Abstract
Phenology refers to the timing of events in the annual cycle of organisms. For temperate-zone mammals, hibernation is one such event, but little is known about its phenology. Hibernation consists of energy-saving torpor bouts interspersed with energetically expensive arousals to normothermic T b, and hibernators should benefit from mechanisms which reduce arousal costs and help them time arousals to coincide with foraging opportunities. In a previous study, we showed that, in contrast to hibernating bats from warmer climates, little brown bats (Myotis lucifugus) from central Canada abandon a circadian pattern to arousal in the middle of winter when there is no chance of feeding. Here, we used temperature telemetry to test whether they would re-synchronize arousals with normal foraging time (i.e. sunset) during late winter as the chance of foraging or emergence opportunities improves, and whether they would synchronize arousals with conspecifics, possibly to exploit social thermoregulation. We also used passive transponders to test whether energy reserves and/or sex differences in reproductive timing influence phenology and the sensitivity of emergence timing to environmental cues. In contrast to patterns in mid-winter, after 7 April 2013, bats synchronized arousals with sunset and with conspecifics. Females emerged earlier than males, and females in the best condition emerged first while body condition had no influence on male emergence timing. Both male and female bats appeared to time emergence with falling barometric pressure, a cue that predicts favourable foraging conditions for bats but which, unlike outside temperature, would have been readily detectable by bats inside the hibernaculum. Our results highlight hibernation traits associated with extreme winter energy limitation for insect-eating bats in cold climates and illustrate the influence of reproductive timing and environmental conditions on hibernation energetics and phenology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phenology refers to the timing of events in the annual cycles of organisms and describes how these events are influenced by seasonal and inter-annual variation in climate (Both et al. 2009). Shifts in phenology associated with climate change have received recent attention as some avian species have failed to advance migration in step with changing peaks in resources (reviewed by Visser and Both 2005). Mammals should also face selection pressure to adjust timing of life cycle events but less is known about their phenology. Few mammals exhibit long-distance latitudinal migrations to avoid winter and, instead, many use hibernation to survive seasonally unfavourable conditions (Geiser 2004, 2013). Some species hibernate for up to 8 months and winter duration likely impacts over-winter survival and fitness in the following reproductive season (Lyman 1982; Twente et al. 1985; Wilkinson and South 2002; Lane et al. 2012; Norquay and Willis 2014). Over-winter survival and reproductive success may also be impacted by emergence phenology. During warmer springs, yellow-bellied marmots (Marmota flaviventris) emerge from hibernation earlier and experience greater reproductive success than in colder years (Ozgul et al. 2010). Spring weather varies inter-annually, and cold years may delay emergence adding additional costs that must be balanced by over-winter energy reserves (Lane et al. 2012).
Before entering hibernation, hibernators accumulate a food cache or fat store to fuel over-winter energy demands and then reduce energetic demands during hibernation via multiday bouts of torpor [i.e. dramatically reduced metabolic rate (MR) and body temperature (T b)] (Geiser 2004). Torpor bouts can last for days to weeks depending on species, and are typically interspersed with brief (typically <24 h) periodic arousals to normothermic T b, which account for up to 90 % of the winter energy budget (Wang 1989; Thomas et al. 1990; Geiser 2004; but see Lovegrove et al. 2014). Hibernators likely face selection pressure to budget the frequency, duration and timing of arousals to ensure they emerge from hibernation in adequate condition to support survival and reproduction (Jonasson and Willis 2011; Norquay and Willis 2014).
Mechanisms underlying the timing of arousal are still not fully understood (reviewed by Körtner and Geiser 2000). Circadian mechanisms are thought to be inhibited during multiday torpor bouts in hibernation (Tøien et al. 2011; Williams et al. 2012a, b). However, in the United Kingdom (U.K.), cave-dwelling, greater horseshoe bats (Rhinolophus ferrumequinum) and Natterer’s bats (Myotis nattereri) maintain a circadian rhythm to arousals in the absence of photoperiod cues and rewarm from torpor at sunset. This is likely because mild winter nights (>10 °C) with flying insects available occur relatively often and bats may emerge to forage (Park et al. 2000; Hope and Jones 2012). In contrast, little brown bats (Myotis lucifugus) hibernating in central Canada where ambient temperature (T a) on winter nights rarely exceeds 0 °C, appear to abandon any circadian rhythmicity to arousals (Czenze et al. 2013).
Despite use of torpor, hibernators may face additional pressure to reduce thermoregulatory costs in winter. Huddling with conspecifics reduces exposed surface area, and therefore, loss of both heat and water (Boyles et al. 2008; Boratyński et al. 2014). Groups of individuals could maximize thermoregulatory benefits of huddling if they synchronize arousals with each other and exploit social rewarming (Arnold 1993). Little brown bats tend to arouse at about the same times as conspecifics but overlap in arousal times has not been quantified in the wild (Czenze et al. 2013). Given the potential sensitivity of hibernating bats to disturbance by other individuals in their hibernaculum (Thomas 1995; Turner et al. 2015), it is unclear whether similarly timed arousals by multiple bats result in energetic benefits via social rewarming or reflect a response to disturbance by colony mates (Czenze et al. 2013; Turner et al. 2015).
Emergence timing is a key phenological trait for fat-storing hibernators and may be influenced by fat reserves and environmental cues (e.g. spring weather patterns) which signal spring conditions and food availability (Murie and Harris 1982; Arlettaz et al. 2001; Schorr et al. 2009; Sheriff et al. 2011; Norquay and Willis 2014). The influence of energy reserves on emergence phenology may also be influenced by sex differences in the timing of reproductive investment. Most fat-storing bats mate before hibernation and delay fertilization until spring. Female little brown bats are thriftier with fat and emerge from hibernation earlier than males presumably to exploit microclimates in maternity roosts (Jonasson and Willis 2011; Norquay and Willis 2014). Warm temperatures expedite gestation (Racey and Swift 1981) and, despite more variable in T a than hibernacula, maternity roosts will be warmer on many spring days than hibernacula. Males, whose reproductive investment takes place before and during early hibernation (Thomas et al. 1979), may remain in hibernation longer and delay emergence until fat reserves are nearly exhausted, reducing exposure to inclement weather and other sources of mortality like predation (Stawski and Geiser 2010; Norquay and Willis 2014).
We used temperature radio-telemetry, and passive transponders (PIT tags) to test hypotheses about links between energetics, torpor-arousal cycles and emergence phenology in hibernating little brown bats. We first revisited Czenze et al.’s (2013) finding that little brown bats exhibit no circadian pattern to arousals during hibernation and tested the hypothesis that, as opposed to during deep winter, opportunities for feeding in late winter/early spring influence arousal timing. We predicted that, as warmer nights with flying insects became more common, bats would reinstate a non-random circadian pattern to arousal. Second, we tested whether hibernation period would affect the propensity of bats to synchronize arousals with conspecifics. We predicted that, as energy reserves decline towards spring and relative benefits of social rewarming increase, individuals would be more likely to rewarm simultaneously to share arousal costs. Finally, we tested the hypothesis that sex, energy reserves, and environmental conditions inside and outside hibernacula influence hibernation phenology. We predicted that females should emerge earlier than males and that energy reserves (assessed based on body condition) should affect hibernation period differently for males and females. Males in the best condition should hibernate longest to minimize exposure to extrinsic mortality while females in the best condition should emerge earliest because they will be best equipped to balance costs of cold, unpredictable spring weather against benefits of early gestation. We also predicted that if bats can detect cues inside the hibernaculum that reflect favourable foraging conditions outside (e.g. warm T a, insect availability), they should adjust emergence timing to coincide with these conditions.
Methods
This study was conducted about 50 km north of the town of Grand Rapids (53°30′N; 99°24′W), in central Manitoba, Canada. Eight caves are known little brown bat hibernacula including our study site, Dale’s Cave. Dale’s Cave houses 80–200 bats and is accessed by a 40–50-cm diameter, 3-m deep sinkhole/tunnel leading to a bell-shaped chamber (approximately 7 m in length, 5 m in breadth with a 4 m ceiling). Most of the bats in Dale’s Cave hibernate in 3–4 large clusters on the ceiling 6–7 m from the entrance with a few smaller clusters of 2–3 bats or solitary bats scattered throughout the chamber (Czenze and Willis unpub. data). Bats in Dale’s cave may experience dim photoperiod cues during winter, especially those roosting close to the entrance tunnel. However, the long, narrow entrance to the cave significantly limits light penetration, and snow and ice cover around the entrance throughout the winter, further reducing the potential for photoperiod cues.
All procedures were approved by the University of Winnipeg Animal Care Committee and conducted under Manitoba Conservation Wildlife Scientific Permit WB0612. Temperature data loggers (HOBO Micro Station—H21-002, Onset Computer Corporation, Cape Cod, Massachusetts, USA) were used to record cave T a and humidity every 2 h. We entered the cave and handled bats during hibernation, but to minimize disturbance, we only entered once and limited the duration of our visit to less than 2 h. Thirty torpid bats were captured by hand on 24 January 2013, sexed and weighed to the nearest 0.01 g (Durascale-100, MyWeigh, Ontario, Canada). Forearm length was measured to the nearest 0.05 mm.
For small bats, skin temperature (T sk) provides a good approximation of T b during torpor (Audet and Thomas 1996; Barclay et al. 1996; Willis and Brigham 2003). To record T sk, we clipped a small (<1 cm2) patch of fur between the shoulders and attached a temperature-sensitive radio-transmitter (0.80–0.90 g, BD-2NT; Holohil Systems Ltd, Carp, Ontario) using a non-toxic, latex-based adhesive (Osto-Bond, Montreal Ostomy, Vaudreuil, Quebec, Canada). Transmitters represented between 6.7 and 10.4 % of body mass, greater than the 5 % guideline suggested by Aldridge and Brigham (1988) but similar to the size which Jonasson and Willis (2012) demonstrated had no negative impact on body condition in hibernating little brown bats. Before use, we calibrated transmitters to the nearest 0.1 °C in a water bath at 5 °C increments from 0.3 to 45 °C against a thermometer traceable to the National Institute of Standards and Technology.
We deployed a data logging receiver (Lotek SRX600; Lotek Engineering Inc Newmarket, Ontario, Canada) powered by two 64 W solar panels (US-64, Uni-solar, Auburn Hills, MI) and connected to a solar charge controller (Sunsaver 10, Morningstar, Newtown, PA) in a weatherproof box outside the cave. The receiver was connected to three yagi antennas positioned inside the cave and recorded T sk of each individual at 10-min intervals. To assess individual emergence date, passive transponders (PIT tags, Trovan Ltd. ID 100-01, <1 g, Douglas, UK) were attached to each transmitter with epoxy. The mouth of the cave was outfitted with two 475 × 400 × 40 mm panel antennas (ANT-612 Large Panel Antenna, Trovan Ltd., Douglas, UK). Panels were placed so that their detection range exceeded the diameter of the cave entrance.
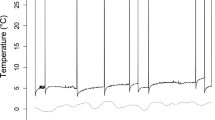
Large differences between cave T a and normothermic T sk made arousals obvious in T sk traces, and we followed Jonasson and Willis (2012) and Czenze et al. (2013) to define phases of torpor and arousal. We defined torpor bouts as periods of reduced stable T sk between the obvious warming and cooling phases associated with each arousal. Occasionally, Holohil BD-2NT transmitters exhibit a gradual increase in temperature after initial entry into a torpor bout, which appears to be an artefact (e.g. Jonasson and Willis 2012; Czenze et al. 2013, Fig. 1) as it does not occur for other types of temperature data loggers attached to hibernating bats (e.g. Warnecke et al. 2012; Reeder et al. 2012). We could readily identify this artefact in our T sk traces, and it did not affect our analyses. Occasionally, T sk also appeared to decline and was occasionally undetectable during deep torpor bouts, especially late in the season presumably as battery power declined (Fig. 1). However, signals were always detectable during arousals so this artefact also had no effect on our analysis. We followed Jonasson and Willis (2012) and classified the warming phase as an abrupt increase in torpid T sk that eventually stabilized at a normothermic level that was readily distinguishable from warming or cooling phases of a torpor bout. The cave’s cold T a appeared to increase ambient cooling of external transmitters, leading to relatively low T sk values during arousal (Willis and Brigham 2003), but this effect was similar across individuals and did not interfere with our ability to detect arousals. The normothermic period was defined as the time between the end of the warming phase and the initiation of cooling which was readily obvious as an abrupt decline in T sk preceding steady-state torpor. We excluded data from the first 24 h after capture to avoid the influence of this disturbance on our results.
Representative T sk traces for six little brown bats (Myotis lucifugus) in Dale’s Cave in Central Manitoba. Dotted lines represent cave T a. Break in the x-axis represents a period between 10 February and 12 March when we were not able to record data due to equipment failure. The gradual decline in torpid T sk represents transmitter battery drain and did not affect our analysis because arousals could still be detected. Dashed lines represent periods when we lost the transmitter signal due to transmitter battery drain
All analyses were conducted in R version 2.10.1 (R Development Core Team 2009). Values are reported as the mean ± SD with N equal to the number of animals and n equal to the number of events/arousals. We recorded the date and time of each arousal from the T sk trace and converted time of arousal from minutes before or after sunset into radians. We then used Rayleigh’s test for circular distributions to determine if the daily distribution of arousal times was significantly different from random. We used an iterative process to determine if arousal timing shifted from a random pattern during mid-winter to a pattern of clustered arousals around the time of sunset during late winter/spring. We began by running Rayleigh’s test on the first ten arousals we recorded after tagging the bats on 24 January 2013 (i.e. Arousal 1 to Arousal 10). We then shifted our analysis window forward by one arousal (i.e. Arousal 2 to Arousal 11) and re-ran the test. We continued this process until our final analysis window included the last 10 arousals we recorded at the end of the winter. This allowed us to investigate potential changes in temporal patterns of arousal from torpor.
To determine which bat-specific variables best predicted emergence phenology, we used a general linear model (GLM) with hibernation period as the response variable and sex and body condition index [BCI; body mass (g)/forearm length (mm)] at capture as predictors. We defined hibernation period as the number of days after 15 September 2012 [i.e. the average recorded immergence date for little brown bats in our study area (Norquay and Willis 2014)]. We used our Hobo data loggers and archived climate data from the Grand Rapids weather station 37 km from Dale’s Cave (53°11′N; 99°16′W) (National Climate Data and Information Archive 2013) to obtain values of weather and microclimate variables that we predicted might influence emergence timing: cave T a (T cave), which will be buffered and much less variable compared to outside T a but could provide a reflection of outside conditions that bats can assess (Perry 2012); and changes in barometric pressure (BP), which reflect passing weather fronts and have been shown to correlate with both insect and bat activity (Paige 1995; Turbill 2008). In our small cave, BP should rapidly equilibrate inside and outside the hibernaculum (Pflitsch et al. 2010) and could be used by bats to predict whether insects might be available (Paige 1995; Turbill 2008). We used a GLM including several temperature and BP variables that we predicted a priori should be most relevant to emerging bats: (1) mean daily T cave for the 24-h period before each night when at least one bat emerged (mean T cave); (2) 24-h change in daily mean T cave prior to nights when at least one bat emerged (ΔT cave = mean T cave on Day 1 − mean T cave on Day 0); and (3) 24-h change in daily mean BP prior to nights when at least one bat emerged (ΔBP). Prior to analysis, we tested for normality with Shapiro–Wilks tests and homogeneity of variances with Levene’s test. We assessed significance at the P < 0.05 level.
Results
Body mass of all bats captured on 24 January 2013 was 9.4 ± 1.1 g, and BCI was 0.25 ± 0.02 g/mm (N = 22 males, 8 females). Males (9.1 ± 0.7 g, N = 22) were lighter (P = 0.02, t = 2.9, df = 9) than females (10.5 ± 1.2 g, N = 8), and average BCI of males (0.24 ± 0.02 g/mm, N = 22) was lower (P = 0.02, t = 2.8, df = 10.2) than females (0.27 ± 0.03 g/mm, N = 8).
We recorded 1276.8 bat days of T sk data from 27 individuals over 81 days (24 January–10 February, 22 February–24 February, 12 March–13 May) following transmitter attachment (mean 48.2 ± 18.5 days per bat). Gaps in the data occurred during deep snowfalls when we could not access the system, snow accumulated on the solar panels and the SRX600 battery failed. During periods when we did record un-interrupted T sk data, bats exhibited long bouts of torpor averaging 20.07 ± 14.55 days with the longest continuous bout lasting 60.25 days (Fig. 1). Torpor bouts were interspersed with periodic arousals averaging 190 ± 90 min (Fig. 2). We recorded 46 arousals before the final transmitter battery failed on 13 May 2013.
We detected a clear shift in the timing of arousals relative to sunset (Fig. 3). Prior to 7 April (i.e. 25 January–6 April), 92 % (11/12) of the 10-arousal analyses, we performed indicated that arousal timing was not significantly different from a random distribution. After 7 April (i.e. between 7 April and 13 May), 100 % (13/13) of our 10-arousal analyses indicated that arousal timing was significantly different from a random distribution (Fig. 3). We, therefore, combined all arousals prior to 7 April and all arousals after 7 April for two subsequent analyses. Before 7 April, bats aroused within 5.3 ± 3.6 h of sunset, and the circular frequency distribution for the timing of all arousals relative to sunset was not significantly different from a random distribution (Fig. 4a, b, Z = 2.02, P = 0.13, mean vector = 0.31, N = 17 bats, n = 21 arousals). We recorded multiple arousals from some individuals so this analysis was technically pseudoreplicated, but we still found no relationship when we calculated average arousal time for each individual (Z = 0.83, P = 0.44, mean vector = 0.24) or limited the analysis to each individual’s first arousal (Z = 1.14, P = 0.32, mean vector = 0.29). In contrast, there was a relationship between arousal times and sunset when we combined all arousals recorded after 7 April during late winter/spring. During this period, bats aroused within 0.6 ± 3.3 h of sunset, and the circular distribution of arousal times relative to sunset was significantly non-random (Fig. 4c, d, Z = 14.8, P < 0.01, mean vector = 0.77, N = 15, n = 25). As above, this analysis included multiple arousals for some individuals, but we still found the relationship when we only used each individual’s first arousal during this period (Z = 10.6, P < 0.01, mean vector = 0.77) or calculated the average arousal time for each individual (Z = 12.8, P < 0.01, mean vector = 0.84).
P values generated from repeated analyses testing whether the timing of ten consecutive arousals by hibernating little brown bats, relative to sunset, differed from a random circular distribution. Arousal timing relative to sunset was calculated for each arousal and converted to radians prior to analysis with Rayleigh’s test. Horizontal axis points represent the time of the 10th arousal in each ten-arousal sample. See “Methods” for details
Histograms showing the timing of arousals relative to sunset for little brown bats during hibernation before (a) and after (b) 7 April 2013. Clock faces represent circular distributions of arousals (dotted line represents mean sunset time) before (c) (mean sunset = 19:02, Z = 2.02, P = 0.13, mean vector = 0.31, N = 17, n = 21) and after (d) (mean sunset = 20:47, Z = 14.8, P < 0.01, mean vector = 0.77, N = 15, n = 25) 7 April 2013
Prior to 7 April, 35 % (6/17) of individuals aroused at least once with a conspecific, and about 38 % (8/21) of arousals were at least partially overlapping in time with those of other individuals. Arousals tended to occur closer together in time during late winter/spring compared to winter. After 7 April, the arousals of 80 % (12/15) of individuals were at least partly overlapping with those of at least one conspecific, and 80 % (20/25) of all arousals were at least partially overlapping in time with arousals of other individuals, a significantly greater proportion than that before 7 April (Z = −2.4, P = 0.02, N = 46). Five bats were detected rewarming both singularly and synchronously. Four of these five bats aroused by themselves prior to 7 April but alongside conspecifics after 7 April. We also observed three instances of what we term “arousal cascades” during which one individual aroused followed by more than one additional individual. All arousal cascades occurred during late winter and involved up to eight individuals. Despite apparent arousal synchrony, bats did not necessarily completely synchronize their arousals. During early winter, only 12.5 % (1/8) of the overlapping arousals referred to above were completely synchronized in time meaning that bats would not have maximized thermoregulatory benefits of social thermoregulation. During late winter, the number of arousals which was completely synchronized in time increased but was still less than half of all overlapping arousals (45 %, 9/20).
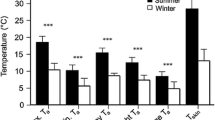
Males emerged from hibernation 22.7 ± 6.9 days later than females (Fig. 5a, P < 0.01, t = 8.4, N = 17). The effect of an interaction between sex and BCI on hibernation period approached significance (P = 0.09), so we examined the effect of BCI for each sex separately. We found no effect for males (P = 0.41, t = 0.8, N = 12 bats), but females with better BCI emerged significantly earlier (Fig. 5b, P = 0.03, t = −3.7, N = 5 bats).
Histogram (a) of recorded emergence dates for individual little brown bats. White bars represent females, and black bars represent males (N = 5 females, 12 males) and b Ordinary least squares regression line of the body condition [body mass (g)/forearm length (mm)] and emergence timing of hibernating female little brown bats. Hibernation period represents the number of days since 15 September 2012
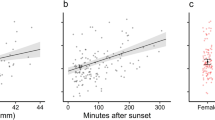
During the emergence period (27 April–16 June), average daily BP ranged from 96.75 to 101.23 kPa, T cave ranged from 2.5 to 4.5 °C, and outside T a ranged from −15.7 to 26.6 °C. ΔT a measured outside the cave resulted in a temperature signal inside the cave, reflected by a positive correlation between ΔT a and ΔT cave (r = 0.45, t = 3.5, df = 49, Fig. 6a) although the range of ΔT cave values was small (Fig. 6a). ΔBP measured outside the cave was negatively correlated with ΔT a such that rapidly falling BP signalled large increases in T a (r = −0.63, t = −0.57, df = 49, Fig. 6b), Mean T cave gradually increased throughout the emergence period reflected by a positive correlation between T cave and hibernation period for all bats (P < 0.01, t = 14.2, N = 17). Interactions between sex and ΔT cave (P = 0.03, t = −2, N = 17) and sex and ΔBP (P < 0.01, t = 5.1, N = 17) on hibernation period were significant so we examined effects of these predictors separately for each sex.
We found no correlation between ΔT cave and hibernation period for females (P = 0.14, t = −1.9, N = 5 bats). Male hibernation period was positively correlated with ΔT cave (P = 0.05, t = 2.25, N = 12), but the effect was strongly influenced by one outlier and disappeared when we removed this data point (P = 0.39, t = 0.89, N = 11). The effect of ΔBP appeared stronger. All females emerged on days when ΔBP was negative (i.e. falling) and females that emerged latest in the spring (i.e. those with the lowest BCI, see above), tended to emerge on days with the greatest fall in BP (Fig. 7a, P = 0.04, t = −3.4, N = 5). Males emerging early in their emergence period, when conditions would have been relatively harsh, also tended to emerge on days with falling BP (Fig. 7b, P < 0.01, t = 4.3, N = 12).
Ordinary least squares regression lines are shown here to illustrate the relationship, which makes up part of a larger model (see “Results”), between the change in barometric pressure (kPa) over 24 h and hibernation period for a female and b male little brown bats. Hibernation period represents the number of days since 15 September 2012
Discussion
We found strong support for the hypotheses that declining energy stores in late hibernation influence arousal timing and arousal synchrony, and that the timing of investment in reproduction influences hibernation phenology. As we reported previously for little brown bats from a different cave a year before this study was undertaken (Czenze et al. 2013), hibernating little brown bats exhibited no circadian pattern to arousal during the coldest months of the year (January–March). Here, we also found that between 24 January and 6 April, bats aroused randomly with respect to sunset suggesting that they were either no longer sensitive to, or had abandoned maintenance of, their circadian rhythm. During this period, mean daily T a outside the cave ranged from −31.4 to −1.7 °C (National Climate Data and Information Archive 2013), eliminating any opportunity for bats to forage. This could help explain the exceptionally long torpor bout (60.25 days) we observed. Costs of maintaining a circadian rhythm in the absence of an energetic benefit could explain the random timing of arousals with respect to sunset (Czenze et al. 2013). After 7 April, however, bats resumed a circadian pattern with arousals clustered after sunset. Some mammalian hibernators appear to maintain a circadian rhythm of arousals (Zervanos et al. 2009; Grahn et al. 1994; Heller and Ruby 2004; Park et al. 2000; Hope and Jones 2012), but, to our knowledge, this is the first report of a hibernator abandoning a circadian arousal pattern and then later resuming that pattern prior to emergence.
The resumption of the circadian rhythm was closely correlated with increasing air temperature and, during this period, mean T a outside the cave ranged from −12.2 to 13.2 °C (National Climate Data and Information Archive 2013). Although there were many nights during which T a was below 10 °C, a threshold thought to be predictive of flying insect availability (Paige 1995), on 21 % (7/33) of nights between 7 April and 13 May, outside T a at sunset exceeded 10 °C. This suggests that, during late winter/early spring, there is at least some opportunity for bats to exploit flying insects and individuals may gain an energetic benefit from the chance they might arouse on a warm evening.
Mechanisms underlying suppression and subsequent resumption of a circadian rhythm could be found in the supra-chiasmatic nucleus (SCN) of the hypothalamus (Ruby et al. 2002). The expression of circadian rhythms in bats may be similar to that of hibernating European hamsters (Cricetus cricetus) for which the expression of SCN clock genes is halted during low T b associated with deep torpor (Revel et al. 2007) possibly because protein synthesis is depressed during long torpor bouts (Storey and Storey 2004). Alternatively, like in arctic ground squirrels (Urocitellus parryii), SCN oscillators may still function rhythmically during hibernation, but output pathways may be inhibited even if the animal experiences photoperiod cues prior to emergence (Williams et al. 2012a, b). We observed arousals during the day throughout the winter and, although bats are unlikely to obtain detectable photoperiod cues where they roost in the cave, it is possible photoperiod was detectable especially near the entrance. Thus, bats may have aroused before flying insects are readily available to obtain photoperiod cues and begin the process of resuming their circadian rhythm in time for the active season.
In addition to scheduling arousals around sunset, we also found that bats synchronized arousals with each other, especially after 7 April when the proportion of arousals that were partially overlapping in time nearly tripled. Part of this effect could reflect the fact that bats were synchronizing their arousals with sunset and, therefore, their arousals overlapped in time. However, rewarming at sunset does not necessarily require them to arouse on the same days, and it is unlikely that the arousal synchrony we observed is completely explained by resumption of the circadian rhythm. We hypothesized that synchronized arousals would result from energy limitation during late winter when savings provided by arousal synchrony and huddling could become especially important. For bats to realize potential energy savings of social thermoregulation, multiple bats should rewarm and remain normothermic at precisely the same times to share the costs of arousal and normothermia. We could not tell which individual bats were huddling together in the same clusters, but we did find that some evidence bats were sharing arousal costs, especially after 7 April when 45 % of group arousals overlapped completely with those of another individual. This suggests that social thermoregulation provides at least a partial, although perhaps not complete explanation for greater arousal synchrony in late winter.
An alternative explanation for arousals by multiple bats at about the same time is disturbance by conspecifics. Some hibernating bats are sensitive to non-tactile disturbance, arousing from torpor when human visitors enter caves (Thomas 1995). Interestingly, Thomas (1995) found that individual bats continued arousing for hours, even after a human visitor left the hibernaculum, which suggests some bats rewarmed because of disturbance by colony mates. Even if only a few bats are sensitive to non-tactile disturbance, their subsequent re-entry into huddles could result in tactile disturbances to huddle mates, un-scheduled arousals, and increased energy expenditure (Speakman et al. 1991; Turner et al. 2015). This suggests that part of the arousal synchrony effect we observed could reflect a maladaptive consequence of colonial hibernation in bats—the potential for increased energy expenditure as a result of disturbance from conspecifics (Turner et al. 2015). Additional studies are needed, potentially using thermal infrared imaging, to help tease out potential costs of disturbance from benefits of social thermoregulation.
In addition to a resumption of a circadian pattern to arousals and increased arousal synchrony among individuals in late winter, we found support for the hypothesis that sex, energy reserves, and environmental conditions influence emergence phenology. Female bats emerged earlier than males, likely to exploit more variable but potentially warmer microclimates in maternity roosts and occasional warm spring nights for foraging, both of which could expedite gestation and, in turn, benefit sub-adult survival (Racey and Swift 1981; Frick et al. 2010). This strategy may be risky, however, as weather during early spring is unpredictable with a high likelihood of cold T a and spring snowstorms. Despite a small sample size, we found a strong relationship between female BCI and emergence date, with females in the best condition emerging first. Norquay and Willis (2014) reported the same pattern during a different winter, at a different hibernaculum 200 km south of our study site. Females in better condition may be better equipped to tolerate unpredictable harsh spring weather and balance risks of early emergence against the benefit of an earlier start to gestation (Jonasson and Willis 2011; Norquay and Willis 2014).
Emergence phenology differed for male bats. We predicted that males in the best condition should emerge later because larger fat reserves would permit longer hibernation, reducing their risk of predation or harsh spring weather (Stawski and Geiser 2010; Geiser and Brigham 2012; Bieber et al. 2014) and because of relaxed selective pressure for early emergence (Norquay and Willis 2014). We found no significant relationship between BCI and hibernation period even though our sample size for males, while small was larger than that for females. High inter-individual variation, combined with a small sample size, may have masked a potentially weak relationship between BCI and emergence phenology of males. We recommend future studies tagging larger samples of male bats to determine potential effects of BCI, and other variables, on emergence patterns of males.
Emergence timing of hibernators can be affected by exogenous cues like winter severity, snow cover, snow depth, food availability, and T a (Murie and Harris 1982; Inouye et al. 2000; Arlettaz et al. 2001; Schorr et al. 2009; Sheriff et al. 2011). For bats, outside T a will influence thermoregulatory costs and flying insect availability (Anthony et al. 1981; Negraeff and Brigham 1995). Although hibernaculum microclimates are typically much more stable than conditions above ground, large, short-term (i.e. 24 h) changes in T a outside a cave could still result in small changes within a cave (Perry 2012) that could be detectable by bats. Outside ΔT a and ΔT cave were correlated in our study (Fig. 6a). However, the magnitude of ΔT cave values we measured was extremely small (i.e. tenths to hundredths of °C, Fig. 6a), and we found no evidence that bats used this subtle signal of outside conditions to time their emergence.
Changes in BP, unlike temperature, can quickly equilibrate inside and outside a hibernaculum particularly if the cave volume is small and openings are un-obstructed (Perry 2012; Pflitsch et al. 2010). The magnitude of ΔBP can predict approaching weather fronts (Linacre and Geerts 1997) with falling BP predictive of passing warm fronts, warmer T a and lower chance of precipitation (Lutgens and Tarbuck 2013). During winter, sub-tropical Australian bats timed periods of activity with falling BP, which predicted warm nights (Turbill 2008), while periods of low BP corresponded with the highest activity of both flying insects and North American tri-colored bats (Perimyotis subflavus) (Paige 1995). In our study, falling BP was a strong predictor of increasing T a (i.e. warm fronts) (Fig. 6b) and could have signalled favourable conditions for emergence to bats. All female bats emerged during warm fronts when BP was falling, and the latest-emerging females, which had the lowest energy reserves and, therefore, may have faced the greatest threat from harsh spring conditions, emerged on days when the drop in BP was greatest (Fig. 7a). Males also appeared to respond to ΔBP. Males that emerged earliest, and thus during periods when the weather was likely to be less favourable overall, ended hibernation during warm fronts when BP was falling. Taken together, our results suggest that BP, but not T cave, provides a signal which bats can detect within a hibernaculum and use to improve the chance of emergence during favourable conditions.
We found that little brown bats abandoned a circadian pattern to arousal during the coldest parts of the winter but resumed this pattern in late winter presumably because of increased opportunity for energy acquisition. Bats synchronized arousals with conspecifics more often during late hibernation which could enhance energy savings via social rewarming or reflect energetically costly disturbance. Despite a small sample, we found that females in the best body condition during winter emerged earliest in spring but that male condition did not affect emergence timing. Both males and females appeared to fine-tune the timing of emergence based on BP which signalled warm fronts and favourable conditions. Our findings highlight adaptations of little brown bats for extreme energy limitation in winter (Jonasson and Willis 2012) while also highlighting the strong influence of sex differences in reproductive timing on hibernation energetics and emergence phenology (Jonasson and Willis 2011; Norquay and Willis 2014).
References
Aldridge H, Brigham RM (1988) Load carrying and maneuverability in an insectivorous bat: a test of the 5% “rule” of radio-telemetry. J Mamm 69:379–382
Anthony ELP, Stack MH, Kunz TH (1981) Night roosting and the nocturnal time budget of the little brown bat, Myotis lucifugus: effects of reproductive status, prey density, and environmental conditions. Oecologia 51:151–156
Arlettaz R, Christe P, Lugon A, Perrin N, Vogel P (2001) Food availability dictates the timing of parturition in insectivorous mouse-eared bats. Oikos 95:105–111
Arnold W (1993) Energetics of social hibernation. In: Carey C, Florant GL, Wunder BA, Horowitz B (eds). Life in the cold. Ecological, physiological, and molecular mechanisms. Westview Press, Inc. Boulder, CO, pp 65–80
Audet D, Thomas DW (1996) Evaluation of the accuracy of body temperature measurement using external radio transmitters. Can J Zool 74:1778–1781
Barclay RMR, Kalcounis MC, Crampton LH, Stefan C, Vonhof MJ, Wilkinson L, Brigham RM (1996) Can external radiotransmitters be used to assess body temperature and torpor in bats? J Mamm 77:1102–1106
Bieber C, Lebl K, Stalder G, Geiser F, Ruf T (2014) Body mass dependent use of hibernation: why not prolong the active season, if they can? Funct Ecol 28:167–177
Boratyński JS, Willis CKR, Jefimow M, Wojciechowski MS (2014) Huddling enhances survival of hibernating bats by reducing evaporative water loss. Comp Biochem Physiol A 179:125–132
Both C, Van Asch M, Bijlsma RG, Van Den Burg AB, Visser ME (2009) Climate change and unequal phenological changes across four trophic levels: constraints or adaptations? J Anim Ecol 78:73–83
Boyles JG, Storm JJ, Brack V Jr (2008) Thermal benefits of clustering during hibernation—a field test of competing hypotheses on Myotis sodalis. Funct Ecol 22:632–6369
Czenze ZJ, Park AD, Willis CKR (2013) Staying cold through dinner: cold-climate bats rewarm with conspecifics but not sunset during hibernation. J Comp Physiol B 183:1–8
Frick WF, Reynolds DS, Kunz TH (2010) Influence of climate and reproductive timing on demography of little brown myotis (Myotis lucifugus). J Anim Ecol 79:128–136
Geiser F (2004) Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu Rev Physiol 66:239–274
Geiser F (2013) Hibernation. Curr Biol 23:R188–R193
Geiser F, Brigham RM (2012) The other functions of torpor. In: Ruf T, Arnold W, Bieber C, Millesi E (eds) Living in a seasonal world. Springer, Berlin Heidelberg, pp 109–121
Grahn DA, Miller JD, Houng VS, Heller HC (1994) Persistence of circadian rhythmicity in hibernating ground squirrels. Am J Physiol Regul Integr Comp Physiol 266:251–1258
Heller HC, Ruby NF (2004) Sleep and circadian rhythms in mammalian torpor. Annu Rev Physiol 66:275–289
Hope PR, Jones G (2012) Warming up for dinner: torpor and arousal in hibernating Natterer’s bats (Myotis nattereri) studied by radio telemetry. J Comp Physiol B 182:569–578
Inouye DW, Barr WA, Armitage KB, Inouye BD (2000) Climate change is affecting altitudinal migrants and hibernating species. PNAS 97:1630–1633
Jonasson KA, Willis CKR (2011) Changes in body condition of hibernating bats support the thrifty female hypothesis and predict consequences for populations with white-nose syndrome. PLoS One 6:e21061
Jonasson KA, Willis CKR (2012) Hibernation energetics of little brown bats. J Exp Biol 215:2141–2149
Körtner G, Geiser F (2000) The temporal organization of daily torpor and hibernation: circadian and circannual rhythms. Chronobiol Intern 17:103–128
Lane JE, Kruuk LE, Charmantier A, Murie JO, Dobson FS (2012) Delayed phenology and reduced fitness associated with climate change in a wild hibernator. Nature 489:554–557
Linacre E, Geerts B (1997) Climate and weather explained 1997. Routledge, London
Lovegrove BG, Lobban KD, Levesque DL (2014) Mammal survival at the Cretaceous-Palaeogene boundary: metabolic homeostasis in prolonged tropical hibernation in tenrecs. Proc Roy Soc B. doi:10.1098/rspb.2014.1304
Lutgens FK, Tarbuck EJ (2013) The atmosphere an introduction to meterology, 12th edn. Pearson
Lyman CP (1982) Who is who among the hibernators. In: Lyman CP, Willis JS, Malan A, Wang LCH (eds) Hibernation and torpor in mammals and birds. Academic Press, New York, pp 2–36
Murie JO, Harris MA (1982) Annual variation of spring emergence and breeding in Columbian ground squirrels (Spermophilus columbianus). J Mamm 63:431–439
National Climate Data and Information Archive (2013) Environment Canada. http://www.climate.weatheroffice.gc.ca. Accessed 21 Oct 2013
Negraeff OE, Brigham RM (1995) The influence of moonlight on the activity of little brown bats (Myotis lucifugus). Z Saugetierkd 60:330–336
Norquay KJO, Willis CKR (2014) Hibernation phenology of Myotis lucifugus. J Zool Lon. (In press)
Ozgul A, Childs DZ, Oli MK, Armitage KB, Blumstein DT, Olson LE, Tuljapurkar Sand Coulson T (2010) Coupled dynamics of body mass and population growth in response to environmental change. Nature 466:482–485
Paige KN (1995) Bats and barometric pressure: conserving limited energy and tracking insects from the roost. Funct Ecol 9:463–467
Park KJ, Jones G, Ransome RD (2000) Torpor, arousal and activity of hibernating greater horseshoe bats (Rhinolophus ferrumequinum). Funct Ecol 14:580–588
Perry RW (2012) A review of factors affecting cave climates for hibernating bats in temperate North America. Environ Rev 21:28–39
Pflitsch A, Wiles M, Horrocks R, Piasecki J, Ringeis J (2010) Dynamicclimatologic processes of barometric cave systems using the example of Jewel Cave and Wind Cave in South Dakota, U.S.A. Acta Carsologica 39:449–462
Racey PA, Swift SM (1981) Variations in gestation length in a colony of pipistrelle bats (Pipistrellus pipistrellus) from year to year. J Reprod Fertil 61:123–129
R Development Core Team (2009) R: a language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria ISBN 3-900051-07-0, http://www.R-project.org
Reeder DM, Frank CL, Turner GG, Meteyer CU, Kurta A et al (2012) Frequent arousal from hibernation linked to severity of infection and mortality in bats with white-nose syndrome. PLoS One 7:e38920
Revel FG, Herwig A, Garidou ML, Dardente H, Menet JS, Masson-Pévet M, Simonneaux V, Saboureau M, Pévet P (2007) The circadian clock stops ticking during deep hibernation in the European hamster. Proc Nat Acad Sci USA 104:13816–13820
Ruby NF, Dark J, Burns DE, Heller HC, Zucker I (2002) The suprachiasmatic nucleus is essential for circadian body temperature rhythms in hibernating ground squirrels. J Neurosci 221:357–364
Schorr RA, Lukacs PM, Florant GL (2009) Body mass and winter severity as predictors of overwinter survival in Preble’s meadow jumping mouse. J Mamm 90:17–24
Sheriff MJ, Kenagy GJ, Richter M, Lee T, Tøien Ø, Kohl F, Buck CL, Barnes BM (2011) Phenological variation in annual timing of hibernation and breeding in nearby populations of Arctic ground squirrels. Proc R Soc Biol Sci Ser B 278:2369–2375
Speakman JR, Webb PI, Racey PA (1991) Effects of disturbance on the energy expenditure of hibernating bats. J Appl Ecol 28:1087–1104
Stawski C, Geiser F (2010) Fat and fed: frequent use of summer torpor in a subtropical bat. Naturwissenschaften 97:29–35
Storey KB, Storey JM (2004) Metabolic rate depression in animals: transcriptional and translational controls. Biol Rev 79:207–233
Thomas DW (1995) Hibernating bats are sensitive to nontactile human disturbance. J Mamm 76:940–946
Thomas DW, Fenton BM, Barclay RMR (1979) Social behavior of the little brown bat, Myotis lucifugus: I. Mating behavior. Behav Ecol and Sociobiol 6:129–136
Thomas DW, Dorais M, Bergeron JM (1990) Winter energy budgets and cost of arousals for hibernating little brown bats, Myotis lucifugus. J Mamm 71:475–479
Tøien Ø, Blake J, Edgar DM, Grahn DA, Heller HC, Barnes BM (2011) Hibernation in black bears: independence of metabolic suppression from body temperature. Science 331:906–909
Turbill C (2008) Winter activity of Australian tree-roosting bats: influence of temperature and climatic patterns. J Zool 276:285–290
Turner JM, Warnecke L, Wilcox A, Baloun D, Bollinger TK, Misra V, Willis CKR (2015) Conspecific disturbance contributes to altered hibernation patterns in bats with white-nose syndrome. Physiol Behav 140:71–78
Twente JW, Twente J, Brack V Jr (1985) The duration of the period of hibernation of three species of vespertilionid bats. II. Laboratory studies. Can J Zool 63:2955–2961
Visser ME, Both C (2005) Shifts in phenology due to global climate change: the need for a yardstick. Proc R Soc B 272:2561–2569
Wang LCH (1989) Ecological, physiological, and biochemical aspects of torpor in mammals and birds. In: Wang LCH (ed) Advances in comparative and environmental physiology. Springer Verlag, Berlin, pp 361–401
Warnecke L, Turner JM, Bollinger TK, Lorch JM, Misra V, Cryan PM, Wibbelt G, Blehert DS, Willis CKR (2012) Inoculation of a North American bat with European Geomyces destructans supports the novel pathogen hypothesis for the origin of white-nose syndrome. Proc Nat Acad Sci USA 109:6999–7003
Wilkinson GS, South JM (2002) Life history, ecology and longevity in bats. Aging Cell 1:124–131
Williams CT, Barnes BM, Buck CL (2012a) Body temperature rhythms persist under the midnight sun but are absent during hibernation in free-living arctic ground squirrels. Biol Lett 8:31–34
Williams CT, Barnes BM, Richter M, Buck CL (2012b) Hibernation and circadian rhythms of body temperature in free-living arctic ground squirrels. Physiol Biochem Zool 85:397–404
Willis CKR, Brigham RM (2003) Defining torpor in free-ranging bats: experimental evaluation of external temperature-sensitive radiotransmitters and the concept of active temperature. J Comp Physiol B 173:379–389
Zervanos SM, Salsbury CM, Brown JK (2009) Maintenance of biological rhythms during hibernation in eastern woodchucks (Marmota monax). J Comp Physiol B 179:411–418
Acknowledgments
We thank Q. Webber, D. Baloun, A. Menzies, M.A. Collis and H. Mayberry for essential help with fieldwork and L. McGuire for statistical advice. We also thank M. Wiegand, K. Campbell and three anonymous reviewers for excellent comments on an earlier version of this manuscript. Funding was provided by Grants to CKRW from the Natural Sciences and Engineering Research Council (NSERC), the Canada Foundation for Innovation, Manitoba Research and Innovation Fund, Manitoba Hydro Forest Enhancement Program and the University of Winnipeg Chancellor’s Research Chair.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier.
Rights and permissions
About this article
Cite this article
Czenze, Z.J., Willis, C.K.R. Warming up and shipping out: arousal and emergence timing in hibernating little brown bats (Myotis lucifugus). J Comp Physiol B 185, 575–586 (2015). https://doi.org/10.1007/s00360-015-0900-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-015-0900-1