeletal muscle is warm enough to shiver (Fons et al. 1997; Choi et al. 1998). Differences in the warm-up rates of T b and T sk (Table 2) support the hypothesis that brown fat and/or flight muscle shivering cause disproportionate warming of external transmitters. Over the course of individual warming bouts, T b warmed more quickly than T sk, but during the first 15 min of warming T sk increased significantly more rapidly than T b, likely due to brown fat metabolism early in the warming bout. Overall, warm-up rates of big brown bats in our experiment were comparable to those reported for bats of similar size (e.g., Nyctimene albiventer, 28.8 g, 0.50 °C/min; Bartholomew et al. 1970) and are consistent with allometric predictions of warm-up rates for a ca. 20-g mammal (Stone and Purvis 1992).
T b and T sk did not differ between periods when bats were housed colonially or in isolation. Thus, for colonial species, external transmitters reliably measure T sk of the individual despite the potential for clustering to influence transmitter temperature. Indeed most temperature-sensitive radiotransmitters are affixed with the temperature sensor in direct contact with the skin. This supports the use of T sk as a measure of T b when comparing thermoregulation in solitary and colonial roosting species if other sources of variation, such as ambient cooling, are controlled. This result also reinforces our comparison of T act between hoary bats and big brown bats. The variation in the relationship between T b and T sk, however, suggests that considerable caution is required when defining torpor in the field based on T sk, and particularly when comparing species. Comparison between mammals, which have brown fat, and birds, which do not (Eckert et al. 1988; Saarela et al. 1991), may be particularly challenging.
Effect of flight on T b vs. T sk
Comparison of overall warming rates revealed no significant difference between T b and T sk. However, this analysis concealed the fact that T b and T sk warming rates differed significantly at different stages of warm-up; T sk was greater than T b during the first half of warming while T b was greater than T sk during the second half. This corroborates our results for bats in outdoor cages and supports the hypothesis that brown fat metabolism and/or shivering thermogenesis could elevate T sk early during the warming phase. It also highlights a potential source of error associated with the use of active temperature. If T act is recorded during a warming bout just prior to dusk departure then T sk could overestimate T b resulting in an overestimate of T act and torpor.
Flight significantly increased T b and T sk during the first half of warming bouts and for overall warming bouts. This is almost certainly due to heat released as a by-product of flight muscle activity meaning that bats could leave roosts at T bs below normothermia. We argue that bats may exploit heat generated during flight to complete the final stages of warming. The costs of rewarming constrain total energy savings during torpor. Therefore, selection should favor mechanisms that reduce warming costs. This hypothesis is supported by the comparison of field T acts with temperatures recorded during flight trials. Active temperatures of free-ranging bats were significantly lower than T sks associated with normal flight in the lab but not different than T sks associated with initial straight-line flight. Bats were capable of straight-line flights at a T b well below most definitions for torpor (Barclay et al. 2001). Previous studies have demonstrated "good flight" by Poorwills (Phalaenoptilus nuttalli) at T bs as low as 27.4 °C, weak flight at T bs as low as 24 °C (Austin and Bradley 1969) and flight by bats at T bs as low as 20 °C (Bradley and O'Farrell 1969). A similar pattern has been observed in non-flying mammals. Free-ranging echidnas actively forage at T bs as low as 21 °C (Augee 1969; L. Kuchel, unpublished data). Active temperature, then, could easily be recorded while animals are technically torpid. Indeed, these findings raise questions about the use of any behavioral boundary to differentiate torpor from normothermia and demonstrate a potential conflict between temperature-based and behavior-based definitions of torpor.
Surprisingly, despite identical treatment during 6–7 months of captivity, Cypress Hills bats could fly at significantly lower T sk than bats from Regina. Climate differences between the two areas could account for this apparent geographic variation. Relative to Regina, where daily summer temperatures vary by only ca. 10 °C, the Cypress Hills are characterized by dramatic diurnal fluctuations in temperature with maxima of 30–35 °C and minima of 5–10 °C common (C. Willis, unpublished observation). Bats in the Cypress Hills must regularly warm at sunset when T a is lower than that experienced by bats in Regina. If bats do occasionally leave their roosts while still rewarming, the ability to fly with relatively cold flight muscles would represent an advantage. Intraspecific geographic variation in thermal physiology is a promising avenue for future research.
Warm-up rates we recorded during flight experiments were surprisingly high. Geiser and Baudinette (1990) and Stone and Purvis (1992) both found a negative relationship between body mass and warm-up rates for mammals. Consistent with this, the ca. 2.5-g Etruscan shrew (Suncus etruscus) is reported to have the highest T b warm-up rates of any mammal (1.25 °C/min; Fons et al. 1997). However, the warm-up rates of bats in our flight experiments were greater than 1.25 °C (Table 3). T sk warm-up rates were particularly high. During flight trials these approached 2-2.5 °C per min, almost two-fold greater than the shrew (Table 3). Brown fat thermogenesis and flight muscle shivering likely explain much of the difference in warm-up rates between T b and T sk, but the training protocol we employed may also have had an influence. Bats were always torpid (T sk~18 °C) and re-warmed prior to being hand-fed each day for the 6-month period leading up to experiments. This daily entrainment may have altered warming ability relative to free-ranging bats. Given that captivity can influence torpor use by mammals and birds (Geiser et al. 2000), it is reasonable to postulate that it could also affect warm-up rates. Pressure to minimize energy expenditure during warming was likely relaxed for bats conditioned to regular feeding. In addition to this, bats were at T sk <18 °C when experiments began, below the flight room T a of 21 °C. Thus, some ambient heating of our thermocouple may have occurred. Despite these potential sources of variation, we contend that the patterns of warming we report (e.g., differences between T b and T sk and the effect of flight) are representative of free-ranging bats. For example, warm-up patterns we observed during the flight experiment were identical to those of bats held for short periods in outdoor cages during the T b vs. T sk experiment (i.e., T sk greater than T b early during warm-up but T sk less than or equal to T b toward the end of warm-up).
Our findings raise questions about the concept of degree-minutes or degree-hours, proposed by Barclay et al. (2001) and Lausen (2001) as a measure of energy savings associated with torpor. This metric multiplies the length of time an animal spends below T act with the magnitude of T sk reduction below T act. The concept is problematic, first because it relies on T act, which does not necessarily represent an energetically relevant definition of torpor; and second because it fails to acknowledge Q10 effects which mean that initial reductions in T b result in greater energy savings than reductions of the same increment at lower T b (e.g., Studier 1981). For example, 6 h at T sk 10 °C below, and 12 h at T sk 5 °C below a T act of 34 °C, both equal 6 degree-hours of torpor. However, at a T a of 20 °C, a 6-h reduction in T b of 10 °C from 34 °C would save a little brown bat ca. 0.42 kJ/g while a 12-h reduction in T b of 5 °C from 34 °C would result in a 22% greater energy savings (ca. 0.54 kJ/g; Studier 1981). The concept of degree-hours has little relevance for the energy budgets of animals in the field because it masks differences in energetic savings associated with different depths and durations of torpor.
Conclusions
Studies of the use of heterothermy by small, free-ranging endotherms have been enhanced by the use of T sk-telemetry. Implanted radiotransmitters are not practical for studies of many small species and we contend that, despite its limitations, T sk does provide valuable information about heterothermy. However, the limitations of T sk as a measure of T b must be addressed if we are to take these studies further and use free-ranging animals to address proximate and ultimate questions about the energetics and evolution of torpor. T sk may be higher or lower than T b depending on T a and on whether animals are rewarming, torpid, or normothermic. Furthermore, T act does not improve dramatically on arbitrary definitions of torpor. Some animals are capable of "activity" and even flight below T bs that qualify as torpor. Indeed, our data strongly suggest that bats may leave their roosts while torpid and exploit flight muscle thermogenesis to finish rewarming. Using flight activity as a threshold for torpor obscures the energetic implications of heterothermy and is no more biologically relevant than an arbitrary T b or T sk, such as 30 °C.
We propose several measures to mitigate limitations of T sk and challenges associated with defining torpor. Most importantly, field studies must assess torpor in terms of energy savings and not just reduced T sk or inactivity, which are merely symptoms of torpor. As demonstrated repeatedly in the laboratory, bouts of heterothermy begin not when endotherms reach a certain T b or when certain behaviors begin or end but when energy is saved as a result of a reduced T b set-point. This distinction is important if field studies employing T sk are to address detailed questions. The ultimate solution is to obtain a direct measure of oxygen consumption in the field. This is logistically challenging even under ideal conditions, but not impossible for some species (e.g., Schmid 1996). Another alternative is to develop species-specific models, based on laboratory data, quantifying the relationship between metabolic rate, T b and T sk over a range of T a, during steady-state torpor, steady-state normothermia, warm-up, and cooling. These models could be used in conjunction with field T sk data to infer metabolic rate and quantify energy budgets and torpor use more precisely. The resulting energy budgets could be verified in the field using doubly labeled water techniques to calculate field metabolic rates. A final and perhaps more attractive possibility, given the intraspecific variation in thermal physiology we found between bats from different study sites, would be to record T sk and oxygen consumption for each study animal prior to its release. These preliminary trials, performed over a wide T a range during normothermia, warming, cooling and torpor would, in essence, calibrate each study animal/T sk combination, allowing inferences about MR to be made from T sk in the field while controlling for inter-individual variation in the relationship between T sk and MR. The findings of studies employing this technique could also be verified using techniques designed for estimating field metabolic rates.
Abbreviations
- BAT :
-
brown adipose tissue
- MR :
-
metabolic rate
- T a :
-
ambient temperature
- T act :
-
active temperature
- T b :
-
body temperature
- T sk :
-
skin temperature
References
Aldridge HDJN, Brigham RM (1988) Load carrying and maneuverability in an insectivorous bat: a test of the 5% "rule" of radio telemetry. J Mammal 69:379–383
Audet D, Thomas DWT (1996) Evaluation of the accuracy of body temperature measurement using external radio transmitters. Can J Zool 74:1778–1781
Augee ML (1969) Temperature regulation and adrenal function in the echidna. Ph.D. Thesis. Department of Zoology, Monash University, Clayton Victoria, Australia
Austen GT, Bradley WG (1969) Additional responses of the poor-will to low temperatures. Auk 86:717–725
Barclay RMR, Kalcounis MC, Crampton LH, Stefan C, Vonhof MJ, Wilkinson L, Brigham RM (1996) Can external radiotransmitters be used to assess body temperature and torpor in bats. J Mammal 77:1102–1106
Barclay RMR, Lausen CL, Hollis L (2001) What's hot and what's not: defining torpor in free-ranging birds and mammals. Can J Zool 79:1885–1890
Barnes BM (1989) Freeze avoidance in a mammal: body temperatures below 0°C in an arctic hibernator. Science 241:1521–1616
Bartholomew GA, Dawson WR, Lasiewski RC (1970) Thermoregulation and heterothermy in some of the smaller flying foxes (Megachiroptera) of New Guinea. Z Vgl Physiol 70:196–209
Bradley WG, O'Farrell MJ (1969) Temperature relationships in the Western Pipistrelle, (Pipistrellus hesperus). In: Hoff CC, Riedesel ML (eds). Physiological systems in semiarid environments. University of New Mexico Press, Albequerque, New Mexico, pp 85–96
Brigham RM (1992) Daily torpor in a free-ranging goatsucker, the common poorwill (Phaelaenoptilus nuttallii). Physiol Zool 65:457–472
Brigham RM, Körtner G, Geiser F (2000) Seasonal use of torpor by free-ranging Australian owlet nightjars. Physiol Biochem Zool 73:613–620
Choi I, Cho Y, Oh YK, Jung N-P, Shin H-C (1998) Behaviour and muscle performance in heterothermic bats. Physiol Zool 71:257–266
Chruszcz BJ, Barclay RMR (2002) Thermoregulatory ecology of a solitary bat, Myotis evotis, roosting in rock crevices. Funct Ecol 16:18–26
The Commission for Thermal Physiology of the International Union of Physiological Sciences (2001) Glossary of terms for thermal physiology. Jap J Physiol 51:245–280
Eckert R, Randall D, Augustine G (1988) Animal physiology: mechanisms and adaptations. WH Freeman, New York
Fons R, Sender S, Peters T, Jürgens D (1997) Rates of rewarming, heart and respiratory rates and their significance for oxygen transport during arousal from torpor in the smallest mammal, the etruscan shrew, Suncus etruscus. J Exp Biol 200:1451–1458
Geiser F, Baudinette RV (1990) The relationship between body mass and rate of rewarming from hibernation and daily torpor in mammals. J Exp Biol 151:349–359
Geiser F, Brigham RM (2000) Torpor, thermal biology, and energetics in Australian long-eared bats (Nyctophilus). J Comp Physiol B 170:153–162
Geiser F, Ferguson C (2001) Intraspecific differences in behaviour and physiology: effects of captive breeding on patterns of torpor in feathertail gliders. J Comp Physiol B 171:569–576
Geiser F, Ruf T (1995) Hibernation vs. daily torpor in mammals and birds: physiological variables and classification of torpor patterns. Physiol Zool 68:935–966
Geiser F, Coburn DK, Körtner G (1996) Thermoregulation, energy metabolism, and torpor in blossom-bats, Syconycteris australis (Megachiroptera). J Zool (Lond) 239:583–590
Geiser F, Holloway JC, Körtner G, Maddocks TA, Turbill C, Brigham RM (2000) Do patterns of torpor differ between free-ranging and captive mammals and birds? In: Heldmaier G, Klingenspor M (eds) Life in the Cold. Proceedings of the 11th International Hibernation Symposium, 13–18 August 2000, Jungolz Austria. Springer, Berlin Heidelberg New York, pp 95–102
Genoud M (1993) Temperature regulation in subtropical tree bats. Comp Biochem Physiol A 104:321–331
Hamilton IM, Barclay RMR (1994) Patterns of daily torpor and day roost selection by male and female big brown bats (Eptesicus fuscus). Can J Zool 72:744–749
Hickey MBC, Fenton MB (1996) Behavioural and thermoregulatory responses of female hoary bats, Lasiurus cinereus (Chiroptera: Vespertilionidae), to variations in prey availability. Ecoscience 3:414–422
Kalcounis MC, Brigham RM (1998) Secondary use of aspen cavities by tree-roosting big brown bats. J Wildlife Manage 62:603–611
Kunz TH (1982) Roosting ecology. In: Kunz TH (ed) Ecology of bats. Plenum, New York, pp 151–200
Lausen CL (2001) Thermoregulation and roost selection by reproductive female big brown bats (Eptesicus fuscus) roosting in rock crevices in the South Saskatchewan River Valley, Alberta. Abstracts of the 31st Annual North American Symposium on Bat research, Victoria, BC Canada, 24–27 October 2001
Lausen CL, Barclay RMR (2003) Thermoregulation and roost selection by reproductive female big brown bats (Epesicus fuscus) roosting in rock crevices. J Zool (Lond) (in press)
Ortmann S, Shmid J, Ganzhorn JU, Heldmaier G (1996) Body temperature and torpor in a Malagasy small primate, the mouse Lemur. In: Geiser F, Hurlbert AJ, Nicol SC (eds) Adaptations to the Cold. Tenth International Hibernation Symposium. University of New England Press, Armidale Australia, pp 55–61
Saarela S, Keith JS, Hohtola AE, Trayhurn P (1991) Is the mammalian brown fat specific mitochondrial uncoupling protein present in adipose tissue of birds? Comp Biochem Physiol B 100:45–50
Sauchyn DJ (1993) Quaternary and late tertiary landscape evolution in the western Cypress Hills. In: Sauchyn DJ (ed) Quaternary and late tertiary landscapes of Southwestern Saskatchewan and adjacent areas. Canadian Plains Research Centre, Regina Canada
Schmid J (1996) Oxygen consumption and torpor in mouse lemurs (Microcebus murinus and M. myoxinus): preliminary results of a study in western Madagascar. In: Geiser F, Hurlbert AJ, Nicol SC (eds) Adaptations to the Cold. Tenth International Hibernation Symposium. University of New England Press, Armidale Australia, pp 47–54
Stone GN, Purvis A (1992) Warm-up rates during arousal from torpor in heterothermic mammals: physiological correlates and a comparison with heterothermic insects. J Comp Physiol B 162:284–295
Studier EH (1981) Energetic advances of slight drops in body temperature in little brown bats, Myotis lucifugus. Comp Biochem Physiol A 70:537–540
Wang LCH (1989) Ecological, physiological and biochemical aspects of torpor in mammals and birds. In: Wang LCH (ed) Advances in comparative and environmental physiology. Springer, Berlin Heidelberg New York, pp 361–401
Wang LCH, Wolowyk MW (1988) Torpor in mammals and birds. Can J Zool 66:133–137
Willis CKR (2003) Daily heterothermy by temperate bats using natural roosts. In: Akbar Z, McCracken GF, Kunz TH (eds) Functional and evolutionary ecology of bats. Proceedings of the 12th International Bat Research Conference. Oxford University Press, New York (In press)
Willis CKR, Kolar KA, Karst AL, Kalcounis-Rueppell MC, Brigham RM (2003) Medium- and long-term reuse of trembling aspen cavities as roosts by big brown bats (Eptesicus fuscus). Acta Chiropterol (In press)
Zar JH (1999) Biostatistical analysis. Prentice Hall, New Jersey
Acknowledgements
Despite our disagreement with him on some of the issues raised above, we wish to express our admiration for the important contributions of Robert Barclay and his students to the study of torpor in free-ranging animals. We also thank Dr. Barclay for comments that improved an early draft of the manuscript. Andrew McKechnie, Don Thomas, and Chris Woods also provided helpful comments. Field and laboratory assistance was provided by Quinn Fletcher, Amanda Karst, Brianna Dobson, Renee Bendig, Desiree Idt, Christine Voss, Seb Martinez, Ryan Fisher, and Julie Adams. Jim Rusak provided invaluable statistical suggestions. This research was funded by Mountain Equipment Co-op, Saskatchewan Environment and Resource Management and by a Natural Sciences and Engineering Research Council (NSERC, Canada) research grant to R.M.B. and postgraduate scholarship to C.K.R.W.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L.C.H. Wang
Rights and permissions
About this article
Cite this article
Willis, C.K.R., Brigham, R.M. Defining torpor in free-ranging bats: experimental evaluation of external temperature-sensitive radiotransmitters and the concept of active temperature. J Comp Physiol B 173, 379–389 (2003). https://doi.org/10.1007/s00360-003-0343-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-003-0343-y